Polio
For patient information click here Template:DiseaseDisorder infobox
|
Polio Microchapters |
|
Causes |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Polio On the Web |
|
American Roentgen Ray Society Images of Polio |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Pathophysiology & Etiology
| Outcome | Proportion of cases[1] |
|---|---|
| Asymptomatic | 90–95% |
| Minor illness | 4–8% |
| Non-paralytic aseptic meningitis |
1–2% |
| Paralytic poliomyelitis | 0.1–0.5% |
| — Spinal polio | 79% of paralytic cases |
| — Bulbospinal polio | 19% of paralytic cases |
| — Bulbar polio | 2% of paralytic cases |
Etiologic agent
Poliovirus is a member of the enterovirus subgroup, family Picornaviridae. Enteroviruses are transient inhabitants of the gastrointestinal tract, and are stable at acid pH. Picornaviruses are small, ether-insensitive viruses with an RNA genome.
There are three poliovirus serotypes (P1, P2, and P3). There is minimal heterotypic immunity between the three serotypes. That is, immunity to one serotype does not produce significant immunity to the other serotypes. The poliovirus is rapidly inactivated by heat, formaldehyde, chlorine, and ultraviolet light.
Pathogenesis

Poliovirus enters the body through the mouth, infecting the first cells it comes into contact with—follicular dendritic cells residing within the germinal centers of the tonsils and intestinal M cells—by binding to a immunoglobulin-like receptor known as the poliovirus receptor (CD155) on the cell surface. Once inside a human cell the virus hijacks the host cell's own machinery, and begins to replicate. Poliovirus divides within gastrointestinal cells for about one week before penetrating the intestinal lining. Following penetration, the virus is absorbed into the blood via the mesentery, and into the lymphatic system via the Peyer's patches.
Once the virus enters the bloodstream it becomes a viremia and is widely-distributed throughout the body. Poliovirus can survive and multiply within the blood and lymphatics for long periods of time, sometimes as long as 17 weeks. In a small percentage of cases the virus spreads and replicates in other sites such as brown fat, the reticuloendothelial tissues, and muscle. This sustained replication causes a secondary major viremia, and leads to the development of minor influenza-like symptoms.
Rarely, the major viremia progresses and the virus invades the central nervous system (CNS), causing a local inflammatory response. In most cases this causes a self limiting inflammation of the meninges, the layers of tissue surrounding the brain, causing non-paralytic aseptic meningitis. Penetration of the CNS provides no known benefit to the virus, and is quite possibly an "accidental" deviation of a normal gastrointestinal infection. The mechanisms by which poliovirus spreads to the CNS are poorly understood, but it appears to be primarily a chance event—largely independent of the age, gender, or socioeconomic position of the individual.
Paralytic polio

In approximately 1% of infections poliovirus spreads along certain nerve fiber pathways, preferentially replicating in and destroying motor neurons within the spinal cord, brain stem, or motor cortex, which leads to the development of paralytic poliomyelitis. The various forms of paralytic poliomyelitis (spinal, bulbar, and bulbospinal) vary only with the amount of neuronal damage and inflammation that occurs, and the region of the CNS that is affected.
The destruction of neuronal cells produces lesions within the spinal ganglia; lesions can also be found in the reticular formation, vestibular nuclei, cerebellar vermis, and deep cerebellar nuclei. Inflammation associated with nerve cell destruction often alters the color and appearance of the gray matter in the spinal column, causing it to appear reddish and swollen. Other changes associated with paralytic disease occur in the hypothalamus and thalamus. The molecular mechanisms by which poliovirus causes paralytic disease are poorly understood.
Early symptoms of paralytic polio include a high fever, headache, stiffness in the back and neck, asymmetrical weakness of various muscles, sensitivity to touch, difficulty swallowing, muscle pain, loss of superficial and deep reflexes, paresthesia, irritability, constipation, or difficulty urinating. Paralysis generally develops 1 to 10 days after early symptoms begin, and progresses for 2 to 3 days. Paralysis is usually complete when the fever breaks.
The likelihood of developing paralytic polio and the extent of paralysis increase with age. In children non-paralytic meningitis is the most likely consequence of CNS involvement, and paralysis occurs in only 1 in 1000 cases. In adults paralysis occurs in 1 in 75 cases. In children under 5 years of age paralysis of one leg is most common, while in adults extensive paralysis in the trunk and muscles of the chest and abdomen and affecting all four limbs—quadriplegia—is more likely. Paralysis rates also vary depending on the serotype of the infecting poliovirus. The highest rates of paralysis (1 in 200) are associated with poliovirus type 1, the lowest rates (1 in 2,000) are associated with type 2.
