Congenital heart disease: Difference between revisions
No edit summary |
No edit summary |
||
| Line 38: | Line 38: | ||
===[[Congenital heart disease causes | Causes]]=== | ===[[Congenital heart disease causes | Causes]]=== | ||
===[[Congenital heart disease differential diagnosis| Differential diagnosis]]=== | ===[[Congenital heart disease differential diagnosis|Differential diagnosis]]=== | ||
* [[Alcohol abuse|Alcohol use of the mother]] | * [[Alcohol abuse|Alcohol use of the mother]] | ||
* [[Chemotherapeutic]]s | * [[Chemotherapeutic]]s | ||
Revision as of 19:09, 28 June 2011
|
Congenital heart disease Microchapters |
|
Differentiating Congenital heart disease from other Disorders |
|---|
|
Diagnosis |
|
Treatment |
|
Case Studies |
|
Congenital heart disease On the Web |
|
American Roentgen Ray Society Images of Congenital heart disease |
|
Risk calculators and risk factors for Congenital heart disease |
| Congenital heart disease | ||
| ICD-10 | I01.0, I09.2, I30-I32 | |
|---|---|---|
| ICD-9 | 420.90 | |
| DiseasesDB | 9820 | |
| MedlinePlus | 000182 | |
| eMedicine | med/1781 emerg/412 | |
| MeSH | heart disease&field=entry#TreeC14.280.720 C14.280.720 | |
For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Keri Shafer, M.D. [2]
Associate Editor-In-Chief::Atif Mohammad, M.D.
Please Take Over This Page and Apply to be Editor-In-Chief for this topic:
There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
Causes
Differential diagnosis
- Alcohol use of the mother
- Chemotherapeutics
- Down's Syndrome
- Ellis-van Creveld Syndrome
- Genetic disorders
- Immunosuppressives
- Hypoxia (Lack of oxygen)
- Marfan's Syndrome
- Noonan Syndrome
- Radiation
- Retinoic acid
- Rubella
- Thalidomide
- Trisomy 13
- Turner's Syndrome
Classification
Congenital heart diseases are classified into 3 basic types
- Septal
- Obstructive
- Cyanotic
Obstruction defects
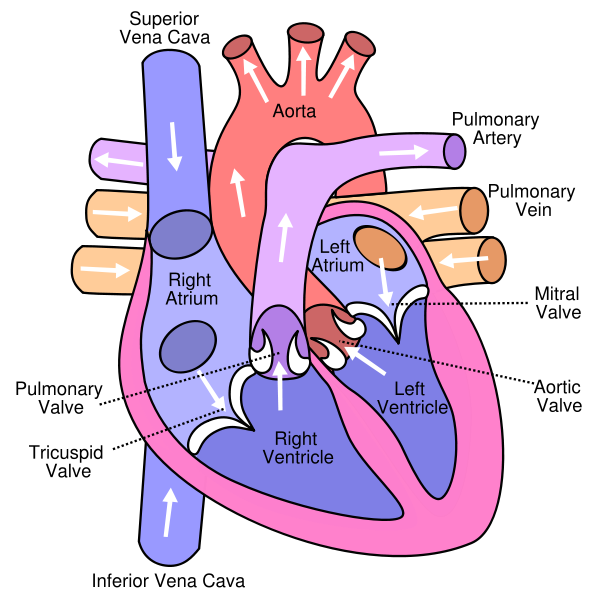
Obstruction defects occur when heart valves, arteries, or veins are abnormally narrow or blocked. Common obstruction defects include pulmonary valve stenosis, aortic valve stenosis, and coarctation of the aorta, with other types such as bicuspid aortic valve stenosis and subaortic stenosis being comparatively rare. Any narrowing or blockage can cause heart enlargement or hypertension.
Septal defects
The septum is a wall of tissue which separates the left heart from the right heart. It is comparatively common for defects to exist in the interatrial septum or the interventricular septum, allowing blood to flow from the left side of the heart to the right, reducing the heart's efficiency. Ventricular septal defects are collectively the most common type of CHD, although approximately 30% of adults have a type of atrial septal defect called probe patent foramen ovale. Septal defects may or may not cause cyanosis depending on the severity of the defect.
Cyanotic defects
Cyanotic heart defects are called such because they result in cyanosis, a bluish-grey discoloration of the skin due to a lack of oxygen in the body. Such defects include persistent truncus arteriosus, total anomalous pulmonary venous connection, tetralogy of Fallot, transposition of the great vessels, and tricuspid atresia.
Antenatal Detection and Diagnosis
Before birth, an obstetric ultrasound scan may be used to screen pregnant women for signs of CHD in their unborn babies. This screening scan is often performed around 20 weeks of pregnancy when the fast moving structures of the fetal heart are large enough to be more easily imaged. If CHD is suspected, a mother will be referred for a fetal echocardiograph, which is a more detailed, diagnostic ultrasound scan by a specialist cardiologist. It is increasingly possible for specialists to screen for CHD as early as 14 weeks, if CHD is suspected from other factors, such as a family history.
Postnatal Detection and Diagnosis
After delivery, if congenital heart disease is present but has not been detected, then a newborn baby may appear blue or breathless. Signs of CHD are sometimes mistaken for an infection or illness, so it is important to rule this out. Blueness and/or breathlessness may take some time to present, depending on the type of congenital heart disease and whether there is a duct-dependent lesion (i.e. one relying on an open ductus arteriosis for blood flow). This duct usually closes within the first three days of life in babies born at term (i.e. at nine months gestation).
Detection and Diagnosis in Adulthood
Although the majority of congenital heart disease diagnoses are made in childhood, there are significant congenital heart defects which may be go undetected until adulthood. These typically include defects that do not cause cyanosis ("blueness") in childhood but may cause problems over time, such as certain kinds of valve problems, transposition disorders, holes in the heart, and abnormalities of the heart's major veins and arteries. Congenital heart defects are most commonly diagnosed through an echocardiogram - an ultrasound of the heart which shows the heart's structure. Cardiac magnetic resonance(MRI) are used to confirm CHD when signs or symptoms occur in the physical examination. An echocardiograph displays images of the might also be used to confirm the problem, particularly in complex defects in which anatomy is hard to determine with echocardiography. It also finds abnormal rhythms or defects of the heart present with CHD. A chest x-ray may also be issued to look at the anatomical position of the heart and lungs. A Cat Scan(CT) can also be used to visualize CHD. All of these tests are ways to diagnose CHD by a physician.
