Endocarditis: Difference between revisions
No edit summary |
|||
| Line 16: | Line 16: | ||
{{SI}} | {{SI}} | ||
{{CMG}} | {{CMG}} | ||
| Line 30: | Line 28: | ||
==[[Endocarditis overview|Overview]]== | ==[[Endocarditis overview|Overview]]== | ||
==[[Endocarditis historical background|Historical Background]] | |||
== Epidemiology and Demographics == | == Epidemiology and Demographics == | ||
Revision as of 14:22, 20 March 2011
| Endocarditis | |
 | |
|---|---|
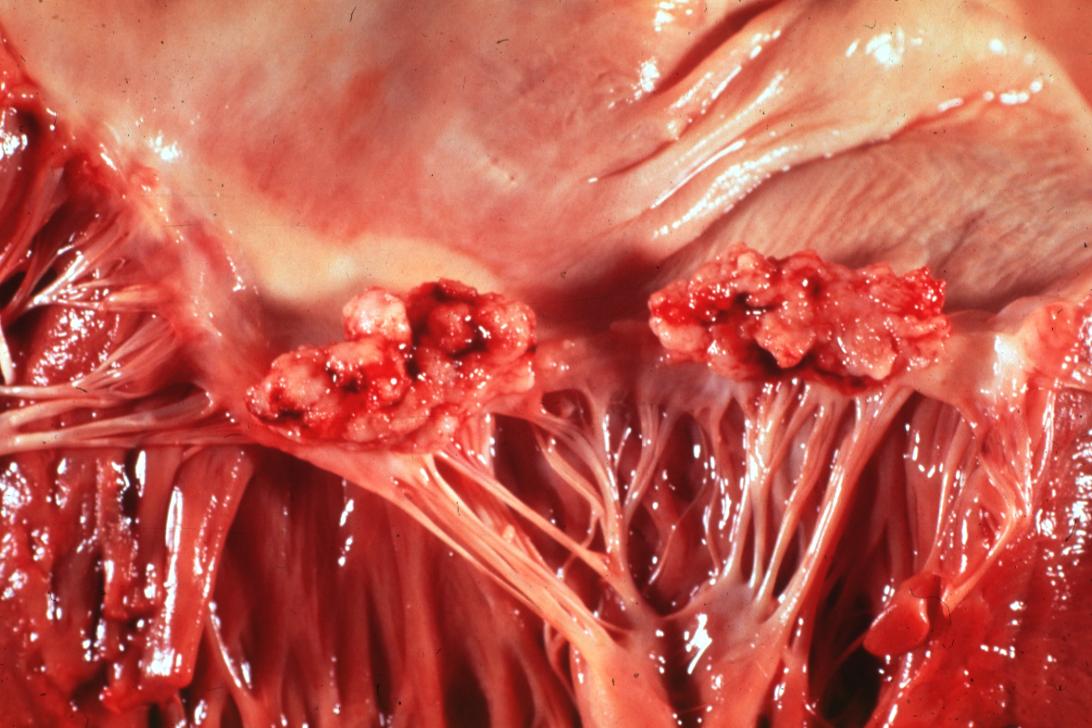
| Mitral valve vegetations in a patient with bacterial endocarditis. | |
| ICD-10 | I33 |
| ICD-9 | 421 |
| DiseasesDB | 4224 |
| MedlinePlus | 001098 |
| eMedicine | emerg/164 med/671 ped/2511 |
| MeSH | D004696 |
|
WikiDoc Resources for Endocarditis |
|
Articles |
|---|
|
Most recent articles on Endocarditis Most cited articles on Endocarditis |
|
Media |
|
Powerpoint slides on Endocarditis |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Endocarditis at Clinical Trials.gov Clinical Trials on Endocarditis at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Endocarditis
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Endocarditis Discussion groups on Endocarditis Patient Handouts on Endocarditis Directions to Hospitals Treating Endocarditis Risk calculators and risk factors for Endocarditis
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Endocarditis |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Join in Editing This Page and Apply to be an Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Related Key Words and Synonyms: Infective endocarditis, IE, subacute bacterial endocarditis, acute bacterial endocarditis, fungal endocarditis, nosocomial infective endocarditis, NIE, intravenous drug abuse endocarditis, intravenous drug abuse infective endocarditis, IVDA endocarditis, IVDA IE, prosthetic valve endocarditis, PVE, pacemaker endocarditis, PM infective endocarditis, PM IE, endocardial infection, native valve endocarditis, NVE, HACEK infection, bloodstream infection, marantic endocarditis
Overview
Epidemiology and Demographics
The incidence of IE is approximately 2-4 cases per 100,000 persons per year worldwide. This rate has not changed in the past 5-6 decades.
IE may occur in a person of any age. The frequency is increasing in elderly individuals, with 25-50% of cases occurring in those older than 60 years of age. The occurrence of IE is 3 times more common in males than in females.
Risk Factors
Adults and children with underlying cardiac conditions placing them at highest risk for adverse outcomes of infective endocarditis (IE) including those with:
- Prosthetic cardiac valve or prosthetic cardiac valve repair
- Previous infective endocarditis
- Congenital heart disease (CHD) associated with
- Unrepaired cyanotic CHD, including palliative shunts and conduits
- Completely repaired congenital heart defect with prosthetic material or device, whether placed by surgery or by catheter intervention, during the first 6 months after the procedure
- Repaired CHD with residual defects at the site or adjacent to the site of a prosthetic patch or prosthetic device (which inhibit endothelialization)
- Cardiac transplantation patients who develop cardiac valvulopathy
Screening
Among those patients at high risk, careful monitoring should be undertaken to detect the early development of complications such as:
- Valvular dysfunction, usually insufficiency of the mitral or aortic valves;
- Myocardial or septal abscesses
- Congestive heart failure
- Metastatic infection
- Embolic phenomenon
Pathophysiology & Etiology
As previously mentioned, altered blood flow around the valves is a risk factor for the development of endocarditis. The valves may be damaged congenitally, from surgery, by auto-immune mechanisms, or simply as a consequence of old age. The damaged part of a heart valve becomes covered with a blood clot, a condition known as non-bacterial thrombotic endocarditis (NBTE).
In a healthy individual, a bacteremia (where bacteria get into the blood stream through a minor cut or wound) would normally be cleared quickly with no adverse consequences. If a heart valve is damaged and covered with thrombus, these structures can provide a nidus for bacteria to attach themselves and an infection can be established.
The bacteremia is often caused by dental procedures, such as a cleaning or extraction of a tooth. It is important that a dentist or a dental hygienist therefore be told of any heart problems before beginning the procedure. Prophylactic antibiotics are administered to patients with certain heart conditions as a precaution.
Another cause of infective endocarditis is a scenario in which an excess number of bacteria enter the bloodstream. Colorectal cancer, serious urinary tract infections, and IV drug use can all introduce large numbers of such bacteria. When a large burden of bacteria are introduced, a normal heart valve may be infected. A more virulent organism (such as Staphylococcus aureus, but see below for others) is often responsible for infecting a normal valve.
Infections of the tricuspid valve and less frequently the pulmonic valve tend to occur in intravenous drug users given the high pathogen burden from their introduction in the vein. The diseased valve is most commonly affected when there is a pre-existing disease. In rheumatic heart disease this is the aortic valve and the mitral valves, on the left side of the heart.
Complications of endocarditis can occur as a result of the locally destructive effects of the infection. These complications include perforation of valve leaflets, perforation of fistula between blood vessels or cardiac chambers, abscesses and disruption of conduction system.