Spinal polio

Spinal polio is the most common form of paralytic poliomyelitis; it results from viral invasion of the motor neurons of the anterior horn cells, or the ventral (front) gray matter section in the spinal column, which are responsible for movement of the muscles, including those of the trunk, limbs and the intercostal muscles.[2] Virus invasion causes inflammation of the nerve cells, leading to damage or destruction of motor neuron ganglia. When spinal neurons die, Wallerian degeneration takes place, leading to weakness of those muscles formerly innervated by the now dead neurons.[3] With the destruction of nerve cells, the muscles no longer receive signals from the brain or spinal cord; without nerve stimulation, the muscles atrophy, becoming weak, floppy and poorly controlled, and finally completely paralyzed.[2] Progression to maximum paralysis is rapid (two to four days), and is usually associated with fever and muscle pain.[3] Deep tendon reflexes are also affected, and are usually absent or diminished; sensation (the ability to feel) in the paralyzed limbs, however, is not affected.[3]
The extent of spinal paralysis depends on the region of the cord affected, which may be cervical, thoracic, or lumbar.[4] The virus may affect muscles on both sides of the body, but more often the paralysis is asymmetrical.[5] Any limb or combination of limbs may be affected—one leg, one arm, or both legs and both arms. Paralysis is often more severe proximally (where the limb joins the body) than distally (the fingertips and toes). [6]
Bulbar polio

Comprising about 2% of cases of paralytic polio, bulbar polio occurs when poliovirus invades and destroys nerves within the bulbar region of the brain stem.[1] The bulbar region is a white matter pathway that connects the cerebral cortex to the brain stem. The destruction of these nerves weakens the muscles supplied by the cranial nerves, producing symptoms of encephalitis, and causes difficulty breathing, speaking and swallowing.[7] Critical nerves affected are the glossopharyngeal nerve, which partially controls swallowing and functions in the throat, tongue movement and taste; the vagus nerve, which sends signals to the heart, intestines, and lungs; and the accessory nerve, which controls upper neck movement. Due to the effect on swallowing, secretions of mucus may build up in the airway causing suffocation. [8] Other signs and symptoms include facial weakness, caused by destruction of the trigeminal nerve and facial nerve, which innervate the cheeks, tear ducts, gums, and muscles of the face, among other structures; double vision; difficulty in chewing; and abnormal respiratory rate, depth, and rhythm, which may lead to respiratory arrest. Pulmonary edema and shock are also possible, and may be fatal.[4]
Bulbospinal polio
Approximately 19% of all paralytic polio cases have both bulbar and spinal symptoms; this subtype is called respiratory polio or bulbospinal polio.[1] Here the virus affects the upper part of the cervical spinal cord (C3 through C5), and paralysis of the diaphragm occurs. The critical nerves affected are the phrenic nerve, which drives the diaphragm to inflate the lungs, and those that drive the muscles needed for swallowing. By destroying these nerves this form of polio affects breathing, making it difficult or impossible for the patient to breathe without the support of a ventilator. It can lead to paralysis of the arms and legs and may also affect swallowing and heart functions.[9]
Natural History
Records from antiquity mention crippling diseases compatible with poliomyelitis. Michael Underwood first described a debility of the lower extremities in children that was recognizable as poliomyelitis in England in 1789. The first outbreaks in Europe were reported in the early 19th century, and outbreaks were first reported in the United States in 1843. For the next hundred years, epidemics of polio were reported from developed countries in the Northern Hemisphere each summer and fall. These epidemics became increasingly severe, and the average age of persons affected rose. The increasingly older age of persons with primary infection increased both the disease severity and number of deaths from polio. Polio reached a peak in the United States in 1952, with more than 21,000 paralytic cases. However, following introduction of effective vaccines, polio incidence declined rapidly. The last case of wild-virus polio acquired in the United States was in 1979, and global polio eradication may be achieved within the next decade.