ACC / AHA Guidelines-Recommendations for Permanent Pacing in Children, Adolescents, and Patients With Congenital (DO NOT EDIT) [1]
| “ |
Class I1. Permanent pacemaker implantation is indicated for advanced second- or third-degree AV block associated with symptomatic bradycardia, ventricular dysfunction, or low cardiac output. (Level of Evidence: C) 2. Permanent pacemaker implantation is indicated for SND with correlation of symptoms during age-inappropriate bradycardia. The definition of bradycardia varies with the patient’s age and expected heart rate. (Level of Evidence: B) 3. Permanent pacemaker implantation is indicated for postoperative advanced second- or third-degree AV block that is not expected to resolve or that persists at least 7 days after cardiac surgery. (Level of Evidence: B) 4. Permanent pacemaker implantation is indicated for congenital third-degree AV block with a wide QRS escape rhythm, complex ventricular ectopy, or ventricular dysfunction. (Level of Evidence: B) 5. Permanent pacemaker implantation is indicated for congenital third-degree AV block in the infant with a ventricular rate less than 55 bpm or with congenital heart disease and a ventricular rate less than 70 bpm. (Level of Evidence: C) Class IIa1. Permanent pacemaker implantation is reasonable for patients with congenital heart disease and sinus bradycardia for the prevention of recurrent episodes of intra-atrial reentrant tachycardia; SND may be intrinsic or secondary to antiarrhythmic treatment. (Level of Evidence: C) 2. Permanent pacemaker implantation is reasonable for congenital third-degree AV block beyond the first year of life with an average heart rate less than 50 bpm, abrupt pauses in ventricular rate that are 2 or 3 times the basic cycle length, or associated with symptoms due to chronotropic incompetence. (Level of Evidence: B) 3. Permanent pacemaker implantation is reasonable for sinus bradycardia with complex congenital heart disease with a resting heart rate less than 40 bpm or pauses in ventricular rate longer than 3 seconds. (Level of Evidence: C) 4. Permanent pacemaker implantation is reasonable for patients with congenital heart disease and impaired hemodynamics due to sinus bradycardia or loss of AV synchrony. (Level of Evidence: C) 5. Permanent pacemaker implantation is reasonable for unexplained syncope in the patient with prior congenital heart surgery complicated by transient complete heart block with residual fascicular block after a careful evaluation to exclude other causes of syncope. (Level of Evidence: B) Class IIb1. Permanent pacemaker implantation may be considered for transient postoperative third-degree AV block that reverts to sinus rhythm with residual bifascicular block. (Level of Evidence: C) 2. Permanent pacemaker implantation may be considered for congenital third-degree AV block in asymptomatic children or adolescents with an acceptable rate, a narrow QRS complex, and normal ventricular function. (Level of Evidence: B) 3. Permanent pacemaker implantation may be considered for asymptomatic sinus bradycardia after biventricular repair of congenital heart disease with a resting heart rate less than 40 bpm or pauses in ventricular rate longer than 3 seconds. (Level of Evidence: C) Class III1. Permanent pacemaker implantation is not indicated for transient postoperative AV block with return of normal AV conduction in the otherwise asymptomatic patient. (Level of Evidence: B) 2. Permanent pacemaker implantation is not indicated for asymptomatic bifascicular block with or without first-degree AV block after surgery for congenital heart disease in the absence of prior transient complete AV block. (Level of Evidence: C) 3. Permanent pacemaker implantation is not indicated for asymptomatic type I second-degree AV block. (Level of Evidence: C) 4. Permanent pacemaker implantation is not indicated for asymptomatic sinus bradycardia with the longest relative risk interval less than 3 seconds and a minimum heart rate more than 40 bpm. (Level of Evidence: C) |
” |
Outcomes
It is now estimated that the number of adults in the United States who have congenital heart disease is approaching one million. Because of advances in cardiac surgery, many who would not previously have survived childhood, now lead normal or relatively normal lives. However, some increase in complications has been observed in adults who were previously thought to have had successful repair of heart defects. These complications include cardiac arrhythmia, disorders of heart valves, and heart failure. Regular check-ups by cardiologists are now recommended for patients with histories of congenital heart disease, including those who may have previously been told that their defects were successfully repaired. Since most adult cardiologists have little experience with congenital heart disease, congenital heart disease centers[4] have been developed to care for adult patients with more severe congenital heart disease. It is thought that some patients, especially those with more complex disorders, and women who are pregnant or considering pregnancy, would likely do better if they are followed in specialty centers. Guidelines have been developed regarding which patients may be successfully followed in non-specialized cardiology practices, and which should be seen in adult congenital heart disease centers.
