Lyme disease pathophysiology
| https://https://www.youtube.com/watch?v=rOQvpcpxbCs%7C350}} |
|
Lyme disease Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Lyme disease pathophysiology On the Web |
|
American Roentgen Ray Society Images of Lyme disease pathophysiology |
|
Risk calculators and risk factors for Lyme disease pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1];Associate Editor(s)-in-Chief: Anmol Pitliya, M.B.B.S. M.D.[2]
Overview
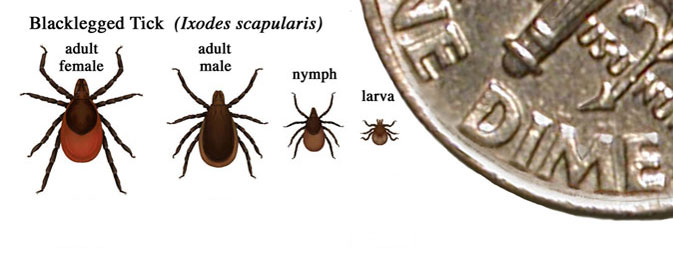
Lyme disease is caused by Borrelia burgdorferi and is transmitted primarily by tick species Ixodes scapularis. Ticks can attach to any part of the human body but are often found in hard-to-see areas such as the groin, armpits, and scalp. In most cases, the tick must be attached for 36 to 48 hours or more before the spirochetes can be transmitted. Very few people affected with Lyme disease recall a tick bite. B. burgdorferi have two morphological forms, a spiral form and a spheroplast form. Survival strategies of B. burgdorferi include: antigenic variation, physical sequestration, intracellular invasion, and immune system supression.
Pathophysiology
Transmission
Primary Vector
|
 |
 | |
| |
Other Potential Vectors
- The lone star tick (Amblyomma americanum), which is found throughout the Southeastern U.S. as far West as Texas and increasingly in Northeastern states, is another potential vector.[9]
- These tick bites usually go unnoticed due to the small size of the tick in its nymphal stage, as well as tick secretions that prevent the host from feeling any itch or pain from the bite.
- It was once thought to be a vector, although recent studies demonstrate that this tick species is not a competent vector of Borrelia burgdorferi sensu lato.[10]
- The lone star tick (Amblyomma americanum) is associated with southern tick-associated rash illness (STARI or Masters' disease), Tularemia, and Ehrlichiosis.[11]
Other Modes of Transmission
- While Lyme spirochetes have been found in insects other than ticks, reports of actual infectious transmission appear to be rare.[12][13]
- Sexual transmission has been reported. Lyme spirochetes have been found in semen and breast milk; however, transmission of the spirochetes by these routes is not known to occur.[14][15][16]
- Congenital transmission of Lyme disease can occur from an infected mother to fetus through the placenta during pregnancy. However, prompt antibiotic treatment appears to prevent fetal harm.[17]
Reservoir host
- The primary reservoir host of B. burgdorferi is rodents. These rodents are infested by I. scapularis.
- The white-footed mouse (Peromyscus leucopus) is the most common rodent infected by B. burgdorferi.[18]
- Other reservoirs may include voles, chipmunks, squirrels, raccoons, skunks, birds, and reptiles such as lizards.
- It is predicted that the density of infected nymphal stage of ticks may be lower in areas where predators of primary reservoir hosts, particularly red foxes (Vulpes vulpes), are more active. The reason for this may include:[19]
- Direct effect: Predation of reservoir hosts.
- Indirect effect: Decreased movement and increased refuge due to presence of active predator.
Coinfection
- Ixodes scapularis is also a vector for Anaplasma phagocytophilum (previously referred to as Ehrlichia phagocytophila) and Babesia microti.[20]
- Anaplasma phagocytophilum causes human granulocytic anaplasmosis (HGA), previously known as human granulocytic ehrlichiosis.
- Babesia microti causes babesiosis.
- Sometimes, patients may be coinfected with two or more pathogens.
- Presence of flu-like symptoms (fever, chills, and headache) in patients of Lyme disease without erythema migrans may indicate concurrent infection with human granulocytic anaplasmosis (HGA) and/or babesiosis.[21]
- Coinfection should be considered in the case of prolonged flu-like symptoms that fail to respond to Lyme disease treatment.
- CBC should be considered as initial investigation for patient thought to have coinfection.