Natural History and Complications
Complications of infective endocarditis include the following:
- Cardiac
- Murmur
- New aortic diastolic murmur suggests dilatation of the aortic annulus or eversion, rupture, or fenestration of an aortic leaflet
- Sudden onset of loud mitral pansystolic murmur suggests rupture of chorda tendineae or fenestration of a mitral valve leaflet
- Congestive heart failure
- Cardiac rhythm disturbances
- Occasionally, pericarditis
- Cutaneous
- Petechiae of the conjunctiva, oropharynx, skin, and legs
- Linear subungual splinter haemorrhages of the lower or middle nail bed
- Oslers nodes
- Janeway lesions
- Musculoskeletal
- Myalgias
- Arthralgias
- Arthritis
- Low back pain
- Rheumatoid factor in up to 50% of patients with endocarditis for > 6 wk
- Clubbing of fingers in < 15% of patients
- Ocular
- Petechial hemorrhages,
- Flame-shaped hemorrhages,
- Roth's spots,
- Cotton-wool exudates in the retina
- Embolic
- Significant arterial emboli occur in 30%–50% of patients, causing the following:
- Stroke
- Monocular blindness
- Acute abdominal pain, ileus, and melena
- Pain and gangrene in the extremities
- CNS emboli are common
- Coronary emboli, often asymptomatic, can cause myocardial infarction
- Pulmonary emboli common in right-sided endocarditis, causing pulmonary infarcts or focal pneumonitis
- Splenic
- Splenomegaly in 15%–30% of patients
- Splenic infarcts in up to 40% of patients
- Splenic abscesses in ~ 5% of patients
- Renal
- Microscopic hematuria in ~ 50% of patients
- Embolic renal infarction
- Diffuse membranoproliferative glomerulonephritis
- Mycotic aneurysms
Occur in any artery in 2%–8% of patients, causing the following:
- Pain or headache
- Pulsatile mass
- Fever
- Sudden expanding hematoma
- Signs of major blood loss
- Neurologic
- Neurologic complications occur in 25%–40% of cases
- Strokes caused by cerebral embolisms in ~ 15% of cases, causing the following:
- Altered level of consciousness
- Seizures
- Fluctuating focal neurologic signs
- Cerebral aneurysms occur in 1%–5% of cases, causing the following:
- Headache
- Focal signs
- Acute intracerebral or subarachnoid hemorrhage caused by rupture
- Mild meningeal irritation resulting from slow leakage
- Brain abscesses may occur in acute endocarditis caused by Staphylococcus aureus
- Seizures
Diagnosis
In general, a patient should fulfill the Duke Criteria[1] in order to establish the diagnosis of endocarditis.
As the Duke Criteria relies heavily on the results of echocardiography, research has addressed when to order an echocardiogram by using signs and symptoms to predict occult endocarditis among patients with intravenous drug abuse[2][3][4] and among non drug abusing patients [5][6]. Unfortunately, this research is over 20 years old and it is possible that changes in the epidemiology of endocarditis and bacteria such as staphylococcus make the following estimates incorrectly low.
Among patients who do not use illicit drugs and have a fever in the emergency room, there is a less than 5% chance of occult endocarditis. Mellors [6] in 1987 found no cases of endocarditis nor of staphylococcal bacteremia among 135 febrile patients in the emergency room. The upper confidence interval for 0% of 135 is 5%, so for statistical reasons alone, there is up to a 5% chance of endocarditis among these patients. In contrast, Leibovici [5] found that among 113 non-selected adults admitted to the hospital because of fever there were two cases (1.8% with 95%CI: 0% to 7%) of endocarditis.
Among patients who do use illicit drugs and have a fever in the emergency room, there is about a 10% to 15% prevalence of endocarditis. This estimate is not substantially changed by whether the doctor believes the patient has a trivial explanation for their fever[4]. Weisse[2] found that 13% of 121 patients had endocarditis. Marantz [4] also found a prevalence of endocarditis of 13% among such patients in the emergency room with fever. Samet [3] found a 6% incidence among 283 such patients, but after excluding patients with initially apparent major illness to explain the fever (including 11 cases of manifest endocarditis), there was a 7% prevalence of endocarditis.
Among patients with staphylococcal bacteremia (SAB), one study found a 29% prevalence of endocarditis in community-acquired SAB versus 5% in nosocomial SAB[7]. However, only 2% of strains were resistant to methicillin and so these numbers may be low in areas of higher resistance.
Common Causes
Many types of organism can cause infective endocarditis. These are generally isolated by blood culture, where the patient's blood is removed, and any growth is noted and identified.
Alpha-haemolytic streptococci, that are present in the mouth will often be the organism isolated if a dental procedure caused the bacteraemia.
If the bacteraemia was introduced through the skin, such as contamination in surgery, during catheterisation, or in an IV drug user, Staphylococcus aureus is common.
A third important cause of endocarditis is Enterococci. These bacteria enter the bloodstream as a consequence of abnormalities in the gastrointestinal or urinary tracts. Enterococci are increasingly recognized as causes of nosocomial or hospital-acquired endocarditis. This contrasts with alpha-haemolytic streptococci and Staphylococcus aureus which are causes of community-acquired endocarditis.
Some organisms, when isolated, give valuable clues to the cause, as they tend to be specific.
- Candida albicans, a yeast, is associated with IV drug users and the immunocompromised. Fungal endocarditis accounts for 5% of cases of native endocarditis and 10% of cases of prosthetic valve endocarditis. A diagnosis of fungal endocarditis is difficult, because many patients are afebrile with a normal white blood cell count (WBC). The fungus is often difficult to culture, and blood cultures typically negative. Fungal infections often result in large vegetations, systemic embolization, myocardial invasion, and are extremely resistant to medical therapy. Early surgical intervention is warranted because medical mortality approaches 100% Anti-fungal therapy for life is required.