Polio Eradication
Following the widespread use of poliovirus vaccine in the mid-1950s, the incidence of poliomyelitis declined rapidly in many industrialized countries. In the United States, the number of cases of paralytic poliomyelitis reported annually declined from more than 20,000 cases in 1952 to fewer than 100 cases in the mid-1960s. The last documented indigenous transmission of wild poliovirus in the United States was in 1979.
In 1985, the member countries of the Pan American Health Organization adopted the goal of eliminating poliomyelitis from the Western Hemisphere by 1990. The strategy to achieve this goal included increasing vaccination coverage; enhancing surveillance for suspected cases (i.e., surveillance for acute flaccid paralysis); and using supplemental immunization strategies such as national immunization days, house-to-house vaccination, and containment activities. Since 1991, when the last wild-virus–associated indigenous case was reported from Peru, no additional cases of poliomyelitis have been confirmed despite intensive surveillance. In September 1994, an international commission certified the Western Hemisphere to be free of indigenous wild poliovirus. The commission based its judgment on detailed reports from national certification commissions that had been convened in every country in the region.
In 1988, the World Health Assembly (the governing body of the World Health Organization) adopted the goal of global eradication of poliovirus by the year 2000. Although this goal was not achieved, substantial progress has been made. One type of poliovirus appears to have already been eradicated. The last isolation of type 2 virus was in India in October 1999.
The Americas were declared polio-free in 1994.[10] In 2000 polio was officially eradicated in 36 Western Pacific countries, including China and Australia.[11][12] Europe was declared polio-free in 2002.[13] Today, polio remains endemic in only four countries: Nigeria, India, Pakistan, and Afghanistan.[14]
Diagnosis
A laboratory diagnosis of poliomyelitis is usually made based on recovery of poliovirus from the stool or pharynx. Neutralizing antibodies to poliovirus can be diagnostic and are generally detected in the blood of infected patients early in the course of infection. Analysis of the patient's cerebrospinal fluid (CSF), which is collected by a lumbar puncture ("spinal tap") reveals an increased number of white blood cells (primarily lymphocytes) and a mildly elevated protein level. Detection of virus from the CSF is diagnostic of paralytic polio, but rarely occurs.
If poliovirus is isolated from a patient experiencing acute flaccid paralysis it is further tested, using oligonucleotide mapping (genetic fingerprinting), or more recently by PCR amplification, to determine if the virus is “wild type” (that is, the virus encountered in nature) or vaccine type (is derived from a strain of poliovirus used to produce polio vaccine). For each reported case of paralytic polio caused by wild poliovirus, it is estimated that another 200 to 3,000 contagious asymptomatic carriers exist. Therefore, isolation of wild poliovirus constitutes a public health emergency, and appropriate efforts to control the spread of the disease must be initiated immediately.
History and Symptoms
The incubation period for poliomyelitis is commonly 6 to 20 days with a range of 3 to 35 days.
The response to poliovirus infection is highly variable and has been categorized on the basis of the severity of clinical presentation.
Up to 95% of all polio infections are inapparent or asymptomatic. Estimates of the ratio of inapparent to paralytic illness vary from 50:1 to 1,000:1 (usually 200:1). Infected persons without symptoms shed virus in the stool and are able to transmit the virus to others.
Approximately 4%–8% of polio infections consist of a minor, nonspecific illness without clinical or laboratory evidence of central nervous system invasion. This clinical presentation is known as abortive poliomyelitis, and is characterized by complete recovery in less than a week. Three syndromes observed with this form of poliovirus infection are upper respiratory tract infection (sore throat and fever), gastrointestinal disturbances (nausea, vomiting, abdominal pain, constipation or, rarely, diarrhea), and influenza-like illness. These syndromes are indistinguishable from other viral illnesses.
Nonparalytic aseptic meningitis (symptoms of stiffness of the neck, back, and/or legs), usually following several days after a prodrome similar to that of minor illness, occurs in 1%–2% of polio infections. Increased or abnormal sensations can also occur. Typically these symptoms will last from 2 to 10 days, followed by complete recovery.
Fewer than 1% of all polio infections result in flaccid paralysis. Paralytic symptoms generally begin 1 to 10 days after prodromal symptoms and progress for 2 to 3 days. Generally, no further paralysis occurs after the temperature returns to normal. The prodrome may be biphasic, especially in children, with initial minor symptoms separated by a 1- to 7-day period from more major symptoms. Additional prodromal signs and symptoms can include a loss of superficial reflexes, initially increased deep tendon reflexes and severe muscle aches and spasms in the limbs or back. The illness progresses to flaccid paralysis with diminished deep tendon reflexes, reaches a plateau without change for days to weeks, and is usually asymmetrical. Strength then begins to return. Patients do not experience sensory losses or changes in cognition.