Pathogenesis
- B. burgdorferi enters the bloodstream through saliva during a tick bite.[22]
- After the incubation period (around 3-30 days), B. burgdorferi migrates outwards in skin, manifesting as erythema chronicum migrans. It is then disseminated to other organs including multiple skin sites manifesting as multiple erythema migrans.[23]
- B. burgdorferi is a very slow growing organism. Its doubling time is 12-24 hours (in contrast with bacterial pathogens such as Streptococcus and Staphylococcus, which have a doubling time of 20-30 minutes).
- B. burgdorferi has an axial filament composed of flagella that runs lengthwise between its cell wall and outer membrane like other spirochetes. This structure allows B. burgdorferi to move efficiently through viscous media (such as connective tissue) in a corkscrew fashion.
- This helps B. burgdorferi disseminate throughout the body in the days to weeks after infection.
- B. burgdorferi penetrates deep into the tissue where the immune system and antibiotics are unable to reach.
- B. burgdorferi has two morphological forms, a spiral form and a spheroplast form (cysts, granules). The existence of B. burgdorferi spheroplasts, which lack a cell wall, has been documented in the following models:
- The spiral form requires energy to convert to the spheroplast form. This shows that the spheroplast form has a survival function, and it is not merely an end stage degeneration product.[24]
- The spheroplasts are virulent and infectious and survive under adverse environmental conditions. Once the conditions are more favorable, they revert back to the spiral form in vitro.[31][32]
- B. burgdorferi spheroplasts play a key factor in the relapsing, persistant nature of Lyme disease due to:
- Spheroplasts have dramatically reduced surface area for immune surveillance as compared to spiral form.
- Spheroplasts express different surface proteins; current tests detect antibodies to surface proteins of the spiral form, which may result in seronegative disease (i.e. false-negative antibody tests).
- B. burgdorferi spheroplasts are generally not susceptible to the antibiotics traditionally used for Lyme disease. They have shown sensitivity in vitro to antiparasitic drugs to which the spiral form of B. burgdorferi is not sensitive. These drugs include:
Mechanisms of persistence
- B. burgdorferi is susceptible to a number of antibiotics in vitro, but the efficacy of antibiotics in vivo has contradictory reports. Especially when treatment is delayed and disease is disseminated, B. burgdorferi has the ability to persist in humans and animals for months to years even after a strong immune response and antibiotic treatment. Numerous studies have demonstrated persistence of infection despite antibiotic therapy.[36][37][38]
- Survival strategies of B. burgdorferi include: [39]
- Antigenic variation
- B. burgdorferi has the ability to vary its surface proteins in response to attack by the immune system.[40]
- This is due to the complex genome of B. burgdorferi which helps in evading the immune system and establishing a persistant infection.[41]
- Physical sequestration
- B. burgdorferi is sequestered in sites such as central nervous system that are inaccessible to the immune system and antibiotics.[42]
- Evidence suggests that B. burgdorferi penetrates the blood-brain barrier by using the fibrinolytic system of the host.[43]
- Intracellular invasion
- B. burgdorferi invades a variety of cells, including:
- B. burgdorferi hides inside these cells and evades the immune system. It is also protected against antibiotics to varying degrees, allowing the infection to persist.[50][51]
- Immune system suppression
- The mechanism of immune system suppression observed in B. burgdorferi includes:[39]
- Complement inhibition[52]
- Induction of anti-inflammatory cytokines such as IL-10
- Formation of immune complexes
- Formation of immune complexes in B. burgdorferi might explain the seronegative disease (i.e. false-negative antibody tests of blood and cerebrospinal fluid). Studies have shown that substantial numbers of seronegative Lyme patients have antibodies bound up in these complexes.[53]
- The mechanism of immune system suppression observed in B. burgdorferi includes:[39]
- Antigenic variation
Role of cytokines
Evidence of a distinct pro-inflammatory immune process has been observed in both acute and antibiotic refractory Lyme disease.
- This pro-inflammatory process is a cell-mediated immunity and results in Th1 upregulation.
- A significant decrease in output of Interleukin-10 (IL-10), an upregulation of Interleukin-6 (IL-6), Interleukin-12 (IL-12), and Interferon-gamma, and dysregulation in TNF-alpha have been observed.[54]
- Host immune response to infection results in increased levels of Interferon-gamma in serum and lesions of Lyme disease patients that correlate with a greater severity of disease.