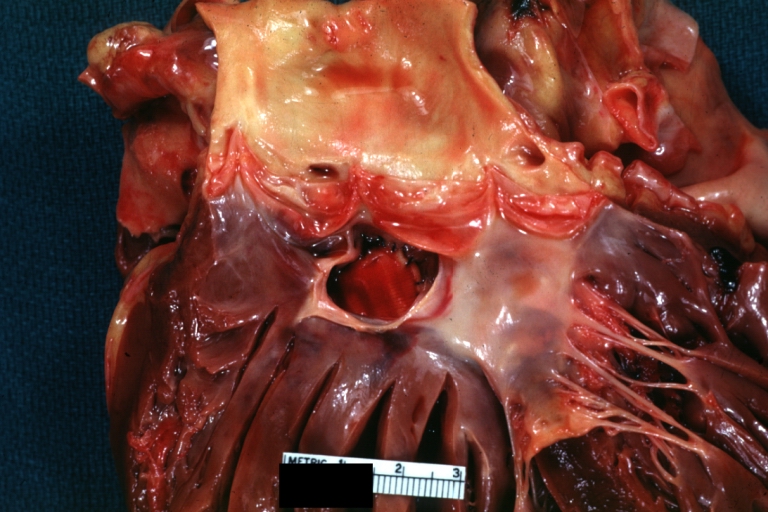
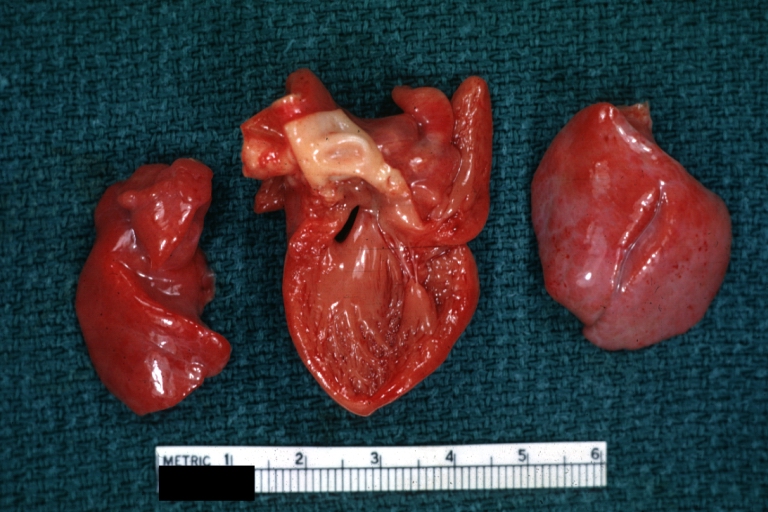
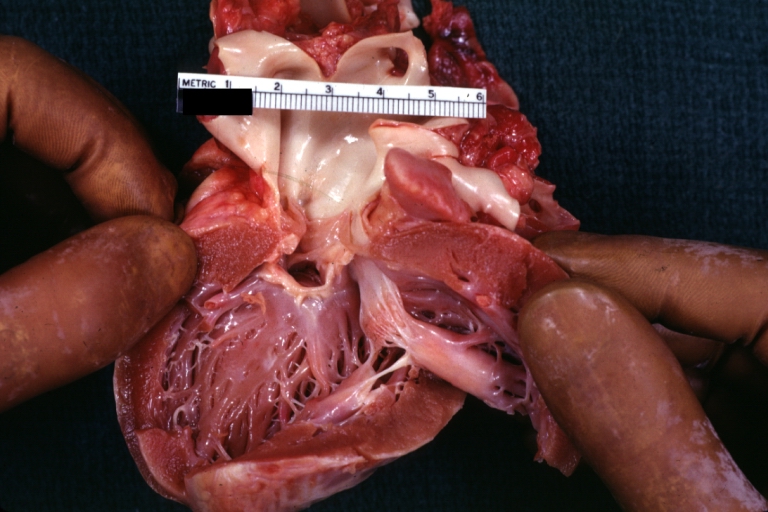
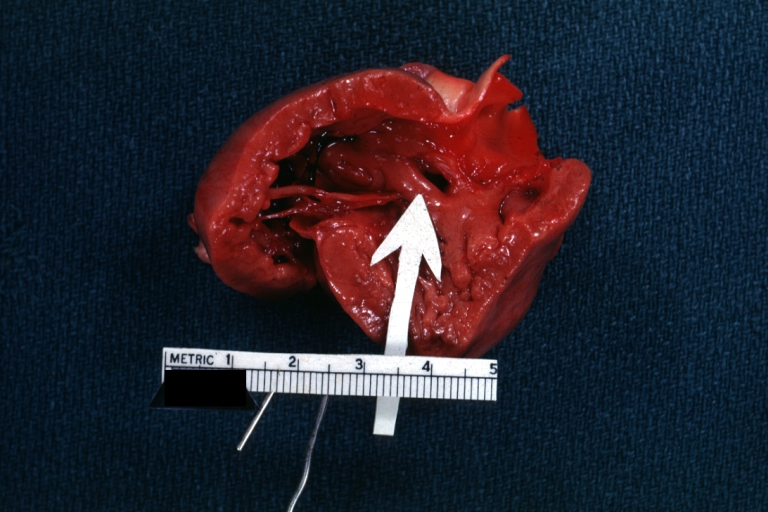
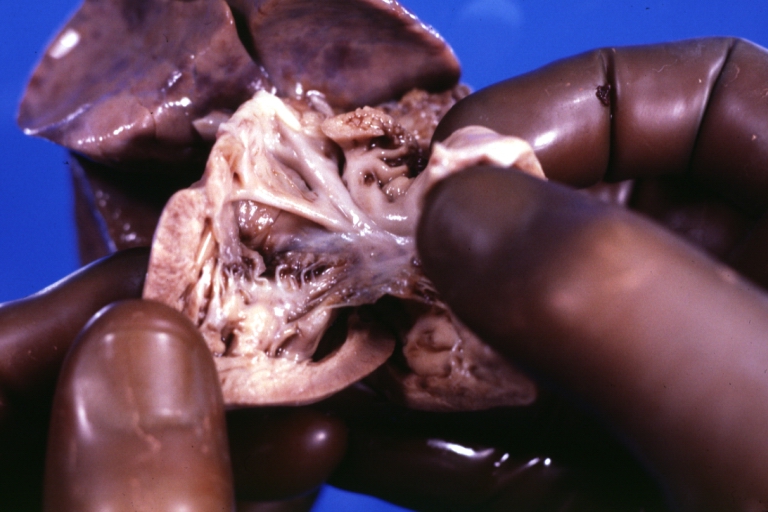