Many persons with paralytic poliomyelitis recover completely and, in most, muscle function returns to some degree. Weakness or paralysis still present 12 months after onset is usually permanent.
Paralytic polio is classified into three types, depending on the level of involvement. Spinal polio is most common, and during 1969–1979, accounted for 79% of paralytic cases. It is characterized by asymmetric paralysis that most often involves the legs. Bulbar polio leads to weakness of muscles innervated by cranial nerves and accounted for 2% of cases during this period. Bulbospinal polio, a combination of bulbar and spinal paralysis, accounted for 19% of cases.
The death-to-case ratio for paralytic polio is generally 2%–5% among children and up to 15%–30% for adults (depending on age). It increases to 25%–75% with bulbar involvement.
Laboratory Findings
Viral Isolation
Poliovirus may be recovered from the stool or pharynx of a person with poliomyelitis. Isolation of virus from the cerebrospinal fluid (CSF) is diagnostic, but is rarely accomplished. If poliovirus is isolated from a person with acute flaccid paralysis, it must be tested further, using oligonucleotide mapping (fingerprinting) or genomic sequencing, to determine if the virus is “wild type” (that is, the virus that causes polio disease) or vaccine type (virus that could derive from a vaccine strain).
Serology
Neutralizing antibodies appear early and may be at high levels by the time the patient is hospitalized; therefore, a fourfold rise in antibody titer may not be demonstrated.
Cerebrospinal Fluid
In poliovirus infection, the CSF usually contains an increased number of white blood cells (10–200 cells/mm3, primarily lymphocytes) and a mildly elevated protein (40–50 mg/100 mL).
Risk Stratification
After an interval of 30–40 years, 25%–40% of persons who contracted paralytic poliomyelitis in childhood experience new muscle pain and exacerbation of existing weakness, or develop new weakness or paralysis. This disease entity is referred to as postpolio syndrome. Factors that increase the risk of postpolio syndrome include increasing length of time since acute poliovirus infection, presence of permanent residual impairment after recovery from the acute illness, and female sex. The pathogenesis of postpolio syndrome is thought to involve the failure of oversized motor units created during the recovery process of paralytic poliomyelitis. Postpolio syndrome is not an infectious process, and persons experiencing the syndrome do not shed poliovirus.
Treatment

No cure for polio exists, and the focus of modern polio treatment has been on increasing comfort, speeding recovery and preventing complications. Supportive measures include: antibiotics to prevent infections in weakened muscles, analgesics for pain, moderate exercise and a nutritious diet. Treatment of polio also often requires long-term rehabilitation including physical therapy, braces, corrective shoes and, in some cases, orthopedic surgery.
Portable ventilators may be required to support breathing. Historically, a noninvasive negative-pressure ventilator (more commonly called an iron lung) was used to artificially maintain respiration during an acute polio infection until a person could breathe independently; generally about one to two weeks. Today many polio survivors with permanent respiratory paralysis use modern jacket-type negative-pressure ventilators that are worn over the chest and abdomen.
Other historical treatments for polio have included hydrotherapy, electrotherapy and surgical treatments such as tendon lengthening and nerve grafting. The use of devices such as rigid braces and body casts—which tended to cause muscle atrophy due to the limited movement of the user—were also touted as effective treatments. Massage, passive motion exercises, and vitamin C were also used to treat polio victims, with varying degrees of success.