- Pseudomonas species, which are very resilient organisms that thrive in water, may contaminate street drugs that have been contaminated with drinking water. P. aeruginosa can infect a child through foot punctures, and can cause both endocarditis and septic arthritis.[8]
- Streptococcus bovis and Clostridium septicum, which are part of the natural flora of the bowel, are associated with colonic malignancies. When they present as the causative agent in endocarditis, it usually call for a concomitant colonoscopy due to worries regarding hematogenous spread of bacteria from the colon due to the neoplasm breaking down the barrier between the gut lumen and the blood vessels which drain the bowel.[9]
- HACEK organisms are a group of bacteria that live on the dental gums, and can be seen with IV drug abusers who contaminate their needles with saliva. Patients may also have a history of poor dental hygiene, or pre-existing valvular disease.[10]
Differential Diagnosis of Risk Factors for or Causes of Endocarditis
| Cardiovascular | • Asymmetric septal hypertrophy • Calcific aortic stenosis • Cardiac catheterization • Cardiac surgery • Congenital Heart Disease • Mitral valve prolapse • Prosthetic heart valve • Septal defects • Valve disease • Previous bacterial endocarditis • Rheumatic Heart Disease • Sclerotherapy • Cardiac myxoma |
| Chemical / poisoning | No underlying causes |
| Dental | • Dental extractions • Dental implants • Root canals • |
| Dermatologic | • Skin infection • |
| Drug Side Effect | • IV drug use • |
| Ear Nose Throat | • Adenoidectomy • |
| Endocrine | No underlying causes |
| Environmental | No underlying causes |
| Gastroenterologic & Genito-Uriner | • Biliary tract surgery • Cystoscopy • Endoscopic retrograde cholangiopancreatography • Urethral dilation • Prostatic surgery • |
| Genetic | • Marfan's Syndrome • |
| Hematologic | No underlying causes |
| Iatrogenic | No underlying causes |
| Infectious Disease | • Diphtheria • Staphylococcus epidermidis • Staphylococcus aureus • Streptococcus bovis • Viridans streptococci • Group A streptococcus • Gram negative rods • Enterococuss • Candida • Tuberculosis • Salmonellosis |
| Musculoskeletal / Ortho | No underlying causes |
| Neurologic | No underlying causes |
| Nutritional / Metabolic | No underlying causes |
| Obstetrics & Gynecology | • Childbirth • |
| Oncologic | No underlying causes |
| Opthalmologic | No underlying causes |
| Overdose / Toxicity | No underlying causes |
| Psychiatric | No underlying causes |
| Pulmonary | • Respiratory infection • Respiratory tract procedures • |
| Renal / Electrolyte | No underlying causes |
| Rheum / Immune / Allergy | • Juvenile rheumatoid arthritis • Polymyalgia rheumatica • Acute rheumatic fever • Polyarteritis nodosa • Systemic lupus erythematosus • Antiphospholipid antibody syndrome • |
| Trauma | No underlying causes |
| Miscellaneous | • Surgical systemic-pulmonary shunts and conduits • |
History and Symptoms
A. Subacute Bacterial Endocarditis (SBE)
- Insidious onset
- Fever
- Sweats
- Weakness
- Myalgias
- Arthralgias
- Malaise
- Anorexia
- Fatigue
- Splenomegaly, clubbing, and Oslers nodes in long-standing SBE
B. Acute Bacterial Endocarditis
C. Endocarditis Associated with Parenteral Drug Use
- High fevers, chills, rigors, malaise, cough, and pleuritic chest pain
- Septic pulmonary emboli causing sputum production, hemoptysis, and signs suggesting pneumonia
- Cardiac murmurs
- Tricuspid insufficiency
- Metastatic infections
- Neurologic manifestations
- Peripheral emboli
D. Prosthetic Valve Endocarditis
- Occurs in 1%–2% of cases at 1 yr and in 4%–5% of cases at 4 yr after implantation
- Infection of perivalvular tissues
- Valvular dysfunction
- Myocardial abscesses
- Fever
- Petechiae, Roth's spots, Osler's nodes, Janeway lesions
- Emboli
Physical Examination
Vital Signs
A fever will likely be present. Rigors may be present.
Skin
- Petechiae 10 - 40%
- Splinter haemorrhage 5 - 15%
- Oslers nodes 7 - 10% (tender subcutaneous nodules in pulp of digits)
- Janeway lesion 6 - 10% (erythematous, nontender lesions on palm or sole)
Eyes
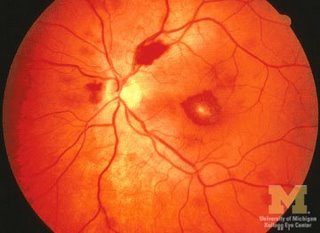
Ear Nose and Throat
In patients in whom there is new acute onset of aortic regurgitation, bobbing of the uvula may be present.
Heart
- Heart Murmurs: 80 - 85%
Lungs
Signs of heart failure may present
Abdomen
- Abdominal pain may be present due to mesenteric embolization or ileus
- Splenomegaly may be found in 15-30% patients. Left upper quadrant (LUQ) pain may be present as a result of a splenic infarct from embolization
- Flank pain may be present as a result of an embolus to the kidney
Extremities
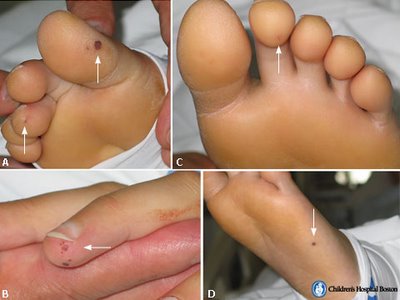
- Janeway lesions (painless hemorrhagic cutaneous lesions on the palms and soles)
- Gangrene of fingers may occur
- The fingers may show splinter haemorrhages
- Osler's nodes (painful subcutaneous lesions in the distal fingers)
Neurologic
Septic emboli may result in stroke and focal neurologic findings
Intracranial hemorrhage may occur
Symptoms Frequency
- Fever 80 - 85%, often spiking
- Chills 42 - 75%
- Anorexia 25 - 55%
- Malaise 25 - 40%
- Weight loss 25 - 35%
- Back pain
- Stroke may be present in 10 - 15% of patients as a result of cerebral embolization
- Chest pain may be present as a result of embolzation in the coronary artery. The infarcts are usually not transmural. Pulmonary emboli, often septic, occur in 75% of patients with tricuspid endocarditis
- Abdominal pain may be present due to mesenteric embolization or ileus
- Blindness may be present due to retinal embolization in 3% of patients
Laboratory Findings
An elevated erythrocyte sedimentation rate is present
A marked leukocytosis is present
A positive serum rheumatoid factor may be present (in approximately 50% of patients with subacute disease). It becomes negative after successful treatment.
The serum BUN and Cr may be elevated if glomerulonephritis is present
Urinalysis
Glomerulonephritis may be present
Electrocardiogram
There is no spesific EKG changes for diagnosis of Infective Endocarditis. EKG may help to detect the 10% of patients who develop a conduction delay during Infective Endocarditis by documenting an increased PR interval.
Chest X Ray
There are no specific chest x-ray findings specific for the diagnosis of endocarditis. Non specific findings would include findings of congestive heart failure.
MRI and CT
- A CT scan of the head should be obtained in patients who exhibit CNS symptoms or findings consistent with a mass effect (eg, macroabscess of the brain).
Echocardiography
Pathology
Differential Diagnosis
- Antiphospholipid syndrome
- Reactive arthritis
- Atrial myxoma
- Systemic lupus erythematosus
- Primary cardiac neoplasms
- Lyme disease
- Polymyalgia rheumatica
- Thrombotic nonbacterial endocarditis
- Vasculitis
- Temporal arteritis
Treatment
High dose antibiotics are administered by the intravenous route to maximize diffusion of antibiotic molecules into vegetation(s) from the blood filling the chambers of the heart. This is necessary because neither the heart valves nor the vegetations adherent to them are supplied by blood vessels. Antibiotics are continued for a long time, typically two to six weeks. Specific drug regimens differ depending on the classification of the endocarditis as acute or subacute (acute necessitating treating for Staphylococcus aureus with oxacillin or vancomycin in addition to gram-negative coverage). Fungal endocarditis requires specific anti-fungal treatment, such as amphotericin B.[11]
Surgical removal of the valve is necessary in patients who fail to clear micro-organisms from their blood in response to antibiotic therapy, or in patients who develop cardiac failure resulting from destruction of a valve by infection. A removed valve is usually replaced with an artificial valve which may either be mechanical (metallic) or obtained from an animal such as a pig; the latter are termed bioprosthetic valves.[11]
Infective endocarditis is associated with a 10-25% mortality.
Primary Prevention (adapted from guidelines from the American Heart Association)
Amoxicillin is the preferred choice for oral therapy because it is well absorbed in the gastrointestinal (GI) tract and provides high and sustained serum concentrations. For individuals who are allergic to penicillins or amoxicillin, the use of cephalexin or another first-generation oral cephalosporin, clindamycin, azithromycin, or clarithromycin is recommended.
Because of possible cross-reactions, a cephalosporin should not be administered to patients with a history of anaphylaxis, angioedema, or urticaria after treatment with any form of penicillin, including ampicillin or amoxicillin.
