Pulmonary embolism natural history, complications and prognosis: Difference between revisions
Iqra Qamar (talk | contribs) |
Iqra Qamar (talk | contribs) |
||
| Line 136: | Line 136: | ||
===Clinical Correlates of Mortality among Patients with PE=== | ===Clinical Correlates of Mortality among Patients with PE=== | ||
==== Hemodynamic Status ==== | ==== Hemodynamic Status ==== | ||
Observational studies such as the International Co-operative Pulmonary Embolism Registry (ICOPER) and the Management and Prognosis in Pulmonary Embolism Trial (MAPPET) have shown that [[shock]] and [[hypotension]] are principal high risk markers of early death in acute PE.<ref name="pmid9350909">{{cite journal| author=Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD et al.| title=Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. | journal=J Am Coll Cardiol | year= 1997 | volume= 30 | issue= 5 | pages= 1165-71 | pmid=9350909 | doi= | pmc=| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9350909 }} </ref> The MAPPET study demonstrated that systemic shock was associated with mortality of 24.5% whereas [[hypotension]] (but not [[shock]]) was associated with a mortality of 15.2%. | Observational studies such as the International Co-operative Pulmonary Embolism Registry (ICOPER) and the Management and Prognosis in Pulmonary Embolism Trial (MAPPET) have shown that [[shock]] and [[hypotension]] are principal high risk markers of early death in [[acute]] PE.<ref name="pmid9350909">{{cite journal| author=Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD et al.| title=Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry. | journal=J Am Coll Cardiol | year= 1997 | volume= 30 | issue= 5 | pages= 1165-71 | pmid=9350909 | doi= | pmc=| url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=9350909 }} </ref> The MAPPET study demonstrated that systemic shock was associated with mortality of 24.5% whereas [[hypotension]] (but not [[shock]]) was associated with a mortality of 15.2%. | ||
A post-hoc analysis of the ICOPER study demonstrated that the 90-day all-cause mortality rate was 52.4% (95% CI,43.3–62.1%) among patients with a [[systolic blood pressure]] less than 90 mm Hg compared to 14.7% (95% CI, 13.3–16.2%) among patients with a normal [[blood pressure]].<ref name="pmid16432055">{{cite journal| author=Kucher N, Rossi E, De Rosa M, Goldhaber SZ| title=Massive pulmonary embolism. | journal=Circulation | year= 2006 | volume= 113 | issue= 4 | pages= 577-82 |pmid=16432055 | doi=10.1161/CIRCULATIONAHA.105.592592 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16432055 }} </ref> | A post-hoc analysis of the ICOPER study demonstrated that the 90-day all-cause mortality rate was 52.4% (95% CI,43.3–62.1%) among patients with a [[systolic blood pressure]] less than 90 mm Hg compared to 14.7% (95% CI, 13.3–16.2%) among patients with a normal [[blood pressure]].<ref name="pmid16432055">{{cite journal| author=Kucher N, Rossi E, De Rosa M, Goldhaber SZ| title=Massive pulmonary embolism. | journal=Circulation | year= 2006 | volume= 113 | issue= 4 | pages= 577-82 |pmid=16432055 | doi=10.1161/CIRCULATIONAHA.105.592592 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16432055 }} </ref> | ||
Revision as of 16:17, 11 October 2017
| Resident Survival Guide |
|
Pulmonary Embolism Microchapters |
|
Diagnosis |
|---|
|
Pulmonary Embolism Assessment of Probability of Subsequent VTE and Risk Scores |
|
Treatment |
|
Follow-Up |
|
Special Scenario |
|
Trials |
|
Case Studies |
|
Pulmonary embolism natural history, complications and prognosis On the Web |
|
FDA on Pulmonary embolism natural history, complications and prognosis |
|
CDC on Pulmonary embolism natural history, complications and prognosis |
|
Pulmonary embolism natural history, complications and prognosis in the news |
|
Blogs on Pulmonary embolism natural history, complications and prognosis |
|
Directions to Hospitals Treating Pulmonary embolism natural history, complications and prognosis |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2] The APEX Trial Investigators; Associate Editor(s)-in-Chief: Rim Halaby, M.D. [3]
Overview
Pulmonary embolism (PE) can be acutely complicated by the development of cardiogenic shock, pulseless electrical activity and sudden cardiac death and chronically by the development of pulmonary hypertension. The medical management of PE often requires the administration of potent parenteral anticoagulants and fibrinolytics and massive bleeding can be a complication of their administration. If left untreated almost one-third of patients with PE die, typically from recurrent PE. However, with prompt diagnosis and treatment, the mortality rate is approximately 2–8%. The true mortality associated with PE may be underestimated as two-thirds of all PE cases are diagnosed by autopsy. Estimates suggest that 60,000-100,000 Americans die of VTE, 10 to 30% of which will die within one month of diagnosis. Sudden death is the first symptom in about one-quarter (25%) of people who have a PE. One-third (about 33%) of people with VTE will have a recurrence within 10 years.[1][2]
Subsegmental pulmonary emboli may[3] or may not[4] have a favorable prognosis.
