Hypertrophic cardiomyopathy diagnostic testing: Difference between revisions
mNo edit summary |
mNo edit summary |
||
| Line 1: | Line 1: | ||
{{Hypertrophic cardiomyopathy}} | {{Hypertrophic cardiomyopathy}} | ||
'''Editor-In-Chief:''' C. Michael Gibson, M.S., M.D. [mailto:mgibson@perfuse.org]; {{AOEIC}} | '''Editor-In-Chief:''' [[C. Michael Gibson, M.S., M.D.]] [mailto:mgibson@perfuse.org]; {{AOEIC}} {{CZ}}; Caitlin J. Harrigan [mailto:charrigan@perfuse.org]; Martin S. Maron, M.D.; Barry J. Maron, M.D.; [[Lakshmi Gopalakrishnan, M.B.B.S.]] | ||
==Overview== | ==Overview== | ||
Revision as of 17:53, 22 December 2011
|
Hypertrophic Cardiomyopathy Microchapters |
|
Differentiating Hypertrophic Cardiomyopathy from other Diseases |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Hypertrophic cardiomyopathy diagnostic testing On the Web |
|
Directions to Hospitals Treating Hypertrophic cardiomyopathy |
|
Risk calculators and risk factors for Hypertrophic cardiomyopathy diagnostic testing |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]; Caitlin J. Harrigan [3]; Martin S. Maron, M.D.; Barry J. Maron, M.D.; Lakshmi Gopalakrishnan, M.B.B.S.
Overview
A diagnosis of hypertrophic cardiomyopathy is based upon a number of features of the disease process. While there is use of echocardiography, cardiac catheterization, or cardiac MRI in the diagnosis of the disease, other important factors include ECG findings and if there is any family history of HCM or unexplained sudden death in otherwise healthy individuals.
Electrocardiogram
Large septal q waves may be present reflective of the septal hypertrophy. In the Yamaguchi variant of apical hypertrophic cardiomyopathy there may be deeply inverted T waves in precordial leads V2-V6 and II, III, aVL (see example).

2011 ACCF/AHA Guideline Recommendations: Electrocardiography [1][2]
| “ |
Class I1. 1. A 12-lead ECG is recommended in the initial evaluation of patients with HOCM. (Level of Evidence: C) 2. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring is recommended in the initial evaluation of patients with HOCM to detect ventricular tachycardia (VT) and identify patients who may be candidates for ICD therapy.[3][4][5][6] (Level of Evidence: B) 3. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring or event recording is recommended in patients with HOCM who develop palpitations or lightheadedness.[3][4][5] (Level of Evidence: B) 4. A repeat ECG is recommended for patients with HOCM when there is worsening of symptoms. (Level of Evidence: C) 5. A 12-lead ECG is recommended every 12 to 18 months as a component of the screening algorithm for adolescent first-degree relatives of patients with HOCM who have no evidence of hypertrophy on echocardiography. (Level of Evidence: C) 6. A 12-lead ECG is recommended as a component of the screening algorithm for first-degree relatives of patients with HOCM. (Level of Evidence: C) Class IIa1. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring, repeated every 1 to 2 years, is reasonable in patients with HOCM who have no previous evidence of VT to identify patients who may be candidates for ICD therapy.[6] (Level of Evidence: C) 2. Annual 12-lead ECGs are reasonable in patients with known HOCM who are clinically stable to evaluate for asymptomatic changes in conduction or rhythm (i.e., AF). (Level of Evidence: C) Class IIb1. Twenty-four–hour ambulatory (Holter) electrocardiographic monitoring might be considered in adults with HOCM to assess for asymptomatic paroxysmal AF/atrial flutter. (Level of Evidence: C) |
” |
Echocardiography
Echo with doppler is the primary proceedure used to diagnose hypertrophic cardiomyopathy. There is a prolonged isovolumic relaxation time, reduced peak E velocity, prolonged deceleration time, increased peak A velocity, and decreased E/A ratio as compared to normal controls. Proper examination should evaluate [7]:
- Left ventricular asymmetric hypertrophy
- Parasternal long axis shows relationshipo of the septal hypertrophy and the outflow tract
- Left ventricular diastolic dysfunction
- LV inflow across the mitral valve
- LA inflow in the pulmonary vein
- Myocardial Doppler tissue velocity
- Isovolumetric relaxation time
- Dynamic outflow tract obstruction
- SAM (systolic anterior motion) of the mitral leaflet
- Mid-systolic closure of the aortic valve
- Late peaking, high velocity flow in the outflow tract
- Variability of obstruction with maneuvers (exercise, amyl nitrate inhalation, and post-PVC beats)
- Doppler Techniques
- Use continuous wave doppler to measure the systolic flow velocity in the LV outflow tract and mid-cavity (both at rest and during maneuvers such as the Valsalva maneuver or during dobutamine administration.