Patients who are unable to tolerate an oral antibiotic may be treated with ampicillin, ceftriaxone, or cefazolin administered intramuscularly or intravenously. For ampicillin-allergic patients who are unable to tolerate an oral agent, therapy is recommended with parenteral cefazolin, ceftriaxone, or clindamycin. [12]
- List of antibiotics can be used for prophylaxis of Infective Endocarditis;
- POTENTIAL HARM: The administration of prophylactic antibiotics is not risk free. Additionally, the widespread use of antibiotic therapy promotes the emergence of resistant microorganisms most likely to cause endocarditis, such as viridans group streptococci and enterococci. The frequency of multidrug-resistant viridans group streptococci and enterococci has increased dramatically during the past 2 decades. This increased resistance has reduced the efficacy and number of antibiotics available for the treatment of infective endocarditis.[12]
- CONTRAINDICATIONS: Cephalosporins should not be used in an individual with a history of anaphylaxis, angioedema, or urticaria with penicillins or ampicillin.[12]
Antibiotic prophylaxis with Respiratory Tract Procedures
Currently there is no published data conclusively demonstrate a link between these procedures and Infective Endocarditis.
Antibiotic prophylaxis is reasonable for high risk patients who undergo an invasive procedure of the respiratory tract that involves incision or biopsy of the respiratory mucosa, such as tonsillectomy and adenoidectomy.
Antibiotic prophylaxis for bronchoscopy is not usually recommended unless the procedure involves incision of the respiratory tract mucosa. For high risk patients who undergo an invasive respiratory tract procedure to treat an established infection, such as drainage of an abscess or empyema, antibiotic regimen should contain an agent active against Streptococcus viridans.
If the infection is known or suspected to be caused by Staphylococcus aureus, the regimen should contain an agent active against S aureus, such as an antistaphylococcal penicillin or cephalosporin, or vancomycin in patients unable to tolerate a beta-lactam. Vancomycin should be administered if the infection is known or suspected to be caused by a methicillin-resistant Staphylococcus aureus.
As a summary; individuals at the highest risk group who need procedures listed below require proper antibiotic prophylaxis;[12]
- Tonsillectomy
- Adenoidectomy
- Rigid bronchoscopic manipulations
- Respiratory mucosa related surgery
Antibiotic prophylaxis with GI or Genitourinary (GU) Tract Procedures
The administration of prophylactic antibiotics solely to prevent endocarditis is not recommended for patients who undergo GU or GI tract procedures, including diagnostic esophagogastroduodenoscopy or colonoscopy.
The significance of the increased frequency of multiresistant strains of enterococci on prevention of IE in patients who undergo GI or GU tract procedures is unknown. The high prevalence of resistant strains of enterococci adds further doubt about the efficacy of prophylactic therapy for GI or GU tract procedures. Patients with infections of the GI or GU tract may have intermittent or sustained enterococcal bacteremia.
For patients with high risk conditions who have an established GI or GU tract infection or for those who receive antibiotic therapy to prevent wound infection or sepsis associated with a GI or GU tract procedure, it may be reasonable that the antibiotic regimen include an agent active against enterococci, such as penicillin,ampicillin, piperacillin, or vancomycin; however, currently no published studies demonstrate that such therapy would prevent enterococcal IE.
For patients with high risk conditions and scheduled for an elective cystoscopy or other urinary tract manipulation who have an enterococcal urinary tract infection or colonization, antibiotic therapy to eradicate enterococci from the urine before the procedure may be reasonable. If the urinary tract procedure is not elective, it may be reasonable that the empiric or specific antimicrobial regimen administered to the patient contain an agent active against enterococci.
Amoxicillin or ampicillin is the preferred agent for enterococcal coverage for these patients. Vancomycin may be administered to patients unable to tolerateampicillin. If infection is caused by a known or suspected strain of resistant enterococcus, consultation with an infectious diseases expert is recommended.
As a summary; individuals at the highest risk group who need procedures listed below require proper antibiotic prophylaxis;[12]
Primary Prevention in Specific Situations and Circumstances
Patients Already Receiving Antibiotics
If a patient is already receiving long-term antibiotic therapy with an antibiotic that is also recommended for Infective Endocarditis prophylaxis for a dental procedure, it is prudent to select an antibiotic from a different class rather than to increase the dosage of the current antibiotic. For example, antibiotic regimens used to prevent the recurrence of acute rheumatic fever are administered in dosages lower than those recommended for the prevention of Infective Endocarditis.
Individuals who take an oral penicillin for secondary prevention of rheumatic fever or for other purposes are likely to have viridans group streptococci in their oral cavity that are relatively resistant to penicillin or amoxicillin. In such cases, the provider should select eitherclindamycin, azithromycin, or clarithromycin for IE prophylaxis for a dental procedure, but only for patients shown in the table above. Because of possible cross-resistance of viridans group streptococci with cephalosporins, this class of antibiotics should be avoided.
If possible, it would be preferable to delay a dental procedure until at least 10 days after completion of the antibiotic therapy. This may allow time for the usual oral flora to be reestablished. [11] [12]
Patients Who Receive Anticoagulants
Intramuscular injections for Infective Endocarditis prophylaxis should be avoided in patients who are receiving anticoagulant therapy. In these circumstances, orally administered regimens should be given whenever possible. Intravenously administered antibiotics should be used for patients who are unable to tolerate or absorb oral medications. [11] [12]
Patients Who Undergo Cardiac Surgery
Patients who undergo surgery for placement of prosthetic heart valves or prosthetic intravascular or intracardiac materials are at risk for the development of infection. Because the morbidity and mortality of infection in these patients are high, perioperative prophylactic antibiotics are recommended.
Early-onset prosthetic valve endocarditis is most often caused by S. aureus, coagulase-negative staphylococci, or diphtheroids. No single antibiotic regimen is effective against all these microorganisms. Prophylaxis at the time of cardiac surgery should be directed primarily against staphylococci and should be of short duration. A first-generation cephalosporin is most often used, but the choice of an antibiotic should be influenced by the antibiotic susceptibility patterns at each hospital. For example, a high prevalence of infection by methicillin-resistant Staphylococcus aureus (MRSA) should prompt the consideration of the use of vancomycin for perioperative prophylaxis.
The majority of nosocomial coagulase-negative staphylococci are methicillin-resistant. Nonetheless, surgical prophylaxis with a first-generation cephalosporin may be recommended for these patients. In hospitals with a high prevalence of methicillin-resistant strains of S. epidermidis, surgical prophylaxis with vancomycin may be reasonable but has not been shown to be superior to prophylaxis with a cephalosporin.
Prophylaxis should be initiated immediately before the operative procedure, repeated during prolonged procedures to maintain serum concentrations intraoperatively, and continued for no more than 48 hours postoperatively to minimize emergence of resistant microorganisms. The effects of cardiopulmonary bypass and compromised renal function on antibiotic concentrations in serum should be considered and dosages adjusted as necessary before and during the procedure. [11] [12]
Pharmacotherapy
Effective treatment requires identification of the etiologic agent and determination of its antimicrobial susceptibility.
Antibiotic therapy for subacute or indolent disease can be delayed until results of blood cultures are known; in fulminant infection or valvular dysfunction requiring urgent surgical intervention, begin empirical antibiotic therapy promptly after blood cultures have been obtained.
For prosthetic valve endocarditis, treatment should be continued for 6–8 weeks.