Complications
Acute Complications
- Atrial flutter
- Heart failure or shock
- Pulmonary hypertension
- Pulseless electrical activity
- Sudden cardiac death
Chronic Complications
- Chronic thromboembolic hypertension (rare - 1%)[5]
- Pulmonary hypertension
- Recurrent pulmonary embolism
Complications of Firbrinolytic Therapy for Pulmonary Embolism[6]
- Severe bleeding can occur as a complication of fibrinolytic treatment:
- Major hemorrhage - 10%
- Non major hemorrhage - 20%
- Intracranial hemorrhage - 0.5%
Prognosis
Pulmonary Embolism Severity Index (PESI) Score
The Pulmonary Embolism Severity Index (PESI) score aims to stratify patients with PE into classes of increasing rate of mortality and adverse outcomes.[7]
Calculation of PESI Score
| Age, per yr | Age, in yr |
| Male sex | 10 |
| Cancer | 30 |
| Heart failure | 10 |
| Chronic lung disease | 10 |
| Pulse ≥110 beat/min | 20 |
| Systolic blood pressure <100 mmHg | 30 |
| Respiratory rate ≥30/min | 20 |
| Temperature <36 | 20 |
| Altered mental status | 60 |
| Arterial oxygen saturation <90% | 20 |
Interpretation of PESI Score
| Class | Score | Class–specific 30-day mortality |
| Class I, very low risk | ≤65 | 1.1% |
| Class II, low risk | 65-85 | 3.1% |
| Class III, intermediate risk | 86-105 | 6.5% |
| Class IV, high risk | 106-125 | 10.4% |
| Class V, very high risk | >125 | 24.5% |
HOPPE risk score
The HOPPE risk score contains[8]:
- Heart rate
- PaO2 (arterial partial pressure of oxygen)
- Systolic blood pressure
- Diastolic blood pressure
- Electrocardiographic score
The HOPPE has not been studied as extensively as PESI; however, initial study suggests it may prove more accurate.[8]
Hestia Clinical Decision Rule
The Hestia Clinical Decision Rule can help guide the decision for hospitalization.[9]
Hospitalization for VTE
- During 2007–2009, an estimated annual average of 547,596 hospitalizations had a diagnosis of VTE for adults aged ≥18 years. Estimates for DVT and PE diagnoses were not mutually exclusive. An estimated annual average of 348,558 adult hospitalizations had a diagnosis of DVT, and 277,549 adult hospitalizations had a diagnosis of PE. An estimated annual average of 78,511 adult hospitalizations (14% of overall VTE hospitalizations) had diagnoses of both DVT and PE.[10]
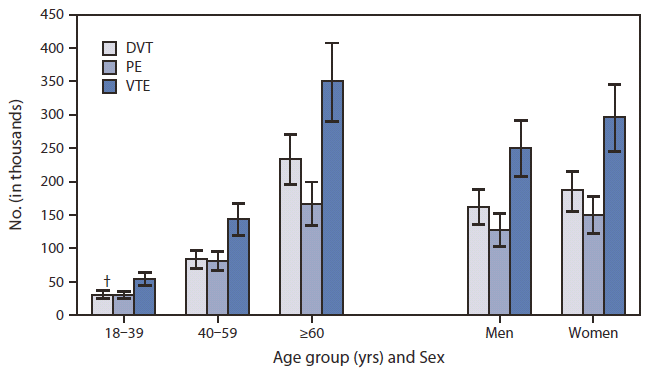
- The estimated average annual number of hospitalizations with VTE was successively greater among older age groups: 54,034 for persons aged 18–39 years; 143,354 for persons aged 40–59 years; and 350,208 for persons aged ≥60 years. The estimated average annual number of hospitalizations with VTE was comparable for men (250,973) and women (296,623).[10] Shown below is an image depicting the estimated average annual number of hospitalization with a diagnosis of DVT, PE, or VTE by age and sex (image courtesy of CDC.gov[10]).