- Interferon-gamma alters gene expression in endothelia exposed to B. burgdorferi in a manner that promotes recruitment of T cells and suppresses that of neutrophils.
- IL-10 is generally regarded as an anti-inflammatory cytokine, as it acts on several different cell types to suppress the production of pro-inflammatory mediators.
- Researchers are also beginning to identify microglia as a previously unrecognized source of inflammatory mediator production following infection with B. burgdorferi.
- Such production may play an important role during the development of cognitive disorders in Lyme neuroborreliosis.
- This effect is associated with induction of nuclear factor-kappa B (NF-KB) by B. burgdorferi.[55][56]
- Dysregulation in production of pro-inflammatory cytokines such as IL-6 and TNF-alpha can lead to neuronal damage in patients infected with B. burgdorferi.[57]
- IL-6 and TNF-Alpha cytokines produce fatigue and malaise, two of the prominent symptoms experienced by patients with post treatment Lyme disease syndrome.[58]
- IL-6 is also significantly linked to cognitive impairment.[59]
Role of Neurotransmitters
- A developing hypothesis is that the chronic secretion of stress hormones (specifically glucocorticoids and catecholamines) as a result of Borrelia infection may reduce the effect of neurotransmitters, or other receptors in the brain by cell-mediated pro-inflammatory pathways, thereby leading to the dysregulation of neurohormones.[60][61]
- This process is mediated via the hypothalamic-pituitary-adrenal axis.
- Additionally, tryptophan, a precursor to serotonin, appears to be reduced within the CNS in a number of patients with Lyme disease.[62]
Pathogenesis of Post treatment Lyme disease syndrome
- It has been found that post treatment Lyme disease syndrome patients have higher amounts of Borrelia-specific forkhead box P3 (FoxP3) than healthy controls, indicating that regulatory T cells, by immunosuppression, might play a role in the development of post treatment Lyme disease syndrome.
- FoxP3 are a specific marker of regulatory T cells.[63]
- The signaling pathway P38 mitogen-activated protein kinases (p38 MAP kinase) has been identified as promoting expression of pro-inflammatory cytokines from Borrelia.[64]
- These new and ongoing immunological studies suggest that cell-mediated immune disruption in Lyme patients amplifies the inflammatory process, often rendering it chronic and self-perpetuating. It is regardless of whether the Borrelia bacterium is still present in the host. It might also suggest an autoimmune pattern.[65]
Microscopic pathology
- Biopsy of erythema migrans shows:
- Dermal and epidermal involvement in center of lesion
- Dermal involvement at the periphery
References
- ↑ 1.0 1.1 1.2 1.3 Rudenko N, Golovchenko M, Grubhoffer L, Oliver JH (2011). "Updates on Borrelia burgdorferi sensu lato complex with respect to public health". Ticks Tick Borne Dis. 2 (3): 123–8. doi:10.1016/j.ttbdis.2011.04.002. PMC 3167092. PMID 21890064.
- ↑ Schwartz, Ira; Fish, Durland; Daniels, Thomas J. (1997). "Prevalence of the Rickettsial Agent of Human Granulocytic Ehrlichiosis in Ticks from a Hyperendemic Focus of Lyme Disease". New England Journal of Medicine. 337 (1): 49–50. doi:10.1056/NEJM199707033370111. ISSN 0028-4793.
- ↑ Falco RC, McKenna DF, Daniels TJ, Nadelman RB, Nowakowski J, Fish D; et al. (1999). "Temporal relation between Ixodes scapularis abundance and risk for Lyme disease associated with erythema migrans". Am J Epidemiol. 149 (8): 771–6. PMID 10206627.
- ↑ Piesman J, Maupin GO, Campos EG, Happ CM (1991). "Duration of adult female Ixodes dammini attachment and transmission of Borrelia burgdorferi, with description of a needle aspiration isolation method". J Infect Dis. 163 (4): 895–7. PMID 2010643.
- ↑ Ohnishi J, Piesman J, de Silva AM (2001). "Antigenic and genetic heterogeneity of Borrelia burgdorferi populations transmitted by ticks". Proc Natl Acad Sci U S A. 98 (2): 670–5. doi:10.1073/pnas.98.2.670. PMC 14646. PMID 11209063.