Acute Pharmacotherapies
Penicillin-Susceptible Viridans and Other Nonenterococcal Streptococci[11]
The minimum inhibitory concentration [MIC] <0.2 µg/ml
- Penicillin G: preferred regimen
Dose: 12–18 million units I.V. daily in divided doses q. 4 hour for 4 weeks
- Penicillin G + gentamicin or ceftriaxone: preferred regimen
Dose: penicillin G, 12–18 million units I.V. daily in divided doses q. 4 hour for 4 weeks; gentamicin, 3 mg/kg I.M. or I.V. daily in divided doses q. 8 hour for 2 weeks (peak serum concentration should be ~ 3 µg/ml and trough concentrations < 1 µg/ml); ceftriaxone, 2 g I.V. daily as a single dose for 2 weeks
- Vancomycin: for patients with history of penicillin hypersensitivity
Dose: 30 mg/kg I.V. daily in divided doses q. 12 hour for 4 weeks
Relatively Penicillin-Resistant Streptococci[11]
A- MIC 0.2–0.5 µg/ml
- Penicillin G + gentamicin: preferred regimen
Dose: penicillin G, 20–30 million units I.V. daily in divided doses q. 4 hour for 4 weeks; gentamicin, 3 mg/kg I.M. or I.V. daily in divided doses q. 8 hr for 2 wk (peak serum concentration should be ~ 3 µg/ml and trough concentrations < 1 µg/ml)
B- MIC > 0.5 µg/ml
- Penicillin G + gentamicin: preferred regimen
Dose: penicillin G, 20–30 million units I.V. daily in divided doses q. 4 hour for 4 week; gentamicin, 3 mg/kg I.M. or I.V. daily in divided doses q. 8 hour for 4 week (peak serum concentration should be ~ 3 µg/ml and trough concentrations < 1 µg/ml)
- Vancomycin: regimen for patients with history of penicillin hypersensitivity
Dose: 30 mg/kg I.V. daily in divided doses q. 12 hour for 4 weeks
Enterococci[11]
- Penicillin G + gentamicin: preferred regimen
Dose: penicillin G, 20–30 million units I.V. daily in divided doses q. 4 hr for 4–6 weeks; gentamicin, 3 mg/kg I.M. or I.V. daily in divided doses q. 8 hour for 4–6 weeks (peak serum concentration should be ~ 3 µg/ml and trough concentrations < 1 µg/ml)
Dose: ampicillin, 12 g I.V. daily in divided doses q. 4 hour for 4–6 weeks; gentamicin, dose as above
- Vancomycin + gentamicin: regimen for patients with history of penicillin hypersensitivity
Dose: vancomycin, 30 mg/kg I.V. daily in divided doses q. 12 hour for 4–6 weeks; gentamicin, dose as above
Staphylococci (Methicillin Susceptible) in the Absence of Prosthetic Material[11]
- Nafcillin or oxacillin + gentamicin (optional): preferred regimen
Dose: nafcillin or oxacillin, 12 g I.V. daily in divided doses q. 4 hour for 4–6 weeks; gentamicin, 3 mg/kg I.M. or I.V. daily in divided doses q. 8 hr for 3–5 days (peak serum concentration should be ~ 3 µg/ml and trough concentrations <1 µg/ml)
- Cefazolin + gentamicin (optional): alternative regimen for patients with history of penicillin hypersensitivity
Dose: cefazolin, 12 g I.V. daily in divided doses q. 4 hour for 4–6 weeks; gentamicin, dose as above
- Vancomycin: alternative regimen for patients with history of penicillin hypersensitivity
Dose: 30 mg/kg I.V. daily in divided doses q. 12 hr for 4–6 weeks
Staphylococci (Methicillin Resistant) in the Absence of Prosthetic Material[11]
Dose: 30 mg/kg I.V. daily in divided doses q. 12 hour for 4–6 weeks
Staphylococci (Methicillin Susceptible) in the Presence of Prosthetic Material[11]
- Nafcillin or oxacillin + rifampin + gentamicin
Dose: nafcillin or oxacillin, 12 g I.V. daily in divided doses q. 4 hour for 6–8 weeks; rifampin, 300 mg p.o., q. 8 hour for 6–8 weeks; gentamicin (administer during the initial 2 weeks), 3 mg/kg I.M. or I.V. daily in divided doses q. 8 hour for 2 weeks
Staphylococci (Methicillin Resistant) in the Presence of Prosthetic Material[11]
Dose: vancomycin, 30 mg/kg I.V. daily in divided doses q. 12 hour for 6–8 weeks; rifampin, 300 mg p.o., q. 8 hour for 6–8 weeks; gentamicin (administer during the initial 2 weeks), 3 mg/kg I.M. or I.V. daily in divided doses q. 8 hour for 2 weeks
HACEK Organisms[11]
- Ceftriaxone or another third-generation cephalosporin
Dose: 2 g I.V. daily as a single dose for 4 weeks
Antithrombotic Therapy[11]
- Anticoagulants can cause or worsen hemorrhage in patients with endocarditis but may be carefully administered when needed
- Prothrombin time should be carefully maintained at INR of 2.0–3.0
- Anticoagulation should be reversed immediately in the event of CNS complications and interrupted for 1–2 wk after acute embolic stroke
- Avoid heparin administration during active endocarditis if possible
Chronic Pharmacotherapies [11]
Surgery and Device Based Therapy
Indications for Surgery
Indications for surgical debridement of vegetations and infected perivalvular tissue, with valve replacement or repair as needed are listed below:[11]
- Moderate to severe congestive heart failure due to valve dysfunction
- Unstable valve prosthesis
- Uncontrolled infection for > 1–3 week despite maximal antimicrobial therapy
- Persistent bacteremia
- Fungal endocarditis
- Relapse after optimal therapy (prosthesis)
- Vegetation in Situ
- Prosthetic valve endocarditis with perivalvular invasion
- Endocarditis caused by Pseudomonas aeruginosa or other gram-negative bacilli that has not responded after 7–10 days of maximal antimicrobial therapy
- Perivalvular extension of infection and abscess formation
- Staphylococcal infection of prosthesis
- Persistent fever (culture negative)
- Large vegetation (>10 mm = increased embolism)
- Relapse after optimal therapy (native valve)
- Vegetations that obstruct the valve orifice
Principles of Surgical Management
- Excise all infected valve tissue
- Drain and debride abscess cavities
- Repair or replace damaged valves
- Repair associated pathology such as septal defect, fistulas [11]
Aortic Valve - Surgical Options
If the infection limited is limited to the leaflets, then replace the aortic valve.
If the infection extends to anulus or beyond, then debride the infected tissues, drain any abscesses to the pericardial sac and replace the aortic root.
Atrioventricular Valve - Surgical Options
If the infection is limited to the leaflets, then perform a vegectomy, repair perforations, and perform a reduction annuloplasty
If the infection extends to the anulus or beyond, then perform valve replacement, debride and abliterate abscesses. In some cases the tricuspid valve may be excised, but 20-30% of patients will develop congestive heart failure.
Surgical Outcomes
Operative mortality is 15 - 20%. The development of an infection of a prosthetic valve during operation for native valve endocarditis is 4%, it is higher (12 - 16%) if active endocarditis is present at the time of the surgery. Late survival at 5 years for native valve endocarditis is 70 - 80% and for prosthetic valve endocarditis is 50 - 80%.[11]
Antibiotic Prophylaxis with Dental Procedures
Background
- Endodontic procedures have been associated with a high incidence of bacteremia since the 1920s. Therefore, dental procedures were implicated as an independent risk factor for the development of bacterial endocarditis.
- However, only 4%-7.5% of all bacterial endocarditis cases are related to endodontic associated bacteremia.[13]
- AHA Guidelines
In 1955, the American Heart Association (AHA) published the first of ten subsequent IE prevention guidelines. Most recent, 2007 guidelines underwent changes intended to clarify patient eligibility criteria for receiving IE prophylaxis.