Primary Prevention
Passive immunization
In 1950, William Hammon at the University of Pittsburgh purified the gamma globulin component of the blood plasma of polio survivors.[15] Hammon proposed that the gamma globulin, which contained antibodies to poliovirus, could be used to halt poliovirus infection, prevent disease, and reduce the severity of disease in other patients who had contracted polio. The results of a large clinical trial were promising; the gamma globulin was shown to be about 80% effective in preventing the development of paralytic poliomyelitis.[16] It was also shown to reduce the severity of the disease in patients that developed polio.[15] The gamma globulin approach was later deemed impractical for widespread use, however, due in large part to the limited supply of blood plasma, and the medical community turned its focus to the development of a polio vaccine.[17]
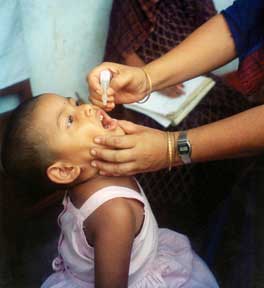
Antibody serum
In 1950 William Hammon at the University of Pittsburgh isolated a serum from the blood of polio survivors. Hammon proposed that the serum, which contained antibodies to poliovirus, could be used to halt poliovirus infection, prevent disease, and reduce the severity of disease in other patients who had contracted polio. The results of a large clinical trial were promising; the serum was shown to be about 80% effective in preventing the development of paralytic poliomyelitis. The serum was also shown to reduce the severity of the disease in patients that developed polio. The antibody approach was later deemed impractical for widespread use, however, due in large part to the limited supply of blood plasma, and the medical community turned its focus to the development of a polio vaccine.
Vaccine
Two polio vaccines are used throughout the world to combat polio. Both vaccines induce immunity to polio, efficiently blocking person-to-person transmission of wild poliovirus, thereby protecting both individual vaccine recipients and the wider community (so-called herd immunity).
The first polio vaccine was developed in 1952 by Jonas Salk at the University of Pittsburgh, and announced to the world on April 12, 1955. The Salk vaccine, or inactivated poliovirus vaccine (IPV), is based on poliovirus grown in a type of monkey kidney tissue culture (Vero cell line), which is chemically-inactivated with formalin. After two doses of IPV, ninety percent or more of individuals develop protective antibody to all three serotypes of poliovirus, and at least 99% are immune to poliovirus following three doses. IPV is currently the vaccine of choice in most countries.
Eight years after Salk's success, Albert Sabin developed an oral polio vaccine (OPV) using live but weakened (attenuated) virus, produced by the repeated passage of the virus through non-human cells at sub-physiological temperatures. Human trials of Sabin's vaccine began in 1957 and it was licensed in 1962. The attenuated poliovirus in the Sabin vaccine replicates very efficiently in the gut, the primary site of wild poliovirus infection and replication, but the vaccine strain is unable to replicate efficiently within nervous system tissue. OPV produces excellent immunity in the intestine, which helps prevent infection with wild virus in areas where the virus is endemic. A single dose of oral polio vaccince produces immunity to all three poliovirus serotypes in approximately 50% of recipients. Three doses of live-attenuated OPV produce protective antibody to all three poliovirus types in more than 95% of recipients.
Prognosis
Patients with abortive polio infections recover completely. In those that develop only aseptic meningitis, the symptoms can be expected to persist for two to ten days, followed by complete recovery.[18] In cases of spinal polio, if the affected nerve cells are completely destroyed, paralysis will be permanent; cells that are not destroyed but lose function temporarily may recover within four to six weeks after onset.[18] Half the patients with spinal polio recover fully, one quarter recover with mild disability and the remaining quarter are left with severe disability.[19] The degree of both acute paralysis and residual paralysis is likely to be proportional to the degree of viremia, and inversely proportional to the degree of immunity.[20]. Spinal polio is rarely fatal.[21]
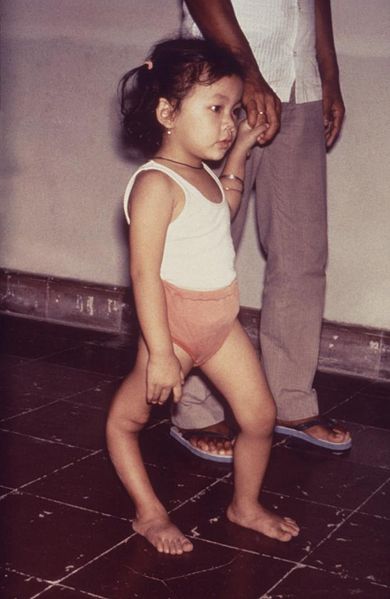
Without respiratory support, consequences of poliomyelitis with respiratory involvement include suffocation or pneumonia from aspiration of secretions.[22] Overall, 5–10% of patients with paralytic polio die due to the paralysis of muscles used for breathing. The mortality rate varies by age: 2–5% of children and up to 15–30% of adults die.[1] Bulbar polio often causes death if respiratory support is not provided;[9] with support, its mortality rate ranges from 25 to 75%, depending on the age of the patient.[1][23] When positive pressure ventilators are available, the mortality can be reduced to 15%.[24]