- ↑ 6.0 6.1 Girard YA, Travinsky B, Schotthoefer A, Fedorova N, Eisen RJ, Eisen L, Barbour AG, Lane RS (2009). "Population structure of the lyme borreliosis spirochete Borrelia burgdorferi in the western black-legged tick (Ixodes pacificus) in Northern California". Appl. Environ. Microbiol. 75 (22): 7243–52. doi:10.1128/AEM.01704-09. PMC 2786521. PMID 19783741.
- ↑ Sui S, Yang Y, Sun Y, Wang X, Wang G, Shan G, Wang J, Yu J (2017). "On the core bacterial flora of Ixodes persulcatus (Taiga tick)". PLoS ONE. 12 (7): e0180150. doi:10.1371/journal.pone.0180150. PMC 5503197. PMID 28692666.
- ↑ Wormser G, Masters E, Nowakowski J; et al. (2005). "Prospective clinical evaluation of patients from missouri and New York with erythema migrans-like skin lesions". Clin Infect Dis. 41 (7): 958–65. PMID 16142659.
- ↑ Clark K (2004). "Borrelia species in host-seeking ticks and small mammals in northern Florida" (PDF). J Clin Microbiol. 42 (11): 5076–86. PMID 15528699.
- ↑ Ledin K, Zeidner N, Ribeiro J; et al. (2005). "Borreliacidal activity of saliva of the tick Amblyomma americanum". Med Vet Entomol. 19 (1): 90–95. PMID 15752182.
- ↑ Wormser GP, Masters E, Nowakowski J, McKenna D, Holmgren D, Ma K; et al. (2005). "Prospective clinical evaluation of patients from Missouri and New York with erythema migrans-like skin lesions". Clin Infect Dis. 41 (7): 958–65. doi:10.1086/432935. PMID 16142659.
- ↑ Magnarelli L, Anderson J (1988). "Ticks and biting insects infected with the etiologic agent of Lyme disease, Borrelia burgdorferi" (PDF). J Clin Microbiol. 26 (8): 1482–6. PMID 3170711.
- ↑ Luger S (1990). "Lyme disease transmitted by a biting fly". N Engl J Med. 322 (24): 1752. PMID 2342543.
- ↑ Bach G (2001). "Recovery of Lyme spirochetes by PCR in semen samples of previously diagnosed Lyme disease patients.". 14th International Scientific Conference on Lyme Disease.
- ↑ Schmidt B, Aberer E, Stockenhuber C; et al. (1995). "Detection of Borrelia burgdorferi DNA by polymerase chain reaction in the urine and breast milk of patients with Lyme borreliosis". Diagn Microbiol Infect Dis. 21 (3): 121–8. PMID 7648832.
- ↑ Steere AC (2003-02-01). "Lyme Disease: Questions and Answers" (PDF). Massachusetts General Hospital / Harvard Medical School. Retrieved 2007-03-22.
- ↑ Walsh CA, Mayer EW, Baxi LV (2007). "Lyme disease in pregnancy: case report and review of the literature". Obstetrical & gynecological survey. 62 (1): 41–50. doi:10.1097/01.ogx.0000251024.43400.9a. PMID 17176487.
- ↑ Anderson JF, Johnson RC, Magnarelli LA, Hyde FW (1986). "Culturing Borrelia burgdorferi from spleen and kidney tissues of wild-caught white-footed mice, Peromyscus leucopus". Zentralbl Bakteriol Mikrobiol Hyg A. 263 (1–2): 34–9. PMID 3577490.
- ↑ Hofmeester, Tim R.; Jansen, Patrick A.; Wijnen, Hendrikus J.; Coipan, Elena C.; Fonville, Manoj; Prins, Herbert H. T.; Sprong, Hein; van Wieren, Sipke E. (2017). "Cascading effects of predator activity on tick-borne disease risk". Proceedings of the Royal Society B: Biological Sciences. 284 (1859): 20170453. doi:10.1098/rspb.2017.0453. ISSN 0962-8452.
- ↑ Wormser GP, Dattwyler RJ, Shapiro ED, Halperin JJ, Steere AC, Klempner MS; et al. (2006). "The clinical assessment, treatment, and prevention of lyme disease, human granulocytic anaplasmosis, and babesiosis: clinical practice guidelines by the Infectious Diseases Society of America". Clin Infect Dis. 43 (9): 1089–134. doi:10.1086/508667. PMID 17029130.