Because of the turbulent, high-velocity jet in the left ventricular outflow tract (LVOT), the anterior mitral leaflet moves anteriorly in systole, exacerbating the outflow tract obstruction, and promoting mitral regurgitation. The following images show classic systolic anterior motion (SAM) of the mitral valve leaflets:
On parasternal long-axis view
{{#ev:googlevideo|-4301014230430356751}}
On parasternal short-axis view
{{#ev:googlevideo|8393870190048992602}}
2011 ACCF/AHA Guideline Recommendations: Electrocardiography [1][2]
| “ |
Class I1. (Level of Evidence: C) |
” |
Cardiac MRI
Late Myocardial Enhancement
Late myocardial enhancement has been associated with myocardial fibrosis and may allow for earlier detection of hypertrophic cardiomyopathy than is currently available with echocardiography and ECG.
- Choudhury et al studied 21 patients with previously diagnosed hypertrophic cardiomyopathy[8]. They noted:
- Late myocardial enhancement following gadolinium administration in a patchy intramyocardial distribution.
- Typically occurred in the hypertrophied regions, predominantly in the middle third of the ventricular wall in a patchy, multi focal distribution.
- If enhancement occurred, it occurred at the junctions of the intraventricular septum and right ventricular free wall.
- Moon et al looked at whether the extent of hyperenhancement on MR in patients with HCM would be associated with the risk of heart failure and sudden death [9] The study involved 53 patient were selected for presence or absence of an increased clinical risk of sudden death and/or progressive adverse left ventricular remodeling.
- Myocardial hyperenhancement was present in 79% of patients.
- They found no evidence of abnormal myocardium on non-contrast images.
- There was more hyperenhancement in patients with progressive disease than without.
- There was greater hyperenhancement in patients with ≥ 2 risk factors for sudden death.
- Patients with diffuse hyperenhancement had ≥ 2 risk factors for sudden death vs patients with confluent hyperenhancement.
Of note, other investigators have discovered that in carriers without signs of hypertrophy on EKG or echocardiography, Cardiac MR can detect the presence of crypts in the LV wall which may progress to hypertrophy.
Left Ventricular Hypertrophy
MR is helpful in visualizing the asymmetric thickening of the interventricular septum in patients with HCM. However, it may be more helpful than other forms of imaging to differentiate the variant types of hypertrophic cardiomyopathy.[10]
Mitral Regurgitation and Systolic Anterior Motion
MR can be helpful in evaluating the extent of systolic anterior motion of the mitral valve.
Obstruction
MR can be help visualize turbulence in left ventricular outflow tract created by obstruction in patients with obstructive hypertrophic cardiomyopathy.
{{#ev:youtube|xP3gvVFGUaU}}
Cardiac CT
Echocardiographic findings reflect the clinical and anatomic findings described above, e.g. LVH, diastolic dysfunction, MR, LA enlargement, elevated PAP.
Characteristic of obstructive HM is systolic anterior motion of the mitral valve (SAM). The anterior leaflet is pulled toward the LVOT during systole via the Venturi effect, leading to obstruction, a gradient and MR.
Positron Emission Tomography
Positron Emission Tomography (PET) studies have demonstrated that coronary flow reserve is reduced in patients with HCM. Those patients who subsequently died had a greater reduction in coronary flow reserve at baseline. It has been hypothesized that this ischemia may mediate in part the higher risk in sudden cardiac death.