- Major changes include, the consideration that frequent exposures to bacteremias associated with daily activities are considered more likely to induce IE then are endodontic-procedural induced bacteremias.
- Optimal oral hygiene is emphasized as an important practice for IE prevention.
- A patient’s lifetime acquisition risk of IE is no longer a consideration for initiating prophylactic antibiotic therapy.
- The AHA now recommends the administration of single-dose prophylactic antibiotics prior to endodontic procedure only to patients with cardiac conditions associated with the highest risk of adverse outcomes following the acquisition of bacterial endocarditis.
2007 AHA Guideline Recommendation for Which patients require Antiobiotic Prophylaxis Prior to Dental Procedure are those with documented
- Prosthetic cardiac valve.
- Previous IE
- Cardiac transplantation recipients who develop cardiac valvulopathy.
- Congential heart disease:
- Unrepaired cyanotic CHD: shunts and conduits
- Completely repaired congential heart defect during 1st 6months following procedure.
- Repaired CHD with residual defects at site of prostetic patch or device.
Endodontic Procedures and Endocarditis risk
- Following dental intervention, the absolute risk for developing IE is estimated at 1 case per 14 million dental procedures.[14]
- IE acquisition risk estimates following an endodontic induced bacteremia in patients with congenital heart disease are 1 per 475,000; rheumatic heart disease, 1 per 142,000; prosthetic heart valve, 1 per 114,000; and previous IE, 1 per 95,000 dental procedures.[15]
- Inocula of 1 x 108 (100 million) colony forming units [cfu]/mL or greater are required to consistently induce experimental endocarditis.
- Recent human quantitative blood culture data support the implication that endodontic associated bacteremia inocula are of insufficient magnitude to induce endocarditis. Bacteremia intensities immediately following invasive human dental procedures are 1.5 cfu/ml-5.9 cfu/ml 10 fold less then necessary to induce IE in the animal model.[16]
- The most recent case-control study of 104 patients with known, high-risk structural heart disease discovered that patients who developed IE were actually less likely to have experienced an endodontic procedure within the 180 days prior to diagnosis than did control patients who did not develop IE (OR 0.2 [95% CI 0.04-0.7]).[17]
- Among high-risk patients with underlying structural heart disease, kidney disease (OR 16.9 [95% CI 1.5-193.0]), diabetes (OR 2.7 [95% CI 1.4-5.2]) and skin flora infection (OR 3.5 [95% CI 0.7-17.0]) were associated with a greater risk for the development of bacterial endocarditis.[18]
- Daily activities such as chewing and oral hygiene practices result in bacteremias more frequently, of longer duration and of greater magnitude in comparison to high-risk endodontic procedures.
These findings have led the AHA to conclude that the cumulative background bacteremia associated with chewing, daily dental hygiene practices, kidney disease, diabetes, and skin colonization present a greater risk of significant bacteremia than any single invasive dental procedure.
Prophylactic Antibiotic Efficacy
- The efficacy of antibiotic regimens for IE prophylaxis has never been assessed under the scrutiny of a randomized controlled trial.
- Evidence supporting pre-endodontic chemoprophylaxis efficacy is extrapolated from data demonstrating reductions in bacteremia magnitudes immediately following the administration of antibiotics.
Antibiotic prophylaxis is reasonable for patients with the conditions who undergo any dental procedure that involves the gingival tissues or periapical region of a tooth and for those procedures that perforate the oral mucosa. Although Infective Endocarditis prophylaxis is reasonable for these patients, its effectiveness is unknown.
Efficacy Assesment
The Cochran Collaboration assessed whether prophylactic administration of penicillin to moderate- to high-risk patients prior to endodontic intervention conferred a mortality, serious illness or endocarditis incidence benefit.[19]
- The pooled, adjusted Odds Ratio across all studies for the development of IE among patients receiving prophylaxis was non-significant (0.56 [95% CI (0.15-2.15)]).
The Cochrane Collaboration concluded; it is unclear whether antibiotic prophylaxis is effective and there is a lack of evidence to support published guidelines using penicillin as chemoprophylaxis for IE.
' To date, only 4 case-control studies has assessed antibiotic efficacy for IE prevention.'
- Strom et al discovered the administration pre-endodontic procedural antibiotics did not provide a protective benefit against the development of IE (OR 0.5 [CI .01-9.6]).
- Van Der Meer et al,[20]8/48 case pts (16%) received antibiotics.26/200 control pts (13%) received antibiotics.Stratified Odds Ratio: 0.51 (0.11-2.29).Protective Efficacy 49%.
- Lacassin et al[21]6/37 case pts (23%) received antibiotics.6/33 control pts (27%) received antibiotics.Matched and Adjusted Odds Ratio: 0.2 (0-0.8) Protective Efficacy 20%.
- Imperiale et al[22]
1/8 case pts (13%) received antibiotics.15/24 control pts (63%) received antibiotics. Matched Odds Ratio: .09 (CI upper limit of 0.93) (p=.025)Protective Efficacy 91%.
Safety Concerns with Antibiotic Prophylaxis
Adverse reactions associated with the administration of beta-lactam antibiotics are common.
- Ranging in severity from purititus to fatal anaphylactic shock, the frequency of all adverse reactions from the administration of penicillin to the general population is 0.7% to 10%.[23]
- Fatal anaphylaxis among patients receiving single-dose penicillin, ampillicin or amoxicillin therapy is approximately 20 cases per 1 million patients treated.[24]
- Single-dose, cephalosporin-associated fatal anaphylaxis risk is estimated at 0.5-5.7 cases per 10 million patients treated.[25]
- Macrolide and clindamycin single-dose fatal anaphylaxis risk is estimated at 0-5 cases per 1 million patients treated.[26]
With the highest associated mortality rate of all the recommended antibiotic regimens, the risk of anaphylaxis with the initiation of beta-lactam IE prophylactic therapy must be considered.
- The risk of mortality associated with the single-dose administration of beta-lactam antibiotics for IE prophylaxis is estimated at 1-3 anaphylactic deaths per 1 million patients treated.
- According to the AHA, single dose administration of a beta-lactam antibiotic for IE prophylactic therapy is a safe practice as it has never resulted in a reportable case of fatal anaphylaxis.
Cost Effectiveness of Oral Antibiotic Prophylaxis
To date, one report has addressed the cost-effectiveness of providing chemoprophylaxis to patients of moderate- and high-risk of IE acquisition prior to endodontic procedure.[27]
- The risk of drug-induced anaphylaxis and associated loss of QALYs with prophylactic oral amoxicillin or ampicillin to moderate-risk patients rendered this practice ineffective and therefore the authors did not complete a cost-effectiveness analysis.
- The estimated cost-effectiveness ratio for the prophylaxis of 10 million moderate-risk patients with clarithromycin, clindamycin or cephalexin, was $88,007, $101,142 and $99,373 per QALY saved, respectively.
- Cost-effectiveness ratio for the use of clarithromycin in patients with the prior diagnosis of endocarditis was $40,334, and in patients with prosthetic valves, $16,818 per QALY saved.
- Cost-effectiveness ratio of treating 10 million high-risk patients administered single dose clindamycin was $46,678 (prior endocarditis) and $19,936 (prosthetic valve) per QALY saved. #Cephalexin was associated with a cost-effectiveness of $37,916 per QALY saved in patients with a history of IE and $14,060 per QALY saved, in patients with a prosthetic valves.