- The average annual rates of hospitalizations with a discharge diagnosis of DVT, PE, or VTE among adults were 152, 121, and 239 per 100,000 population, respectively. For VTE, the average annual rates were 60 per 100,000 population aged 18–39 years, 143 for persons aged 40–49 years, 200 for persons aged 50–59 years, 391 for persons aged 60–69 years, 727 for persons aged 70–79 years, and 1,134 for persons aged ≥80 years. The rates of hospitalization were similar for men and women, and the point estimates increased for both sexes by age.[10]
- On average, 28,726 hospitalized adults with a VTE diagnosis died each year. Of these patients, an average of 13,164 had a DVT diagnosis and 19,297 had a PE diagnosis; 3,735 had both DVT and PE diagnoses.[10]
Recurrence of VTE
- There are mixed results regarding the rate of PE recurrence which ranged in the literature from as low as 2 to 50%.[11][12][13] According to some reports, one-third (about 33%) of people with VTE will have a recurrence within 10 years.[14][2] A follow up period of 2.2 years of subsequent observation of 265 patients reported that the risk of recurrence of VTE in patients diagnosed with first-time VTE to be around 7-8 percent per year. [15]
- Among patients with a first episode of VTE, the risk of recurrence of VTE is elevated in the first 6 to 12 months following the first episode of VTE, particularly in the first week. The risk of recurrent VTE remains up to 10 years, with an estimated cumulative incidence of first overall VTE recurrence of 30 %. Predictors for recurrence of VTE include malignancy, neurological diseases, and paresis.[16]
Mortality
- The hospital mortality rates of PE in untreated PE patients and treated PE patients are approximately 30% and 8%, respectively.[17][18][19] Unfortunately, two-thirds of all PE cases are diagnosed by autopsy. [20] Pulmonary embolism causes death in approximately 16% of hospitalized patients.
- A 26% mortality rate associated with untreated PE is often cited based upon a trial published in 1960 by Barrit and Jordan[21] which compared anti-coagulation against placebo for the management of pulmonary embolism. Barritt and Jordan performed their study in the Bristol Royal Infirmary in 1957. This study is the only placebo controlled trial ever to examine the efficacy of anticoagulants in the treatment of PE. The results of this were so convincing that the trial has not been repeated. On the other hand, the reported mortality rate of 26% in the placebo group may underestimate the true mortality insofar as the sensitivity and specificity of diagnostic technology in 1957 may have only allowed the detection of massive PE.
- Factors that are associated with an elevated rate of mortality in PE are:[13]
- Age >60 years
- Congestive heart failure
- Pulmonary hypertension
- Ischemic heart disease
- Chronic lung disease
- Cancer
- Stroke
- The presence of congestive heart failure or cancer are independent correlates of increased mortality in PE.[13]
- The one year mortality in patients who had an episode of PE was reported to be approximately 24%, only 2.5% of which is due to PE itself (usually within the first two weeks) while the two third of one-year mortality is due to other medical conditions such as heart disease, lung disease, cancer, or sepsis.[13]
- The rate of PE-related mortality varies according to the severity of PE:
- Massive PE, also known as high risk PE, is associated with a rate PE-related early mortality of > 15%.[22]
- Submassive PE, also known as intermediate risk PE, is associated with a rate of PE-related early mortality ranging from 3 to 15%.[22]
- Low risk PE is associated with a rate of PE-related early mortality of <1%.[22]
- Estimates suggest that 60,000-100,000 Americans die of VTE, 10 to 30% of which will die within one month of diagnosis.[23][2]
- An analysis of multiple-cause mortality files compiled by the National Center for Health Statistics from 1979 to 1998 reported that out of 42,932,973 deaths that occurred, almost 600,000 patients (approximately 1.5 percent) had been diagnosed with PE. PE might have caused the death of 200,000 of those patients.[24]
Clinical Correlates of Mortality among Patients with PE
Hemodynamic Status
Observational studies such as the International Co-operative Pulmonary Embolism Registry (ICOPER) and the Management and Prognosis in Pulmonary Embolism Trial (MAPPET) have shown that shock and hypotension are principal high risk markers of early death in acute PE.[25] The MAPPET study demonstrated that systemic shock was associated with mortality of 24.5% whereas hypotension (but not shock) was associated with a mortality of 15.2%.