Recovery
Many cases of poliomyelitis result in only temporary paralysis.[25] Nerve impulses return to the formerly paralyzed muscle within a month, and recovery is usually complete in six to eight months.[18] The neurophysiological processes involved in recovery following acute paralytic poliomyelitis are quite effective; muscles are able to retain normal strength even if half the original motor neurons have been lost.[26] Paralysis remaining after one year is likely to be permanent, although modest recoveries of muscle strength are possible 12 to 18 months after infection.[18]
One mechanism involved in recovery is nerve terminal sprouting, in which remaining brainstem and spinal cord motor neurons develop new branches, or axonal sprouts.[27] These sprouts can reinnervate orphaned muscle fibers that have been denervated by acute polio infection,[28] restoring the fibers' capacity to contract and improving strength.[29] Terminal sprouting may generate a few significantly enlarged motor neurons doing work previously performed by as many as four or five units: [30] a single motor neuron that once controlled 200 muscle cells might control 800 to 1000 cells. Other mechanisms that occur during the rehabilitation phase, and contribute to muscle strength restoration, include myofiber hypertrophy—enlargement of muscle fibers through exercise and activity—and transformation of type II muscle fibers to type I muscle fibers.[28][31]
In addition to these physiological processes, the body possesses a number of compensatory mechanisms to maintain function in the presence of residual paralysis. These include the use of weaker muscles at a higher than usual intensity relative to the muscle's maximal capacity, enhancing athletic development of previously little-used muscles, and using ligaments for stability, which enables greater mobility.[31]
Complications
Residual complications of paralytic polio often occur following the initial recovery process. [32] Muscle paresis and paralysis can sometimes result in skeletal deformities, tightening of the joints and movement disability. Once the muscles in the limb become flaccid, they may interfere with the function of other muscles. A typical manifestation of this problem is equinus foot (similar to club foot). This deformity develops when the muscles that pull the toes downward are working, but those that pull it upward are not, and the foot naturally tends to drop toward the ground. If the problem is left untreated, the Achilles tendons at the back of the foot retract and the foot cannot take on a normal position. Polio victims that develop equinus foot cannot walk properly because they cannot put their heel on the ground. A similar situation can develop if the arms become paralyzed.[33] In some cases the growth of an affected leg is slowed by polio, while the other leg continues to grow normally. The result is that one leg is shorter than the other and the person limps and leans to one side, in turn leading to deformities of the spine (such as scoliosis).[33] Osteoporosis and increased likelihood of bone fractures may occur. Extended use of braces or wheelchairs may cause compression neuropathy, as well as a loss of proper function of the veins in the legs, due to pooling of blood in paralyzed lower limbs.[34][9] Complications from prolonged immobility involving the lungs, kidneys and heart include pulmonary edema, aspiration pneumonia, urinary tract infections, kidney stones, paralytic ileus, myocarditis and cor pulmonale.[34][9]
Post-polio syndrome
Around a quarter of individuals who survive paralytic polio in childhood develop additional symptoms decades after recovering from the acute infection, notably muscle weakness, extreme fatigue, or paralysis. This condition is known as post-polio syndrome (PPS).[35] The symptoms of PPS are thought to involve a failure of the over-sized motor units created during recovery from paralytic disease.[36][37] Factors that increase the risk of PPS include the length of time since acute poliovirus infection, the presence of permanent residual impairment after recovery from the acute illness, and both overuse and disuse of neurons.[35] Post-polio syndrome is not an infectious process, and persons experiencing the syndrome do not shed poliovirus.[1]
References
- ↑ 1.0 1.1 1.2 1.3 1.4 1.5
- ↑ 2.0 2.1 Frauenthal HWA, Manning JVV (1914). Manual of infantile paralysis, with modern methods of treatment. Philadelphia Davis. pp. 79–101. OCLC 2078290.
- ↑ 3.0 3.1 3.2 Cono J, Alexander LN (2002). "Chapter 10, Poliomyelitis.". Vaccine Preventable Disease Surveillance Manual (PDF) (3rd ed. ed.). Centers for Disease Control and Prevention. pp. p. 10–1.
- ↑ 4.0 4.1 Professional Guide to Diseases (Professional Guide Series). Hagerstown, MD: Lippincott Williams & Wilkins. 2005. pp. 243–5. ISBN 1-58255-370-X.