Cardiac Catheterization
Upon cardiac catheterization, catheters can be placed in the left ventricle and the ascending aorta, to measure the pressure difference between these structures. In normal individuals, during ventricular systole, the pressure in the ascending aorta and the left ventricle will equalize, and the aortic valve is open. In individuals with aortic stenosis or with HCM with an outflow tract gradient, there will be a pressure gradient (difference) between the left ventricle and the aorta, with the left ventricular pressure higher than the aortic pressure. This gradient represents the degree of obstruction that has to be overcome in order to eject blood from the left ventricle.
The Brockenbrough–Braunwald–Morrow sign is observed in individuals with HCM with outflow tract gradient. This sign can be used to differentiate HCM from aortic stenosis. In individuals with aortic stenosis, after a premature ventricular contraction (PVC), the following ventricular contraction will be more forceful, and the pressure generated in the left ventricle will be higher. Because of the fixed obstruction that the stenotic aortic valve represents, the post-PVC ascending aortic pressure will increase as well. In individuals with HCM, however, the degree of obstruction will increase more than the force of contraction will increase in the post-PVC beat. The result of this is that the left ventricular pressure increases and the ascending aortic pressure decreases, with an increase in the LVOT gradient.
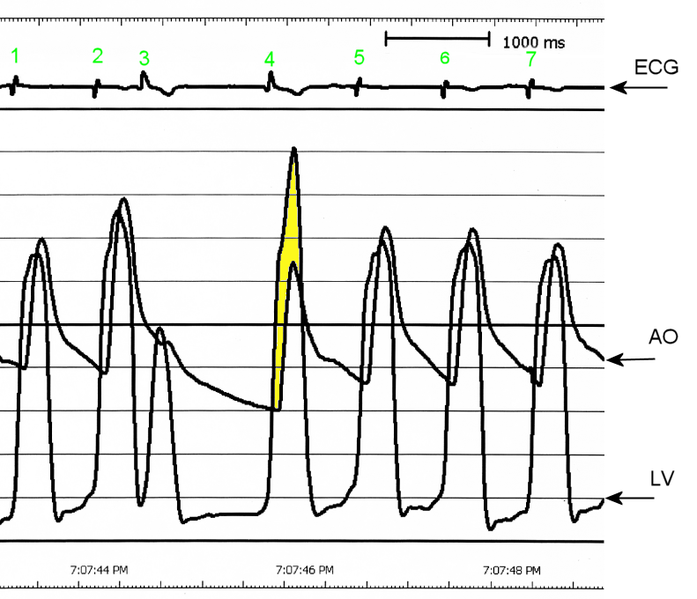
AO = Descending aorta; LV = Left ventricle; ECG = Electrocardiogram.
After the third QRS complex, the ventricle has more time to fill. Since there is more time to fill, the left ventricle will have more volume at the end of diastole (increased preload). Due to the Frank–Starling law of the heart, the contraction of the left ventricle (and pressure generated by the left ventricle) will be greater on the subsequent beat (beat #4 in this picture). Because of the dynamic nature of the outflow obstruction in HCM, the obstruction increases more that the left ventricular pressure increase. This causes a fall in the aortic pressure as the left ventricular pressure rises (seen as the yellow shaded area in the picture).
While the Brockenbrough–Braunwald–Morrow sign is most dramatically demonstrated using simultaneous intra-cardiac and intra-aortic catheters, it can be seen on routine physical examination as a decrease in the pulse pressure in the post-PVC beat in individuals with HCM.
Electrophysiologic study
The prognostic value of electrophysiologic testing in the absence of spontaneous, sustained ventricular tachycardia is limited, and in fact, the study itself may be dangerous. Sustained ventricular tachyarrhythmias, predominantly rapid polymorphic ventricular tachycardia, have been induced in 27 to 43 percent of patients with HCM at electrophysiologic study, but their prognostic significance is controversial. The predictive value of asymptomatic nonsustained ventricular tachycardia is also limited. Paced electrogram fractionation in hypertrophic cardiomyopathy may helpful in determining which patients are at risk for ventricular fibrillation.