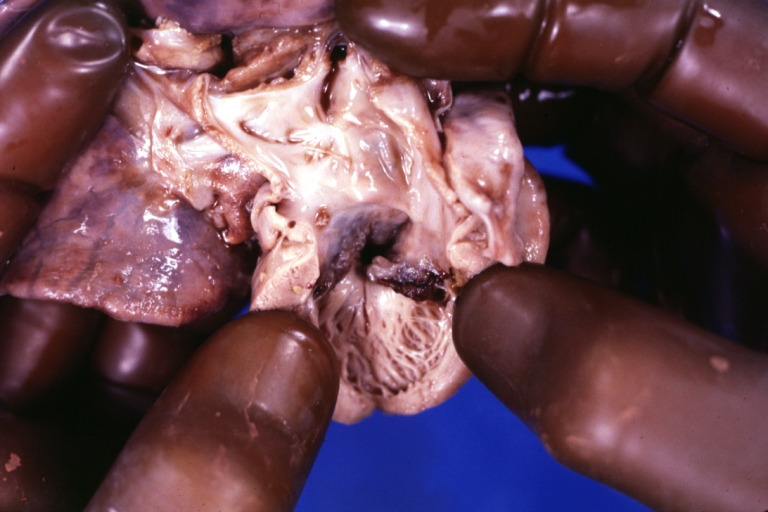
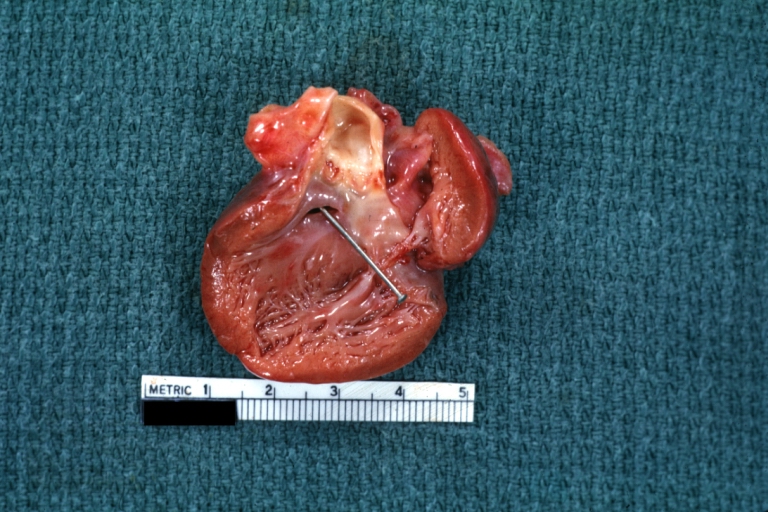
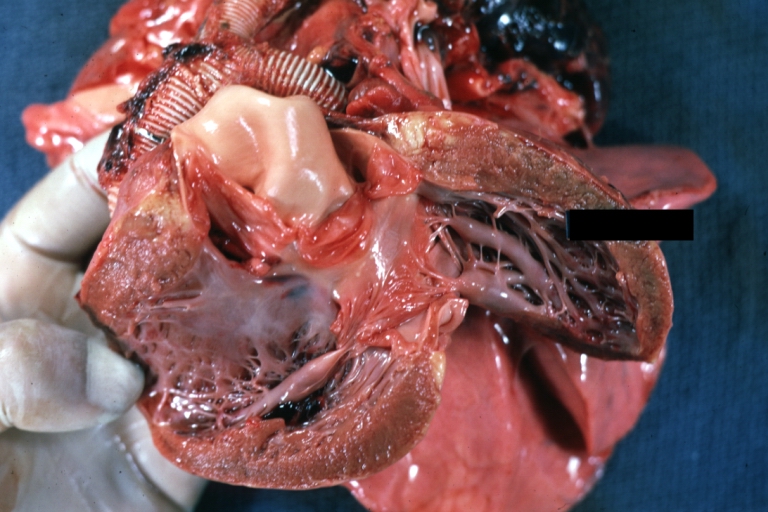
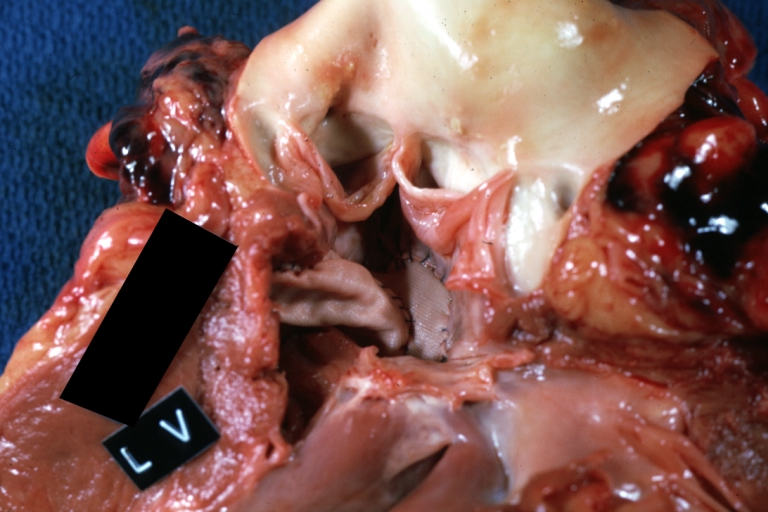
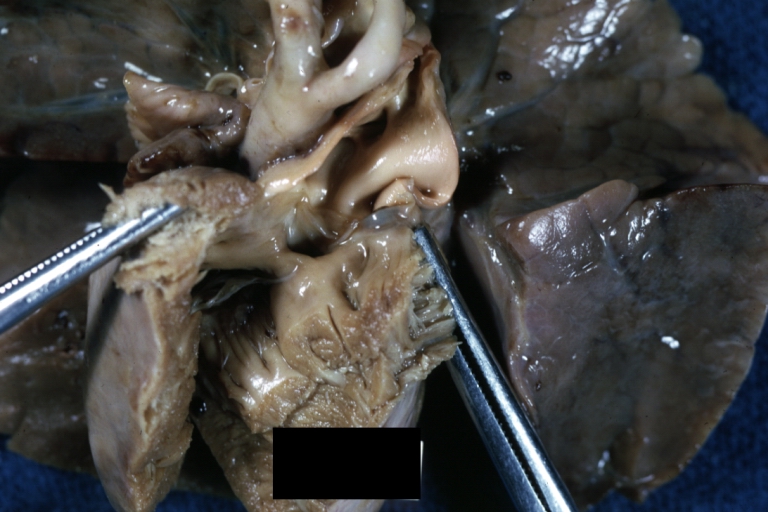