A post-hoc analysis of the ICOPER study demonstrated that the 90-day all-cause mortality rate was 52.4% (95% CI,43.3–62.1%) among patients with a systolic blood pressure less than 90 mm Hg compared to 14.7% (95% CI, 13.3–16.2%) among patients with a normal blood pressure.[26]
Markers of Right Ventricular Dysfunction (RVD)
The presence of right ventricular dysfunction (RVD) on echocardiography has been associated with a higher mortality in the setting of pulmonary embolism.[27][28][29][30][31][32]
Brain Natriuretic Peptide
In patients with a pulmonary embolism, elevated plasma levels of natriuretic peptides (brain natriuretic peptide and N-terminal pro-brain natriuretic peptide) have been associated with higher mortality.[33] Levels of N-terminal pro-brain natriuretic peptide greater than 500 ng/L serve as an indicator of the burden of PE and are associated with death.[34]
Serum Troponin
Elevated serum troponin levels are associated with an increased risk of death among pulmonary embolism patients. The elevation of troponin in patients with a massive pulmonary embolism does not reflect epicadial coronary artery disease but rather transmural RV infarctions on autopsy.[35] [36]
Hyponatremia
Hyponatremia at the time of presentation of PE is associated with increased mortality and hospital readmission.[37]
Electrocardiographic Abnormalities
The electrocardiographic findings in pulmonary embolism lack specificity and sensitivity and their prognostic value is limited. Development of a QR wave in lead V1 has been identified as an independent risk factor for an adverse prognosis.[38]
ESC 2008 Guidelines for Prognostic Assessment (DO NOT EDIT)[22]
| Class I |
| "1. Initial risk stratification of suspected and/or confirmed PE based on the presence of shock and hypotension is recommended to distinguish between patients with high and non-high-risk of PE-related early mortality. (Level of Evidence: B) " |
| Class II |
| "1. In non-high-risk PE patients, further stratification to an intermediate- or low-risk PE subgroup based on the presence of imaging or biochemical markers of RVD and myocardial injury should be considered.(Level of Evidence: B) " |
References
- ↑ CDC- Deep Vein Thrombosis (DVT) / Pulmonary Embolism (PE) — Blood Clot Forming in a Vein
- ↑ 2.0 2.1 2.2 Beckman MG, Hooper WC, Critchley SE, Ortel TL (2010). "Venous thromboembolism: a public health concern". Am J Prev Med. 38 (4 Suppl): S495–501. doi:10.1016/j.amepre.2009.12.017. PMID 20331949.
- ↑ Carrier M, Righini M, Wells PS, Perrier A, Anderson DR, Rodger MA; et al. (2010). "Subsegmental pulmonary embolism diagnosed by computed tomography: incidence and clinical implications. A systematic review and meta-analysis of the management outcome studies". J Thromb Haemost. 8 (8): 1716–22. doi:10.1111/j.1538-7836.2010.03938.x. PMID 20546118.
- ↑ den Exter PL, van Es J, Klok FA, Kroft LJ, Kruip MJ, Kamphuisen PW; et al. (2013). "Risk profile and clinical outcome of symptomatic subsegmental acute pulmonary embolism". Blood. 122 (7): 1144–9, quiz 1329. doi:10.1182/blood-2013-04-497545. PMID 23736701.
- ↑ "Acute pulmonary embolism: clinical outcomes in the International Cooperative Pulmonary Embolism Registry (ICOPER) : The Lancet". Retrieved 2012-10-07.
- ↑ "Thrombolysis Compared With Heparin for the Initial Treatment of Pulmonary Embolism". Retrieved 2012-10-06.
- ↑ Aujesky D, Obrosky DS, Stone RA, Auble TE, Perrier A, Cornuz J; et al. (2005). "Derivation and validation of a prognostic model for pulmonary embolism". Am J Respir Crit Care Med. 172 (8): 1041–6. doi:10.1164/rccm.200506-862OC. PMC 2718410. PMID 16020800.
- ↑ 8.0 8.1 Subramanian M, Gopalan S, Ramadurai S, Arthur P, Prabhu MA, Thachathodiyl R; et al. (2017). "Derivation and Validation of a Novel Prediction Model to Identify Low-Risk Patients With Acute Pulmonary Embolism". Am J Cardiol. 120 (4): 676–681. doi:10.1016/j.amjcard.2017.05.043. PMID 28683900.