The absence of inducible, sustained monomorphic ventricular tachyarrhythmias, absence of nonsustained ventricular tachycardia on ambulatory ECG, and no history of impaired consciousness (i.e., cardiac arrest or syncope) identified a subset (22 percent) of patients with HCM with a low (<1 percent) risk for sudden cardiac death.
Guideline Resources
2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy [1][2]
References
- ↑ 1.0 1.1 1.2 Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011). "2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy: Executive Summary A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Journal of the American College of Cardiology. 58 (25): 2703–38. doi:10.1016/j.jacc.2011.10.825. PMID 22075468. Retrieved 2011-12-19. Unknown parameter
|month=ignored (help) - ↑ 2.0 2.1 2.2 Gersh BJ, Maron BJ, Bonow RO, Dearani JA, Fifer MA, Link MS, Naidu SS, Nishimura RA, Ommen SR, Rakowski H, Seidman CE, Towbin JA, Udelson JE, Yancy CW (2011). "2011 ACCF/AHA Guideline for the Diagnosis and Treatment of Hypertrophic Cardiomyopathy A Report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines Developed in Collaboration With the American Association for Thoracic Surgery, American Society of Echocardiography, American Society of Nuclear Cardiology, Heart Failure Society of America, Heart Rhythm Society, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Journal of the American College of Cardiology. 58 (25): e212–60. doi:10.1016/j.jacc.2011.06.011. PMID 22075469. Retrieved 2011-12-19. Unknown parameter
|month=ignored (help) - ↑ 3.0 3.1 Christiaans I, Birnie E, van Langen IM, van Spaendonck-Zwarts KY, van Tintelen JP, van den Berg MP, Atsma DE, Helderman-van den Enden AT, Pinto YM, Hermans-van Ast JF, Bonsel GJ, Wilde AA (2010). "The yield of risk stratification for sudden cardiac death in hypertrophic cardiomyopathy myosin-binding protein C gene mutation carriers: focus on predictive screening". European Heart Journal. 31 (7): 842–8. doi:10.1093/eurheartj/ehp539. PMID 20019025. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 4.0 4.1 Elliott PM, Gimeno JR, Tomé MT, Shah J, Ward D, Thaman R, Mogensen J, McKenna WJ (2006). "Left ventricular outflow tract obstruction and sudden death risk in patients with hypertrophic cardiomyopathy". European Heart Journal. 27 (16): 1933–41. doi:10.1093/eurheartj/ehl041. PMID 16754630. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 5.0 5.1 Maron BJ, Savage DD, Wolfson JK, Epstein SE (1981). "Prognostic significance of 24 hour ambulatory electrocardiographic monitoring in patients with hypertrophic cardiomyopathy: a prospective study". The American Journal of Cardiology. 48 (2): 252–7. PMID 7196685. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ 6.0 6.1 Monserrat L, Elliott PM, Gimeno JR, Sharma S, Penas-Lado M, McKenna WJ (2003). "Non-sustained ventricular tachycardia in hypertrophic cardiomyopathy: an independent marker of sudden death risk in young patients". Journal of the American College of Cardiology. 42 (5): 873–9. PMID 12957435. Retrieved 2011-12-22. Unknown parameter
|month=ignored (help) - ↑ Otto, Catherine. Textbook of Clinical Echocardiography. 3rd Edition, 2004
- ↑ Choudhury et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am Coll Card. 2002; 40: 2156.
- ↑ Moon et al., Toward Clinical Risk Assessment in Hypertrophic Cardiomyopathy with Gadolinium Cardiovascular Magnetic Resonance. J Am Coll Card. 2003; 41; 1561.
- ↑ Germans, T et al. Structural Abnormalities of the inferoseptal left ventricular wall detected by Cardiac Magnetic Resonance Imaging in carriers of Hypertrophic Cardiomyopathy mutations. J Am Coll Cardiol. 2006: 48; 2518.