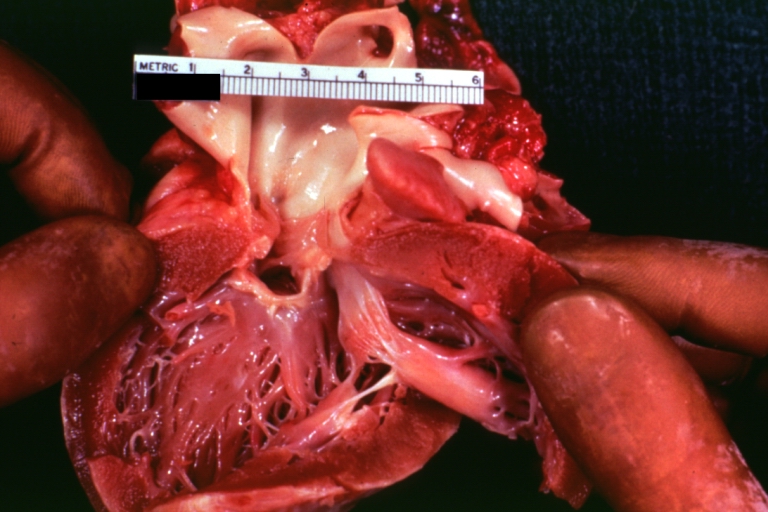
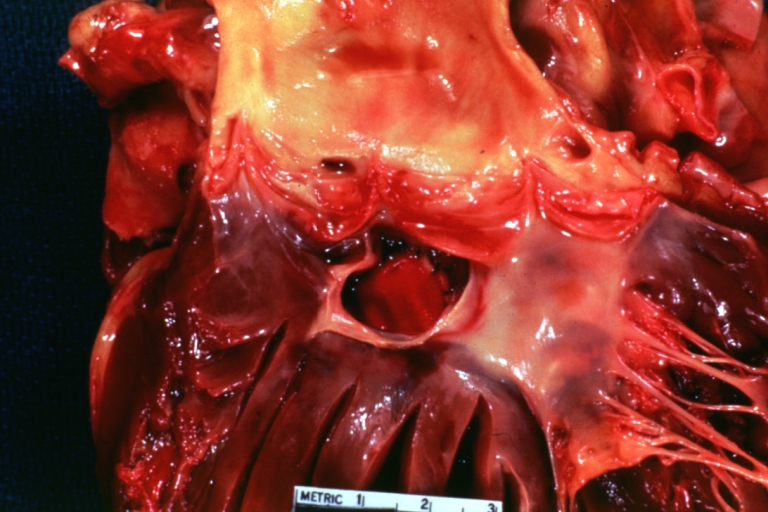
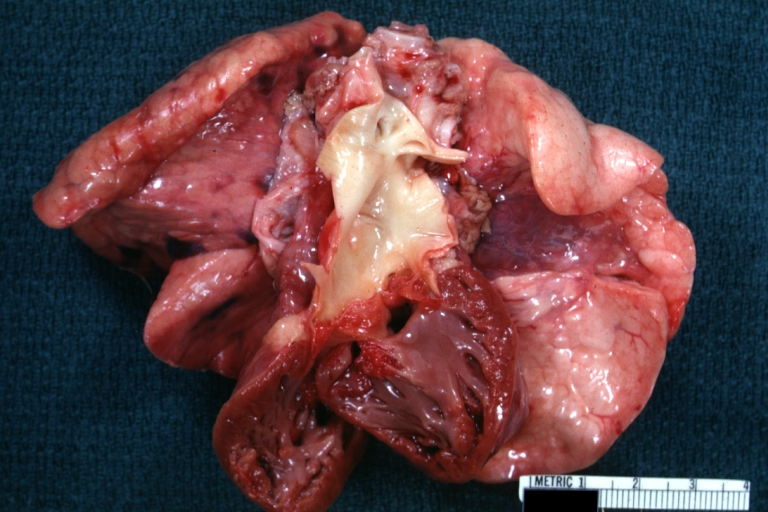
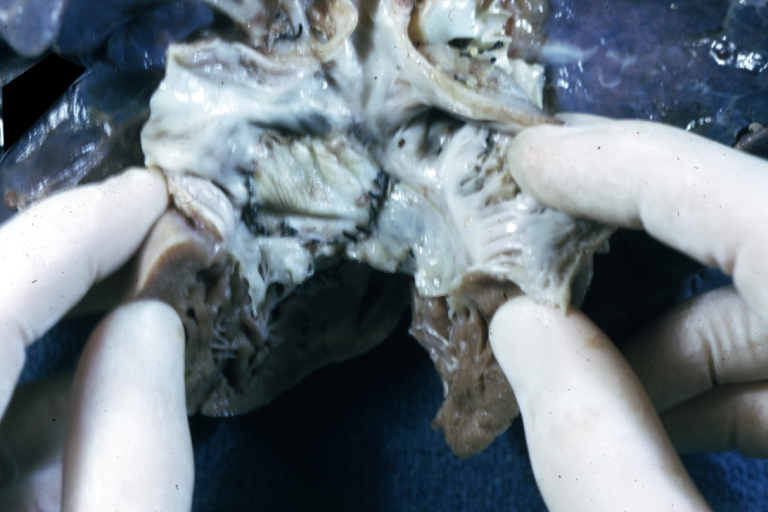
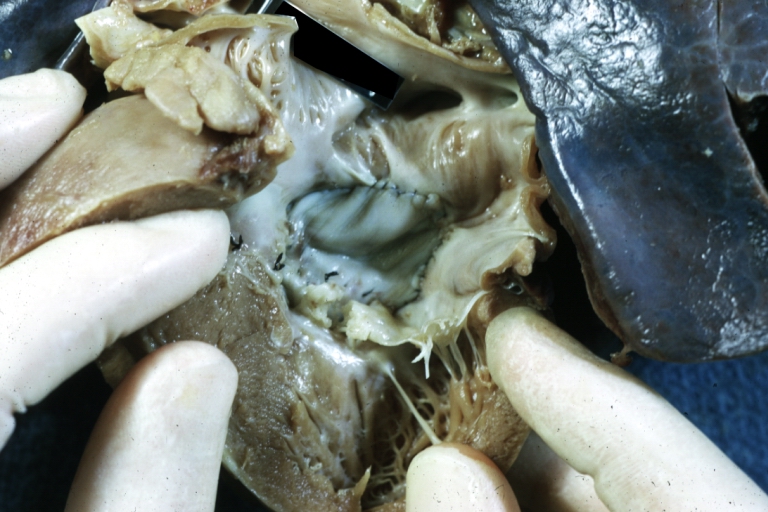