- ↑ den Exter PL, Zondag W, Klok FA, Brouwer RE, Dolsma J, Eijsvogel M; et al. (2016). "Efficacy and Safety of Outpatient Treatment Based on the Hestia Clinical Decision Rule with or without N-Terminal Pro-Brain Natriuretic Peptide Testing in Patients with Acute Pulmonary Embolism. A Randomized Clinical Trial". Am J Respir Crit Care Med. 194 (8): 998–1006. doi:10.1164/rccm.201512-2494OC. PMID 27030891.
- ↑ 10.0 10.1 10.2 10.3 10.4 [1] Hussain R. Yusuf, MD, James Tsai, MD, Hani K. Atrash, MD, Sheree Boulet, DrPH, Scott D. Grosse, PhD, Div of Blood Disorders, National Center on Birth Defects and Developmental Disabilities, CDC. Venous Thromboembolism in Adult Hospitalizations — United States, 2007–2009
- ↑ Paraskos JA, Adelstein SJ, Smith RE, Rickman FD, Grossman W, Dexter L; et al. (1973). "Late prognosis of acute pulmonary embolism". N Engl J Med. 289 (2): 55–8. doi:10.1056/NEJM197307122890201. PMID 4710405.
- ↑ PHEAR D (1960). "Pulmonary embolism. A study of late prognosis". Lancet. 2 (7155): 832–5. PMID 13735229.
- ↑ 13.0 13.1 13.2 13.3 Carson JL, Kelley MA, Duff A, Weg JG, Fulkerson WJ, Palevsky HI; et al. (1992). "The clinical course of pulmonary embolism". N Engl J Med. 326 (19): 1240–5. doi:10.1056/NEJM199205073261902. PMID 1560799.
- ↑ CDC- Deep Vein Thrombosis (DVT) / Pulmonary Embolism (PE) — Blood Clot Forming in a Vein
- ↑ Cushman M, Tsai AW, White RH; et al. (2004). "Deep vein thrombosis and pulmonary embolism in two cohorts: the longitudinal investigation of thromboembolism etiology". Am. J. Med. 117 (1): 19–25. doi:10.1016/j.amjmed.2004.01.018. PMID 15210384. Unknown parameter
|month=ignored (help) - ↑ Heit JA, Mohr DN, Silverstein MD, Petterson TM, O'Fallon WM, Melton LJ (2000). "Predictors of recurrence after deep vein thrombosis and pulmonary embolism: a population-based cohort study". Arch. Intern. Med. 160 (6): 761–8. PMID 10737275. Unknown parameter
|month=ignored (help) - ↑ HERMANN RE, DAVIS JH, HOLDEN WD (1961). "Pulmonary embolism. A clinical and pathologic study with emphasis on the effect of prophylactic therapy with anticoagulants". Am J Surg. 102: 19–28. PMID 13713631.
- ↑ MORRELL MT, TRUELOVE SC, BARR A (1963). "PULMONARY EMBOLISM". Br Med J. 2 (5361): 830–5. PMC 1872984. PMID 14063921.
- ↑ BARRITT DW, JORDAN SC (1960). "Anticoagulant drugs in the treatment of pulmonary embolism. A controlled trial". Lancet. 1 (7138): 1309–12. PMID 13797091.
- ↑ American Heart Association. (2007). Venous Thromboembolism & Pulmonary Embolism - Statistical Fact Sheet: 2007 Update. Retreived from http://stopdvt.org/Documents/AMA%20Fact%20Sheet%20Current%20Research.pdf
- ↑ "Anticoagulant drugs in the treatment of pulmonary embolism: a controlled trial". Lancet. 1: 1309&ndash, 1312. 1960. PMID 13797091. Text " Barritt DW, Jorden SC " ignored (help)
- ↑ 22.0 22.1 22.2 22.3 Torbicki A, Perrier A, Konstantinides S, Agnelli G, Galiè N, Pruszczyk P; et al. (2008). "Guidelines on the diagnosis and management of acute pulmonary embolism: the Task Force for the Diagnosis and Management of Acute Pulmonary Embolism of the European Society of Cardiology (ESC)". Eur Heart J. 29 (18): 2276–315. doi:10.1093/eurheartj/ehn310. PMID 18757870.
- ↑ CDC- Deep Vein Thrombosis (DVT) / Pulmonary Embolism (PE) — Blood Clot Forming in a Vein
- ↑ Horlander KT, Mannino DM, Leeper KV (2003). "Pulmonary embolism mortality in the United States, 1979-1998: an analysis using multiple-cause mortality data". Arch Intern Med. 163 (14): 1711–7. doi:10.1001/archinte.163.14.1711. PMID 12885687.