- ↑ Krause PJ, McKay K, Thompson CA, Sikand VK, Lentz R, Lepore T; et al. (2002). "Disease-specific diagnosis of coinfecting tickborne zoonoses: babesiosis, human granulocytic ehrlichiosis, and Lyme disease". Clin Infect Dis. 34 (9): 1184–91. doi:10.1086/339813. PMID 11941544.
- ↑ Steere AC, Bartenhagen NH, Craft JE, Hutchinson GJ, Newman JH, Rahn DW; et al. (1983). "The early clinical manifestations of Lyme disease". Ann Intern Med. 99 (1): 76–82. PMID 6859726.
- ↑ Wormser GP, McKenna D, Carlin J, Nadelman RB, Cavaliere LF, Holmgren D; et al. (2005). "Brief communication: hematogenous dissemination in early Lyme disease". Ann Intern Med. 142 (9): 751–5. PMID 15867407.
- ↑ 24.0 24.1 Alban PS, Johnson PW, Nelson DR (2000). "Serum-starvation-induced changes in protein synthesis and morphology of Borrelia burgdorferi". Microbiology. 146 ( Pt 1): 119–27. PMID 10658658.
- ↑ 25.0 25.1 Mursic VP, Wanner G, Reinhardt S; et al. (1996). "Formation and cultivation of Borrelia burgdorferi spheroplast-L-form variants". Infection. 24 (3): 218–26. PMID 8811359.
- ↑ Kersten A, Poitschek C, Rauch S, Aberer E (1995). "Effects of penicillin, ceftriaxone, and doxycycline on morphology of Borrelia burgdorferi" (PDF). Antimicrob Agents Chemother. 39 (5): 1127–33. PMID 7625800.
- ↑ Schaller M, Neubert U (1994). "Ultrastructure of Borrelia burgdorferi after exposure to benzylpenicillin". Infection. 22 (6): 401–6. PMID 7698837.
- ↑ 28.0 28.1 Nanagara R, Duray PH, Schumacher HR Jr (1996). "Ultrastructural demonstration of spirochetal antigens in synovial fluid and synovial membrane in chronic Lyme disease: possible factors contributing to persistence of organisms". Hum Pathol. 27 (10): 1025–34. PMID 8892586.
- ↑ Phillips SE, Mattman LH, Hulinska D, Moayad H (1998). "A proposal for the reliable culture of Borrelia burgdorferi from patients with chronic Lyme disease, even from those previously aggressively treated". Infection. 26 (6): 364–7. PMID 9861561.
- ↑ Duray PH, Yin SR, Ito Y; et al. (2005). "Invasion of human tissue ex vivo by Borrelia burgdorferi". J Infect Dis. 191 (10): 1747–54. PMID 15838803.
- ↑ Gruntar I, Malovrh T, Murgia R, Cinco M (2001). "Conversion of Borrelia garinii cystic forms to motile spirochetes in vivo". APMIS. 109 (5): 383–8. PMID 11478686.
- ↑ Murgia R, Cinco M (2004). "Induction of cystic forms by different stress conditions in Borrelia burgdorferi". APMIS. 112 (1): 57–62. PMID 14961976.
- ↑ Brorson O, Brorson SH (1999). "An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to metronidazole". APMIS. 107 (6): 566–76. PMID 10379684.
- ↑ Brorson O, Brorson SH (2004). "An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to tinidazole" (PDF). Int Microbiol. 7 (2): 139–42. PMID 15248163.
- ↑ Brorson O, Brorson SH (2002). "An in vitro study of the susceptibility of mobile and cystic forms of Borrelia burgdorferi to hydroxychloroquine". Int Microbiol. 5 (1): 25–31. PMID 12102233.
- ↑ Bayer ME, Zhang L, Bayer MH (1996). "Borrelia burgdorferi DNA in the urine of treated patients with chronic Lyme disease symptoms. A PCR study of 97 cases". Infection. 24 (5): 347–53. PMID 8923044.
- ↑ Preac-Mursic V, Weber K, Pfister HW; et al. (1989). "Survival of Borrelia burgdorferi in antibiotically treated patients with Lyme borreliosis". Infection. 17 (6): 355–9. PMID 2613324.
- ↑ Oksi J, Marjamaki M, Nikoskelainen J, Viljanen MK (1999). "Borrelia burgdorferi detected by culture and PCR in clinical relapse of disseminated Lyme borreliosis". Ann Med. 31 (3): 225–32. PMID 10442678.