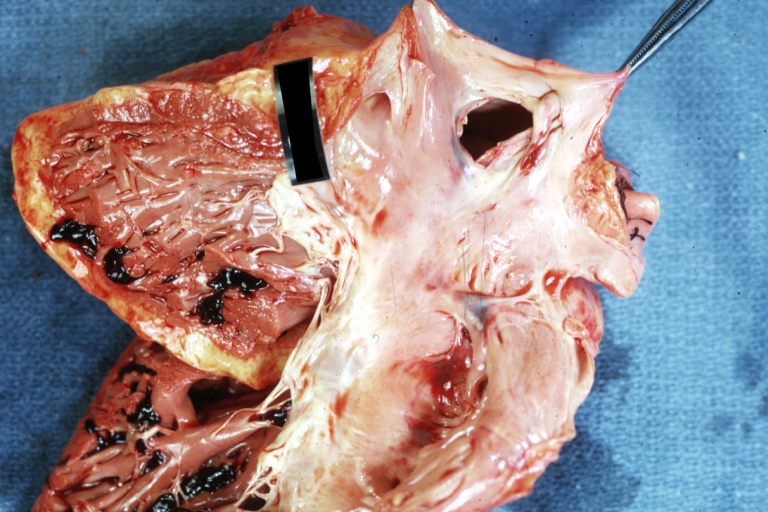
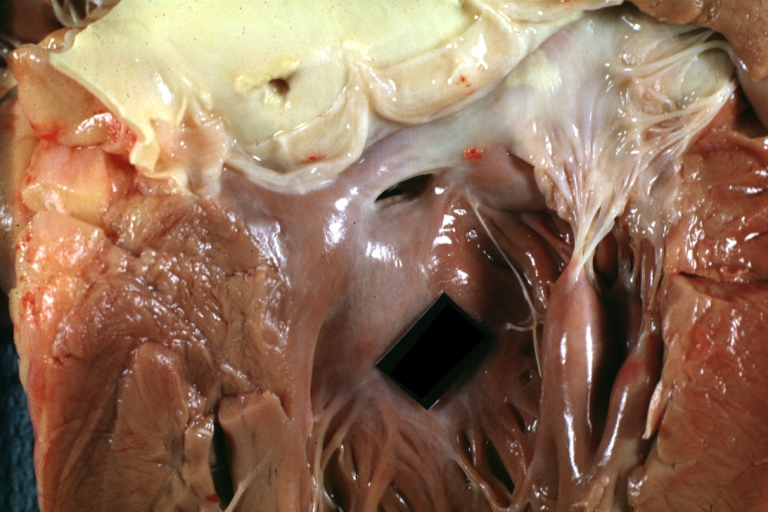
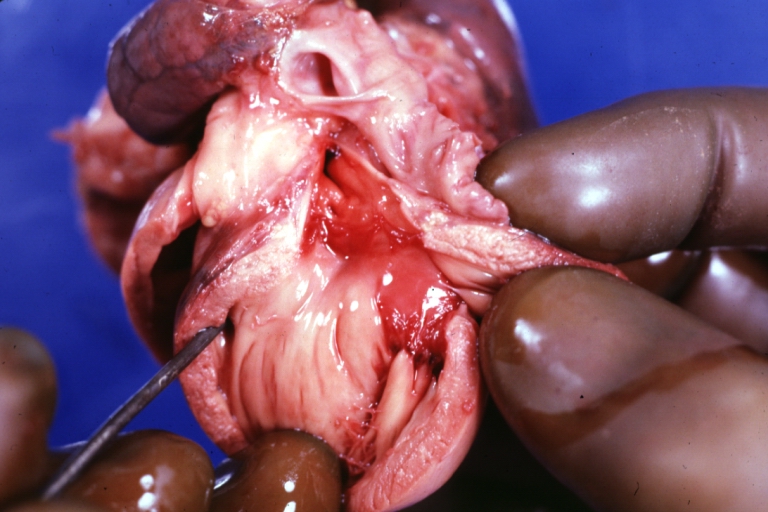
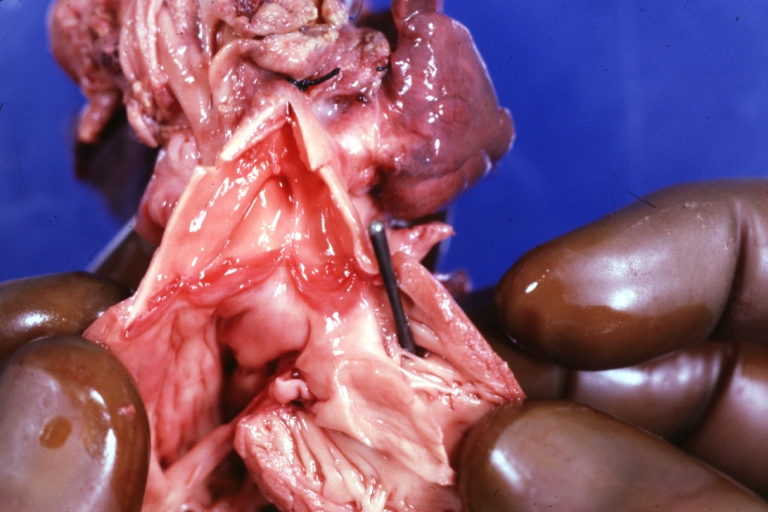
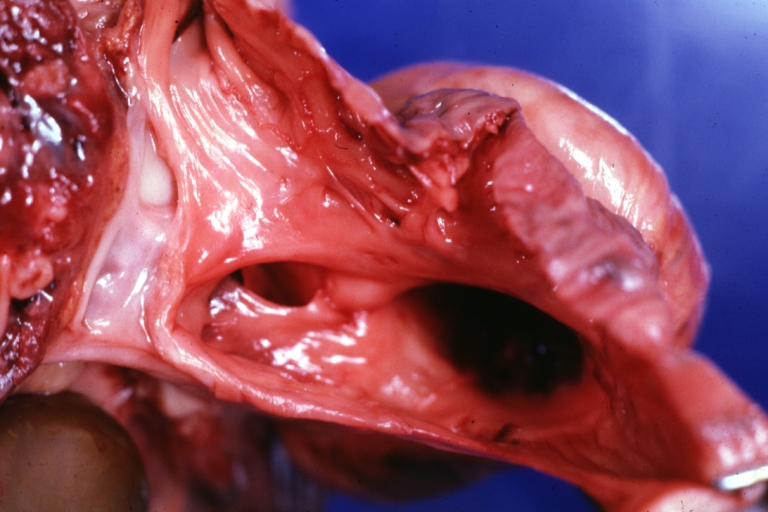