- ↑ Yin-Murphy M, Almond JW (1996). "Picornaviruses: The Enteroviruses: Polioviruses", Baron's Medical Microbiology (Baron S et al, eds.), 4th ed., Univ of Texas Medical Branch, e-text. ISBN 0-9631172-1-1
- ↑ Yin-Murphy M, Almond JW (1996). "Picornaviruses: The Enteroviruses: Polioviruses", Baron's Medical Microbiology (Baron S et al, eds.), 4th ed., Univ of Texas Medical Branch, e-text. ISBN 0-9631172-1-1
- ↑
- ↑ Silverstein A, Silverstein V, Nunn LS (2001). Polio, Diseases and People. Berkeley Heights, NJ: Enslow Publishers, 12. ISBN 0-7660-1592-0.
- ↑ 9.0 9.1 9.2 9.3 Hoyt, William Graves; Miller, Neil; Walsh, Frank (2005). Walsh and Hoyt's clinical neuro-ophthalmology. Hagerstown, MD: Lippincott Williams & Wilkins. pp. 3264–65. ISBN 0-7817-4814-3.
- ↑ "International Notes Certification of Poliomyelitis Eradication—the Americas, 1994". MMWR Morb Mortal Wkly Rep. Centers for Disease Control and Prevention. 43 (39): 720–2. 1994. PMID 7522302.
- ↑ "General News. Major Milestone reached in Global Polio Eradication: Western Pacific Region is certified Polio-Free" (PDF). Health Educ Res. 16 (1): p. 109. 2001.
- ↑ D'Souza R, Kennett M, Watson C (2002). "Australia declared polio free". Commun Dis Intell. 26 (2): 253–60. PMID 12206379.
- ↑ "Europe achieves historic milestone as Region is declared polio-free" (Press release). European Region of the World Health Organization. 2002-06-21. Retrieved 2007-11-07. Check date values in:
|date=(help) - ↑ "Update on vaccine-derived polioviruses" (2006). MMWR Morb Mortal Wkly Rep 55 (40): 1093–7. PMID 17035927
- ↑ 15.0 15.1 Hammon W (1955). "Passive immunization against poliomyelitis". Monogr Ser World Health Organ. 26: 357–70. PMID 14374581.
- ↑ Hammon W, Coriell L, Ludwig E; et al. (1954). "Evaluation of Red Cross gamma globulin as a prophylactic agent for poliomyelitis. 5. Reanalysis of results based on laboratory-confirmed cases". J Am Med Assoc. 156 (1): 21–7. PMID 13183798.
- ↑ Rinaldo C (2005). "Passive immunization against poliomyelitis: the Hammon gamma globulin field trials, 1951–1953". Am J Public Health. 95 (5): 790–9. PMID 15855454.
- ↑ 18.0 18.1 18.2 18.3 Neumann D (2004). "Polio: its impact on the people of the United States and the emerging profession of physical therapy" (PDF). The Journal of orthopaedic and sports physical therapy. 34 (8): 479–92. PMID 15373011. Reproduced online with permission by Post-Polio Health International; retrieved on 2007-11-10.
- ↑ Cuccurullo SJ (2004). Physical Medicine and Rehabilitation Board Review. Demos Medical Publishing. ISBN 1-888799-45-5.
- ↑ Mueller S, Wimmer E, Cello J (2005). "Poliovirus and poliomyelitis: a tale of guts, brains, and an accidental event". Virus Res 111 (2): 175–93. PMID 15885840
- ↑ Silverstein A, Silverstein V, Nunn LS (2001). Polio, Diseases and People. Berkeley Heights, NJ: Enslow Publishers, 12. ISBN 0-7660-1592-0.
- ↑ Goldberg A (2002). "Noninvasive mechanical ventilation at home: building upon the tradition". Chest. 121 (2): 321–4. PMID 11834636.
- ↑ Miller AH, Buck LS (1950). "Tracheotomy in bulbar poliomyelitis". California medicine. 72 (1): 34–6. PMID 15398892.
- ↑ Template:Cite paper
- ↑ Frauenthal HWA, Manning JVV (1914). Manual of infantile paralysis, with modern methods of treatment.. Philadelphia Davis, 79–101. OCLC 2078290
- ↑ Sandberg A, Hansson B, Stålberg E (1999). "Comparison between concentric needle EMG and macro EMG in patients with a history of polio". Clinical Neurophysiology. 110 (11): 1900–8. PMID 10576485.