- ↑ 39.0 39.1 Embers ME, Ramamoorthy R, Philipp MT (2004). "Survival strategies of Borrelia burgdorferi, the etiologic agent of Lyme disease". Microbes Infect. 6 (3): 312–8. PMID 15065567.
- ↑ Liang FT, Yan J, Mbow ML; et al. (2004). "Borrelia burgdorferi changes its surface antigenic expression in response to host immune responses". Infect Immun. 72 (10): 5759–67. PMID 15385475.
- ↑ Gilmore RD, Howison RR, Schmit VL; et al. (2007). "Temporal expression analysis of the Borrelia burgdorferi paralogous gene family 54 genes BBA64, BBA65, and BBA66 during persistent infection in mice". Infect. Immun. 75 (6): 2753–64. doi:10.1128/IAI.00037-07. PMID 17371862.
- ↑ Miklossy J, Khalili K, Gern L; et al. (2004). "Borrelia burgdorferi persists in the brain in chronic lyme neuroborreliosis and may be associated with Alzheimer disease". J Alzheimers Dis. 6 (6): 639–49, discussion 673-81. PMID 15665404.
- ↑ Grab DJ, Perides G, Dumler JS, Kim KJ, Park J, Kim YV, Nikolskaia O, Choi KS, Stins MF, Kim KS (2005). "Borrelia burgdorferi, host-derived proteases, and the blood-brain barrier". Infect Immun. 73 (2): 1014–22. PMID 15664945.
- ↑ Ma Y, Sturrock A, Weis JJ (1991). "Intracellular localization of Borrelia burgdorferi within human endothelial cells" (PDF). Infect Immun. 59 (2): 671–8. PMID 1987083.</ref [[Fibroblasts]]<ref name="Klempner-b">Klempner MS, Noring R, Rogers RA (1993). "Invasion of human skin fibroblasts by the Lyme disease spirochete, Borrelia burgdorferi". J Infect Dis. 167 (5): 1074–81. PMID 8486939.
- ↑ Dorward DW, Fischer ER, Brooks DM (1997). "Invasion and cytopathic killing of human lymphocytes by spirochetes causing Lyme disease". Clin Infect Dis. 25 Suppl 1: S2–8. PMID 9233657.
- ↑ Montgomery RR, Nathanson MH, Malawista SE (1993). "The fate of Borrelia burgdorferi, the agent for Lyme disease, in mouse macrophages. Destruction, survival, recovery". J Immunol. 150 (3): 909–15. PMID 8423346.
- ↑ Aberer E, Kersten A, Klade H, Poitschek C, Jurecka W (1996). "Heterogeneity of Borrelia burgdorferi in the skin". Am J Dermatopathol. 18 (6): 571–9. PMID 8989928.
- ↑ Girschick HJ, Huppertz HI, Russmann H, Krenn V, Karch H (1996). "Intracellular persistence of Borrelia burgdorferi in human synovial cells". Rheumatol Int. 16 (3): 125–32. PMID 8893378.
- ↑ Livengood JA, Gilmore RD (2006). "Invasion of human neuronal and glial cells by an infectious strain of Borrelia burgdorferi". Microbes Infect. [Epub ahead of print]. PMID 17045505.
- ↑ Georgilis K, Peacocke M, Klempner MS (1992). "Fibroblasts protect the Lyme disease spirochete, Borrelia burgdorferi, from ceftriaxone in vitro". J Infect Dis. 166 (2): 440–4. PMID 1634816.
- ↑ Brouqui P, Badiaga S, Raoult D (1996). "Eucaryotic cells protect Borrelia burgdorferi from the action of penicillin and ceftriaxone but not from the action of doxycycline and erythromycin" (PDF). Antimicrob Agents Chemother. 40 (6): 1552–4. PMID 8726038.
- ↑ Zajkowska J, Grygorczuk S, Kondrusik M, Pancewicz S, Hermanowska-Szpakowicz T (2006). "New aspects of pathogenesis of Lyme borreliosis". Przegla̧d epidemiologiczny (in Polish). 60 Suppl 1: 167–70. PMID 16909797.
- ↑ Schutzer SE, Coyle PK, Reid P, Holland B (1999). "Borrelia burgdorferi-specific immune complexes in acute Lyme disease". JAMA. 282 (20): 1942–6. PMID 10580460.