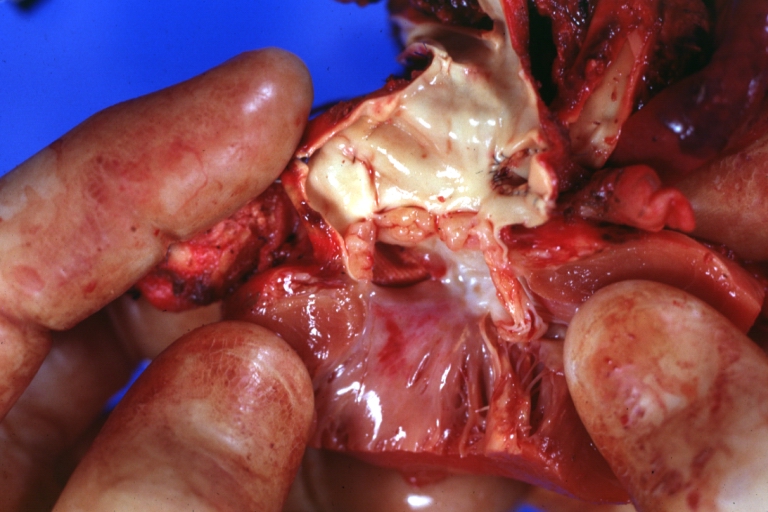
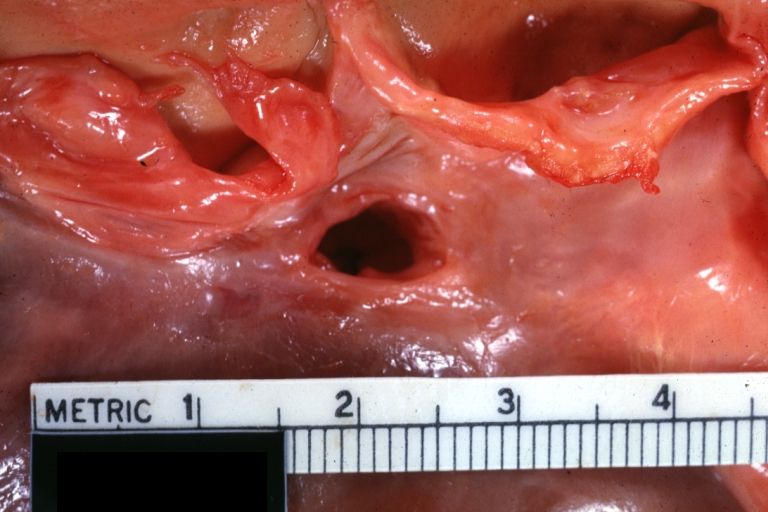
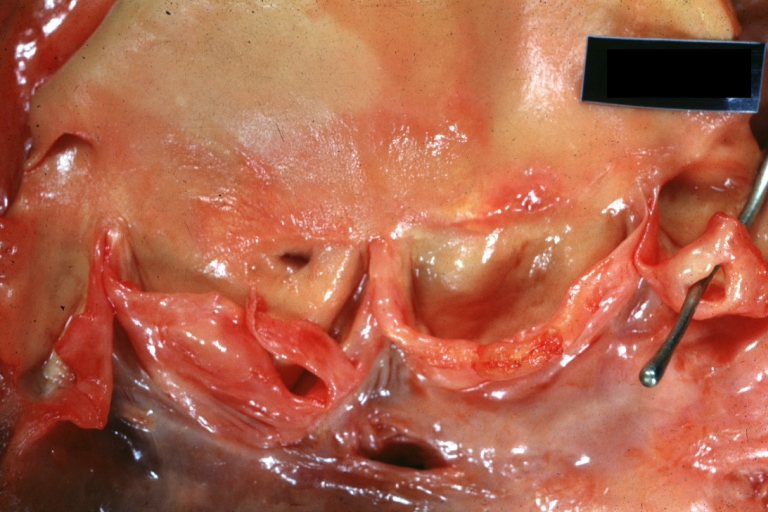
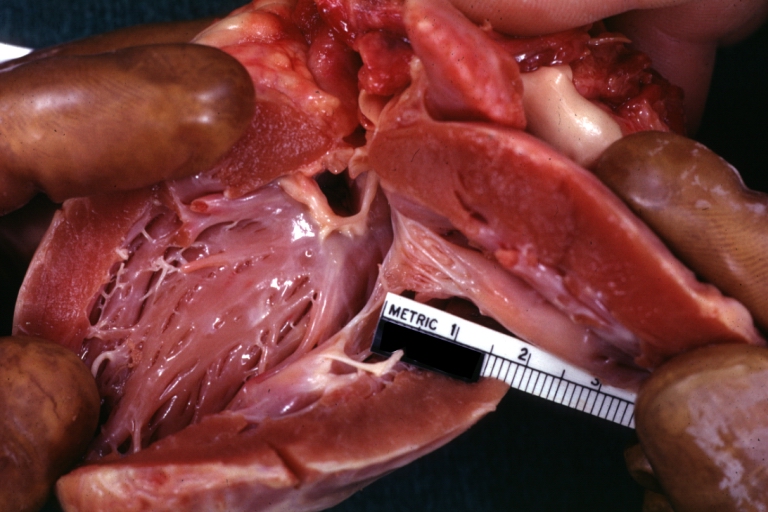
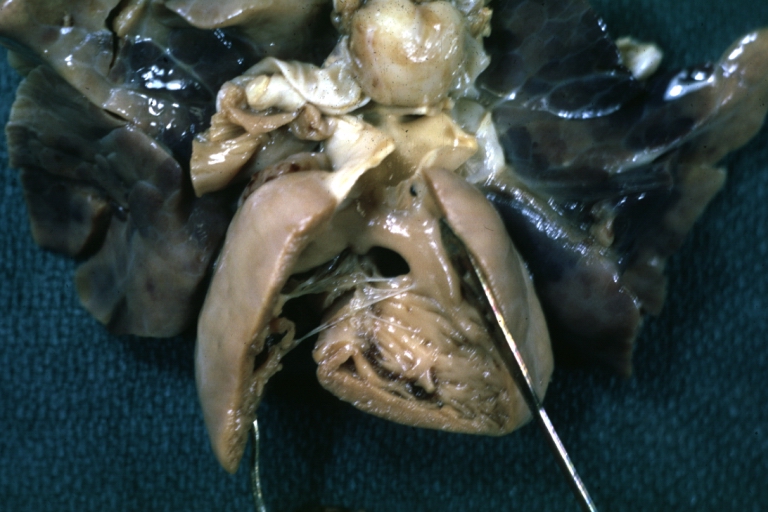