The cost-effective analyses suggest that the 2007 AHA IE prevention guidelines advocating chemoprophylaxis to patients with a high-risk of adverse outcomes upon acquisition of IE is a reasonable, cost-effective practice.
The term of prophylaxis required procedure include biopsies, suture removal, and placement of orthodontic bands, but it does not include routine anesthetic injections through non-infected tissue, the taking of dental radiographs, placement of removable prosthodontic or orthodontic appliances, placement of orthodontic brackets, or adjustment of orthodontic appliances.
SUMMARY for antibiotic prophlaxis prior to endodontic procedure
- The presumed correlation of endodontic induced bacteremia, and new onset IE made pre-procedural antibiotic prophylaxis a reasonable practice for the preceding 60 years. However, there is a paucity of evidence in support of providing chemoprophylaxis for effective IE prevention.
- Chewing, dental hygiene practices, kidney disease, diabetes, and skin colonization present a greater risk of significant bacteremia and greater cumulative IE acquisition risk than any single invasive dental procedure.
- The AHA recommends reducing the incidence of bacteremia with the optimization of oral hygiene in at-risk patients and does not recommend indiscriminant pre-procedural chemoprophylaxis as a safe, IE prevention practice.
- Case-control studies have reported conflicting results on the protective efficacy conferred from providing pre-procedural antibiotic prophylaxis to at-risk patients.
The AHA acknowledges that even if chemoprophylaxis conferred 100% efficacy, few cases of IE would be prevented as the incidence of endodontic induced IE is so low.
The AHA now recommends the administration of pre-endodontic procedural prophylactic antibiotics to patients with the highest risk of adverse outcomes subsequent to the development of IE.
- Acknowledging an estimated 10-20 fold greater risk of single-dose fatal anaphylaxis with amoxicillin compared to single dose cephalosporin, macrolide and clindamycin regimens, the AHA believes prophylaxis with amoxicillin is a safe practice as there have been no reports of fatal anaphylaxis from a single-dose of pre-dental IE prophylaxis oral amoxicillin.
- The 2007, AHA guidelines appropriately advocate against providing chemoprophylaxis to moderate-risk patients and appropriately advocate for chemoprophylaxis to high-risk patients prior to endodontic procedure as this is a cost-effective practice for IE prevention.
Finally, there are other events that are not dental procedures and for which prophylaxis is not recommended, such as shedding of deciduous teeth and trauma to the lips and oral mucosa.
In this limited patient population, prophylactic antimicrobial therapy should be directed against Streptococcus viridans.
As a summary; individuals at the highest risk group who need procedures listed below require proper antibiotic prophylaxis;[12]
- Any type of dental extractions
- Any type of periodontal procedures and gingival surgery
- Placement of dental implants and avulsed teeth replantation
- Dental canal or root surgery
- Antibiotic fibres or strips placement at subgingival area
- Initial placement of orthodontic brackets
- Intraligamentous injection of local anesthetic drugs
- Bleeding during prophylactic cleaning of teeth or implants
Antibiotic prophylaxis with Procedures on Infected Skin, Skin Structure, or Musculoskeletal Tissue
These infections are often polymicrobial, but only staphylococci and beta hemolytic beta-hemolytic streptococci are likely to cause Infective Endocarditis.
For patients with high risk conditions who undergo a surgical procedure that involves infected skin, skin structure, or musculoskeletal tissue, it may be reasonable that the therapeutic regimen administered for treatment of the infection contain an agent active against staphylococci and beta-hemolytic streptococci, such as an antistaphylococcal penicillin or a cephalosporin.
Vancomycin or clindamycin may be administered to patients unable to tolerate a beta-lactam or who are known or suspected to have an infection caused by a methicillin-resistant staphylococcus aureus.[12]
Secondary Prevention and Follow-Up
Short-Term Follow-Up
The majority of patients with IE are cured with appropriate medical and, if necessary, surgical treatment. Before completing antimicrobial therapy, the patient should receive TTE (Class IIb, Level of Evidence: C) to establish a new baseline for subsequent comparison.
A referral to a program to assist in cessation of drug use should be made for IDU patients. Patients should be educated about the signs of endocarditis and urged to seek immediate medical attention should they occur. A thorough dental evaluation should be obtained and all active sources of oral infection should be eradicated.
All catheters used to infuse antimicrobial treatment should be promptly removed at the end of therapy. In the short-term follow-up, patients should be monitored for development of several complications. A relapse of endocardial infection is a primary concern. Patients should be aware that relapses can occur and that new onset of fever, chills, or other evidence of systemic toxicity mandates immediate evaluation, including obtaining ≥3 sets of blood cultures from different phlebotomy sites after a thorough history and physical examination.[11] [12]
Long-Term Follow-Up
Months to years after completion of medical therapy for IE, patients need ongoing observation and education regarding recurrent infection and delayed onset of worsening valvular dysfunction. Ongoing daily dental hygiene should be stressed, with serial evaluations by a dentist who is familiar with this patient population.
Patients should be questioned about the symptoms of decreased cardiac output and CHF. A thorough cardiac examination will be needed. Additional evaluations with TTE will be necessary in selected patients with positive findings from history and physical examination. Patients must be reminded to seek immediate medical evaluation for fever. This is necessary because IE can mimic a panoply of febrile illnesses.
Antibiotic therapy should not be initiated for treatment of undefined febrile illnesses without obtaining previous blood cultures. Antibiotics prescribed for nonspecific or unproved febrile syndromes are the major cause of (blood) culture negative endocarditis and should be strongly discouraged. [11] [12]
Cost-Effectiveness of Therapy
Although cost-effectiveness calculations suggest that TEE should be the first examination in adults with suspected IE, particularly in the setting of staphylococcal bacteriemia, many patients are not candidates for immediate TEE because of oral intake during the preceding 6 hours or because the patients are in institutions that cannot provide 24-hour TEE services. When TEE is not clinically possible or must be delayed, early TTE should be performed without delay.[11] [12]
Future or Investigational Therapies
Considering infective endocarditis to be an "emerging" problem in the 21st century may seem unusual, given that the illness has been well documented over the last 450 years. However, such emergence can be attributed to several factors:
- The emergence of antimicrobial resistance in classic infective endocarditis microflora, namely, the gram-positive cocci;
- The existence of antimicrobial resistance in complex ecologic biofilms;
- The changing pattern of causal agents now regarded as important pathogens of infective endocarditis, e.g., Bartonella spp., T. whipplei, and fungi;
- Changing epidemiologic trends of persons who acquire infective endocarditis, including injection drug users, persons with HIV/AIDS, children with congenital heart defects, and persons undergoing body piercing.
Furthermore, the way we provide inpatient medical care has also been associated with the emergence of nosocomial infective endocarditis, which can result from invasive procedures such as catheterization, although no cardiac surgery has been performed.
The forthcoming years will likely witness the emergence of even more changing trends of infective endocarditis, which as yet have not been well recognized. [28] [12]
"The Way I Like To Do It ..." Tips and Tricks From Clinicians Around The World
Suggested Revisions to the Current Guidelines
References
- ↑ Durack D, Lukes A, Bright D (1994). "New criteria for diagnosis of infective endocarditis: utilization of specific echocardiographic findings. Duke Endocarditis Service". Am J Med. 96 (3): 200–9. PMID 8154507.
- ↑ 2.0 2.1 Weisse A, Heller D, Schimenti R, Montgomery R, Kapila R (1993). "The febrile parenteral drug user: a prospective study in 121 patients". Am J Med. 94 (3): 274–80. PMID 8452151.