- ↑ Shin JJ, Glickstein LJ, Steere AC (2007). "High levels of inflammatory chemokines and cytokines in joint fluid and synovial tissue throughout the course of antibiotic-refractory lyme arthritis". Arthritis Rheum. 56 (4): 1325–35. doi:10.1002/art.22441. PMID 17393419.
- ↑ Rasley A, Anguita J, Marriott I (2002). "Borrelia burgdorferi induces inflammatory mediator production by murine microglia". J. Neuroimmunol. 130 (1–2): 22–31. PMID 12225885.
- ↑ Rasley A, Tranguch SL, Rati DM, Marriott I (2006). "Murine glia express the immunosuppressive cytokine, interleukin-10, following exposure to Borrelia burgdorferi or Neisseria meningitidis". Glia. 53 (6): 583–92. doi:10.1002/glia.20314. PMID 16419089.
- ↑ Ramesh G, Philipp MT (2005). "Pathogenesis of Lyme neuroborreliosis: mitogen-activated protein kinases Erk1, Erk2, and p38 in the response of astrocytes to Borrelia burgdorferi lipoproteins". Neurosci. Lett. 384 (1–2): 112–6. doi:10.1016/j.neulet.2005.04.069. PMID 15893422.
- ↑ Papanicolaou DA, Wilder RL, Manolagas SC, Chrousos GP (1998). "The pathophysiologic roles of interleukin-6 in human disease". Ann. Intern. Med. 128 (2): 127–37. PMID 9441573.
- ↑ Wright CB, Sacco RL, Rundek TR; et al. (2006). "Interleukin-6 is associated with cognitive function: the Northern Manhattan Study". 15 (1): 34–38. doi:10.1016/j.jstrokecerebrovasdis.2005.08.009. PMID 16501663.
- ↑ Elenkov IJ, Iezzoni DG, Daly A, Harris AG, Chrousos GP (2005). "Cytokine dysregulation, inflammation and well-being". Neuroimmunomodulation. 12 (5): 255–69. doi:10.1159/000087104. PMID 16166805.
- ↑ Calcagni E, Elenkov I (2006). "Stress system activity, innate and T helper cytokines, and susceptibility to immune-related diseases". Ann. N. Y. Acad. Sci. 1069: 62–76. doi:10.1196/annals.1351.006. PMID 16855135.
- ↑ Gasse T, Murr C, Meyersbach P; et al. (1994). "Neopterin production and tryptophan degradation in acute Lyme neuroborreliosis versus late Lyme encephalopathy". European journal of clinical chemistry and clinical biochemistry : journal of the Forum of European Clinical Chemistry Societies. 32 (9): 685–9. PMID 7865624.
- ↑ Jarefors S, Janefjord CK, Forsberg P, Jenmalm MC, Ekerfelt C (2007). "Decreased up-regulation of the interleukin-12Rbeta2-chain and interferon-gamma secretion and increased number of forkhead box P3-expressing cells in patients with a history of chronic Lyme borreliosis compared with asymptomatic Borrelia-exposed individuals". Clin. Exp. Immunol. 147 (1): 18–27. doi:10.1111/j.1365-2249.2006.03245.x. PMID 17177959.
- ↑ Ramesh G, Philipp MT (2005). "Pathogenesis of Lyme neuroborreliosis: mitogen-activated protein kinases Erk1, Erk2, and p38 in the response of astrocytes to Borrelia burgdorferi lipoproteins". Neurosci. Lett. 384 (1–2): 112–6. doi:10.1016/j.neulet.2005.04.069. PMID 15893422.
- ↑ Singh SK, Girschick HJ (2006). "Toll-like receptors in Borrelia burgdorferi-induced inflammation". Clin. Microbiol. Infect. 12 (8): 705–17. doi:10.1111/j.1469-0691.2006.01440.x. PMID 16842565.
- CS1 maint: Multiple names: authors list
- CS1 maint: Explicit use of et al.
- CS1 maint: Unrecognized language
- Bacterial diseases
- Insect-borne diseases
- Lyme disease
- Zoonoses
- Spirochaetes
- Disease
- Dermatology
- Emergency medicine
- Intensive care medicine
- Up-To-Date
- Infectious disease
- Ophthalmology
- Neurology
- Cardiology
- Rheumatology