- ↑ Cashman NR, Covault J, Wollman RL, Sanes JR (1987). "Neural cell adhesion molecule in normal, denervated, and myopathic human muscle". Ann. Neurol. 21 (5): 481–9. PMID 3296947.
- ↑ 28.0 28.1 Agre JC, Rodríquez AA, Tafel JA (1991). "Late effects of polio: critical review of the literature on neuromuscular function". Archives of physical medicine and rehabilitation. 72 (11): 923–31. PMID 1929813.
- ↑ Trojan DA, Cashman NR (2005). "Post-poliomyelitis syndrome". Muscle Nerve. 31 (1): 6–19. PMID 15599928.
- ↑ Gawne AC, Halstead LS (1995). "Post-polio syndrome: pathophysiology and clinical management". Critical Review in Physical Medicine and Rehabilitation 7: 147–88. Reproduced online with permission by Lincolnshire Post-Polio Library; retrieved on 2007-11-10.
- ↑ 31.0 31.1 Grimby G, Einarsson G, Hedberg M, Aniansson A (1989). "Muscle adaptive changes in post-polio subjects". Scandinavian journal of rehabilitation medicine. 21 (1): 19–26. PMID 2711135.
- ↑ Leboeuf C (1992). The late effects of Polio: Information For Health Care Providers. (PDF), Commonwealth Department of Community Services and Health. ISBN 1-875412-05-0. Retrieved on 2007-11-10.
- ↑ 33.0 33.1 Sanofi Pasteur. "Poliomyelitis virus (picornavirus, enterovirus), after-effects of the polio, paralysis, deformations". Polio Eradication. Retrieved 2007-07-31.
- ↑ 34.0 34.1 Mayo Clinic Staff (2005-05-19). "Polio: Complications". Mayo Foundation for Medical Education and Research (MFMER). Retrieved 2007-02-26. Check date values in:
|date=(help) - ↑ 35.0 35.1 Trojan D, Cashman N (2005). "Post-poliomyelitis syndrome". Muscle Nerve. 31 (1): 6–19. PMID 15599928.
- ↑ Ramlow J, Alexander M, LaPorte R, Kaufmann C, Kuller L (1992). "Epidemiology of the post-polio syndrome". Am. J. Epidemiol. 136 (7): 769–86. PMID 1442743.
- ↑ Lin K, Lim Y (2005). "Post-poliomyelitis syndrome: case report and review of the literature" (PDF). Ann Acad Med Singapore. 34 (7): 447–9. PMID 16123820.
Further reading
- Frauenthal HWA, Manning JVV (1914). Manual of infantile paralysis, with modern methods of treatment: Pathology. Philadelphia: Davis. pp. pp. 79–101. OCLC 2078290. (Full text available from Google Books, with hundreds of pictures.)
- Huckstep RL (1975). Poliomyelitis: a guide for developing countries - including appliances and rehabilitation for the disabled. Edinburgh: Churchill Livingstone. ISBN 0443013128. (A look at the modern polio patient and polio treatment techniques.)
- http://www.cdc.gov/vaccines/pubs/pinkbook/downloads/polio.pdf
- http://en.wikipedia.org/wiki/Poliomyelitis
- Template:Dmoz
- Polio: A Virus' Struggle – an amusing yet educational graphic novella from the Science Creative Quarterly (co-published by the University of British Columbia, in PDF format).
- Fermín: Making Polio History An article about Luis Fermín Tenorio Cortez, the last case of polio reported in the Americas.
- A UK Polio survivor – An account of John Prestwich, who lived 50 years in an iron lung.
Acknowledgements
The content on this page was first contributed by: C. Michael Gibson, M.S., M.D.
ar:شلل أطفال ca:Poliomielitis da:Polio de:Poliomyelitis eo:Poliomjelito gl:Poliomielite ko:소아마비 io:Poliomielito id:Poliomielitis is:Mænusótt it:Poliomielite he:שיתוק ילדים lt:Poliomielitas nl:Poliomyelitis no:Poliomyelitt nn:Poliomyelitt simple:Poliomyelitis sk:Detská obrna su:Polio fi:Polio sv:Polio te:పోలియో uk:Поліомієліт