- ↑ 3.0 3.1 Samet J, Shevitz A, Fowle J, Singer D (1990). "Hospitalization decision in febrile intravenous drug users". Am J Med. 89 (1): 53–7. PMID 2368794.
- ↑ 4.0 4.1 4.2 Marantz P, Linzer M, Feiner C, Feinstein S, Kozin A, Friedland G (1987). "Inability to predict diagnosis in febrile intravenous drug abusers". Ann Intern Med. 106 (6): 823–8. PMID 3579068.
- ↑ 5.0 5.1 Leibovici L, Cohen O, Wysenbeek A (1990). "Occult bacterial infection in adults with unexplained fever. Validation of a diagnostic index". Arch Intern Med. 150 (6): 1270–2. PMID 2353860.
- ↑ 6.0 6.1 Mellors J, Horwitz R, Harvey M, Horwitz S (1987). "A simple index to identify occult bacterial infection in adults with acute unexplained fever". Arch Intern Med. 147 (4): 666–71. PMID 3827454.
- ↑ Kaech C, Elzi L, Sendi P, Frei R, Laifer G, Bassetti S, Fluckiger U (2006). "Course and outcome of Staphylococcus aureus bacteraemia: a retrospective analysis of 308 episodes in a Swiss tertiary-care centre". Clin Microbiol Infect. 12 (4): 345–52. doi:10.1111/j.1469-0691.2005.01359.x. PMID 16524411.
- ↑ http://wordnet.com.au/Products/topics_in_infectious_diseases_Aug01.htm Topics in Infectious Diseases Newsletter, August 2001, Pseudomonas aeruginosa.
- ↑ Simon S. B. Chew, David Z. Lubowski (2001). "Clostridium septicum and malignancy". Unknown parameter
|source=ignored (help) - ↑ Mirabelle Kelly, MD (June 7, 2005). "HACEK Group Infections".
- ↑ 11.00 11.01 11.02 11.03 11.04 11.05 11.06 11.07 11.08 11.09 11.10 11.11 11.12 11.13 11.14 11.15 11.16 11.17 11.18 11.19 11.20 Baddour Larry M., Wilson Walter R., Bayer Arnold S., Fowler Vance G. Jr, Bolger Ann F., Levison Matthew E., Ferrieri Patricia, Gerber Michael A., Tani Lloyd Y., Gewitz Michael H., Tong David C., Steckelberg James M., Baltimore Robert S., Shulman Stanford T., Burns Jane C., Falace Donald A., Newburger Jane W., Pallasch Thomas J., Takahashi Masato, Taubert Kathryn A. (2005). "Infective Endocarditis: Diagnosis, Antimicrobial Therapy, and Management of Complications: A Statement for Healthcare Professionals From the Committee on Rheumatic Fever, Endocarditis, and Kawasaki Disease, Council on Cardiovascular Disease in the Young, and the Councils on Clinical Cardiology, Stroke, and Cardiovascular Surgery and Anesthesia, American Heart Association-Executive Summary: Endorsed by the Infectious Diseases Society of America". Circulation. 111 (23): 3167–84. PMID 15956145.
- ↑ 12.00 12.01 12.02 12.03 12.04 12.05 12.06 12.07 12.08 12.09 12.10 12.11 12.12 12.13 Wilson W, Taubert KA, Gewitz M, Lockhart PB, Baddour LM, Levison M, Bolger A, Cabell CH, Takahashi M, Baltimore RS, Newburger JW, Strom BL, Tani LY, Gerber M, Bonow RO, Pallasch T, Shulman ST, Rowley AH, Burns JC, Ferrieri P, Gardner T, Goff D, Durack DT (2007). "American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Clinical Cardiology; American Heart Association Council on Cardiovascular Surgery and Anesthesia; Quality of Care and Outcomes Research Interdisciplinary Working Group. Prevention of infective endocarditis: guidelines from the American Heart Association: a guideline from the American Heart Association Rheumatic Fever, Endocarditis, and Kawasaki Disease Committee, Council on Cardiovascular Disease in the Young, and the Council on Clinical Cardiology, Council on Cardiovascular Surgery and Anesthesia, and the Quality of Care and Outcomes Research Interdisciplinary Working Group". Circulation. 116 (15): 1736–54. PMID 17446442.
- ↑ Gendron R, Grenier D, Maheu-Robert L. The oral cavity as a reservoir of bacterial pathogens for focal infections. Microbes Infect. 2000;2:897-906.
- ↑ Pallasch TJ. Antibiotic prophylaxis: problems in paradise. Dent Clin North Am. 2003;47:665-679.
- ↑ Pallasch TJ, Wahl MJ. Focal infection: new age or ancient history? Endodontic Topics. 2003;4:32-45.
- ↑ Roberts GJ. Dentists are innocent! Everyday" bacteremia is the real culprit: A review and assessment of the evidence that dental surgical procedures are a principal cause of bacterial endocarditis in children. Pediatric Cardiology. 1999;20:317.
- ↑ Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, et al. Dental and cardiac risk factors for infective endocarditis: A population-based, case-control study. Ann Int Med. 1998;129:761-769.
- ↑ Strom BL, Abrutyn E, Berlin JA, Kinman JL, Feldman RS, Stolley PD, et al. Risk factors for infective endocarditis: oral hygiene and nondental exposures. Circulation. 2000;102:2842.
- ↑ Oliver R, Roberts GJ, Hooper L. Penicillins for the prophylaxis of bacterial endocarditis in dentistry. Cochrane Database of Systematic Reviews 2004, Issue 2.
- ↑ Van der Meer JT, Thompson J, Valkenburg HA, Michel MF. Epidemiology of bacterial endocarditis in The Netherlands II. Antecedent procedures and use of prophylaxis. Arch Intern Med. 1992;152:1869-1873.
- ↑ Lacassin F, Hoen B, Leport C, Selton-Suty C, Delahaye F, Goulet V, et al. Procedures associated with infective endocarditis in adults - A case control study. Eur Heart J. 1995;16:1968-1974.
- ↑ Imperiale TF, Horwitz RI. Does prophylaxis prevent post-dental infective endocarditis? A controlled evaluation of protective efficacy. Am J Med. 1990;88:131-136.
- ↑ Idsoe O, Guthe T, Willcox RR, De Weck A. Nature and extent of penicillin side-reactions, with particular reference to fatalities from anaphylactic shock. Bull World Health Organ. 1968;38:159-188.
- ↑ The International Collaborative Study of Severe Anaphylaxis. Risk of anaphylaxis in a hospital population in relation to the use of various drugs: an international study. Pharmacoepidemiology and drug safety. 2003;12:195-202.
- ↑ Kelkar P,James T. Current concepts: cephalosporin allergy. N Engl J Med. 2001;345:804-9.
- ↑ Mazur N, Greenberger P, Regalado J. Clindamycin hypersensitivity appears to be rare. Ann Allergy Asthma Immunol. 1999;82:443–5.
- ↑ Agha Z, Lofgren RP, VanRuiswyk JV. Is antibiotic prophylaxis for bacterial endocarditis cost-effective? Med Decis Making. 2005;25:308-320.
- ↑ Millar BC, Moore JE. Emerging issues in infective endocarditis. Emerg Infect Dis 2004 Jun [Access date: 03-03-2008]. Available from: http://www.cdc.gov/ncidod/EID/vol10no6/03-0848.htm
External Links and Patient Resources
- Brief Communication: Treatment of Enterococcus faecalis Endocarditis with Ampicillin plus Ceftriaxone
- Infective Endocarditis in Adults
- Temporal Trends in Infective Endocarditis
For Patients:
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
Contributors