- ↑ Kasper W, Konstantinides S, Geibel A, Olschewski M, Heinrich F, Grosser KD; et al. (1997). "Management strategies and determinants of outcome in acute major pulmonary embolism: results of a multicenter registry". J Am Coll Cardiol. 30 (5): 1165–71. PMID 9350909.
- ↑ Kucher N, Rossi E, De Rosa M, Goldhaber SZ (2006). "Massive pulmonary embolism". Circulation. 113 (4): 577–82. doi:10.1161/CIRCULATIONAHA.105.592592. PMID 16432055.
- ↑ Konstantinides S (2005). "Pulmonary embolism: impact of right ventricular dysfunction". Curr Opin Cardiol. 20 (6): 496–501. PMID 16234620.
- ↑ Goldhaber SZ, Haire WD, Feldstein ML, Miller M, Toltzis R, Smith JL; et al. (1993). "Alteplase versus heparin in acute pulmonary embolism: randomised trial assessing right-ventricular function and pulmonary perfusion". Lancet. 341 (8844): 507–11. PMID 8094768.
- ↑ Ribeiro A, Lindmarker P, Juhlin-Dannfelt A, Johnsson H, Jorfeldt L (1997). "Echocardiography Doppler in pulmonary embolism: right ventricular dysfunction as a predictor of mortality rate". Am Heart J. 134 (3): 479–87. PMID 9327706.
- ↑ Kasper W, Konstantinides S, Geibel A, Tiede N, Krause T, Just H (1997). "Prognostic significance of right ventricular afterload stress detected by echocardiography in patients with clinically suspected pulmonary embolism". Heart. 77 (4): 346–9. PMC 484729. PMID 9155614.
- ↑ Grifoni S, Olivotto I, Cecchini P, Pieralli F, Camaiti A, Santoro G; et al. (2000). "Short-term clinical outcome of patients with acute pulmonary embolism, normal blood pressure, and echocardiographic right ventricular dysfunction". Circulation. 101 (24): 2817–22. PMID 10859287.
- ↑ Kucher N, Rossi E, De Rosa M, Goldhaber SZ (2005). "Prognostic role of echocardiography among patients with acute pulmonary embolism and a systolic arterial pressure of 90 mm Hg or higher". Arch Intern Med. 165 (15): 1777–81. doi:10.1001/archinte.165.15.1777. PMID 16087827.
- ↑ Cavallazzi R, Nair A, Vasu T, Marik PE (2008). "Natriuretic peptides in acute pulmonary embolism: a systematic review". Intensive Care Med. 34 (12): 2147–56. doi:10.1007/s00134-008-1214-5. PMID 18626627.
- ↑ Alonso-Martínez JL, Urbieta-Echezarreta M, Anniccherico-Sánchez FJ, Abínzano-Guillén ML, Garcia-Sanchotena JL (2009). "N-terminal pro-B-type natriuretic peptide predicts the burden of pulmonary embolism". Am J Med Sci. 337 (2): 88–92. doi:10.1097/MAJ.0b013e318182d33e. PMID 19214022.
- ↑ Becattini C, Vedovati MC, Agnelli G (2007). "Prognostic value of troponins in acute pulmonary embolism: a meta-analysis". Circulation. 116 (4): 427–33. doi:10.1161/CIRCULATIONAHA.106.680421. PMID 17606843.
- ↑ Jiménez D, Uresandi F, Otero R, Lobo JL, Monreal M, Martí D; et al. (2009). "Troponin-based risk stratification of patients with acute nonmassive pulmonary embolism: systematic review and metaanalysis". Chest. 136 (4): 974–82. doi:10.1378/chest.09-0608. PMID 19465511.
- ↑ Scherz N, Labarère J, Méan M, Ibrahim SA, Fine MJ, Aujesky D (2010). "Prognostic importance of hyponatremia in patients with acute pulmonary embolism". Am J Respir Crit Care Med. 182 (9): 1178–83. doi:10.1164/rccm.201003-0481OC. PMC 3001260. PMID 20595225.
- ↑ Kucher N, Walpoth N, Wustmann K, Noveanu M, Gertsch M (2003). "QR in V1--an ECG sign associated with right ventricular strain and adverse clinical outcome in pulmonary embolism". European Heart Journal. 24 (12): 1113–9. PMID 12804925. Retrieved 2011-12-05. Unknown parameter
|month=ignored (help)