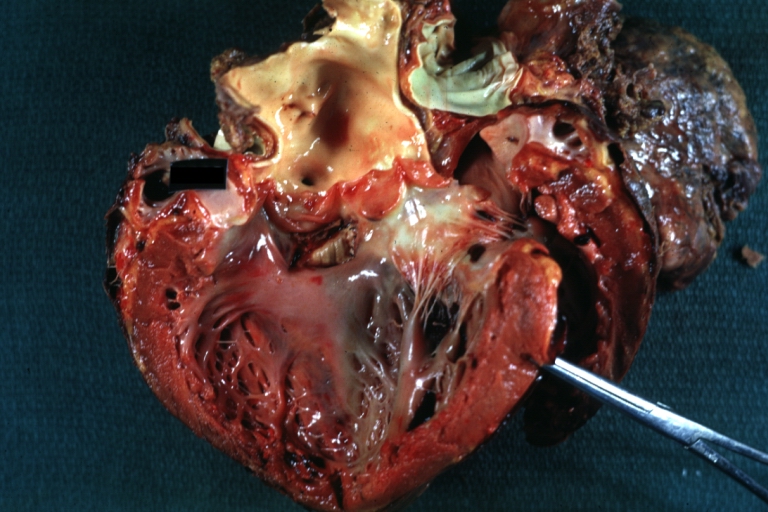
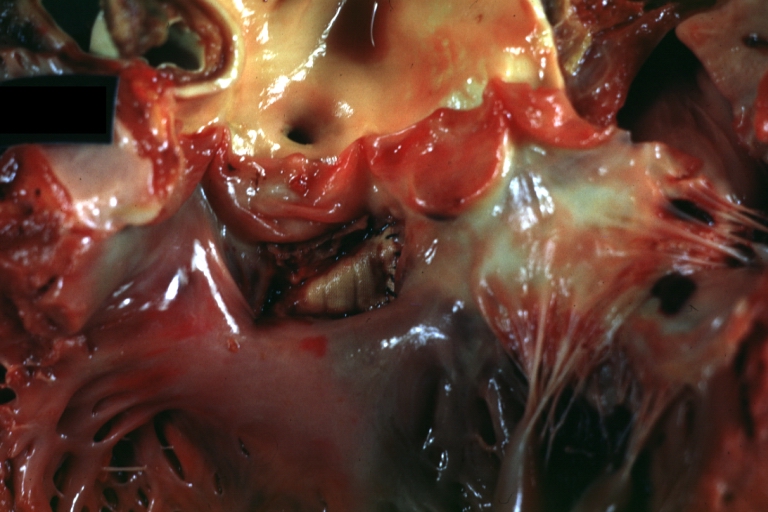
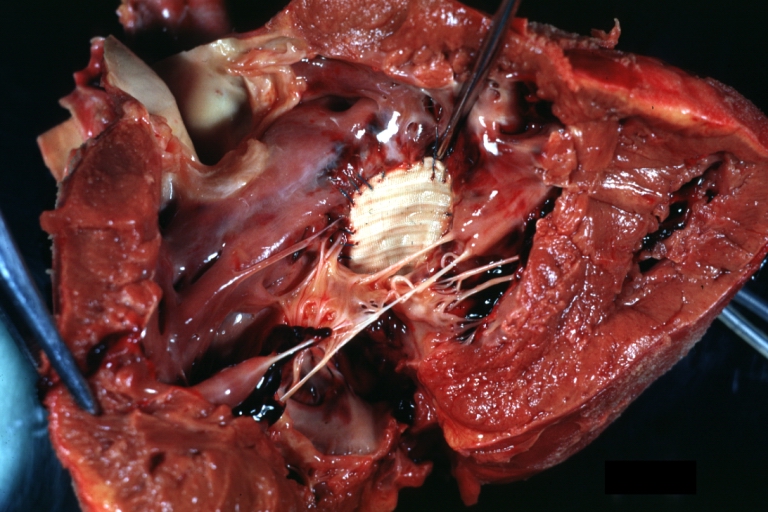
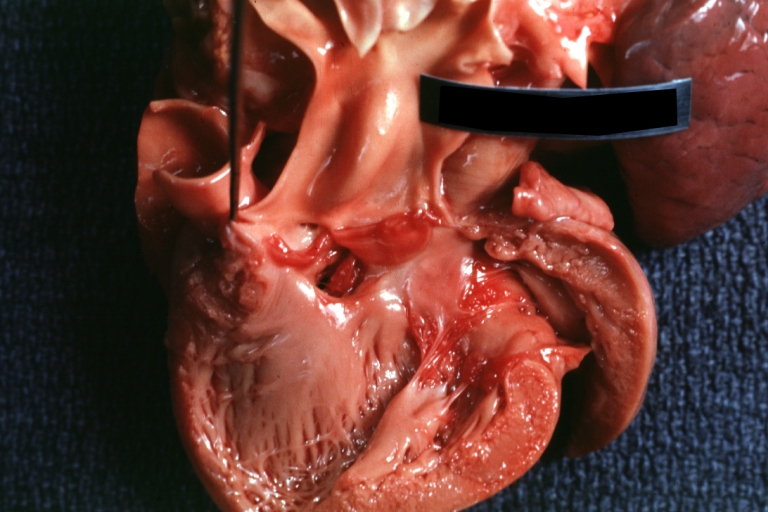
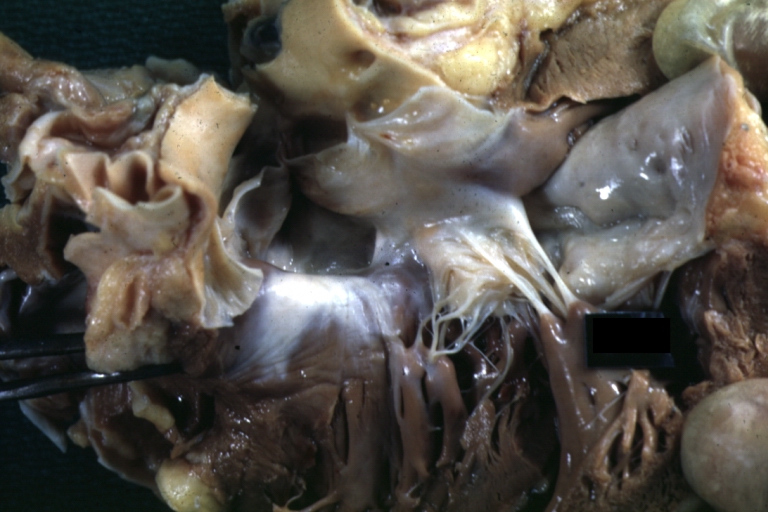
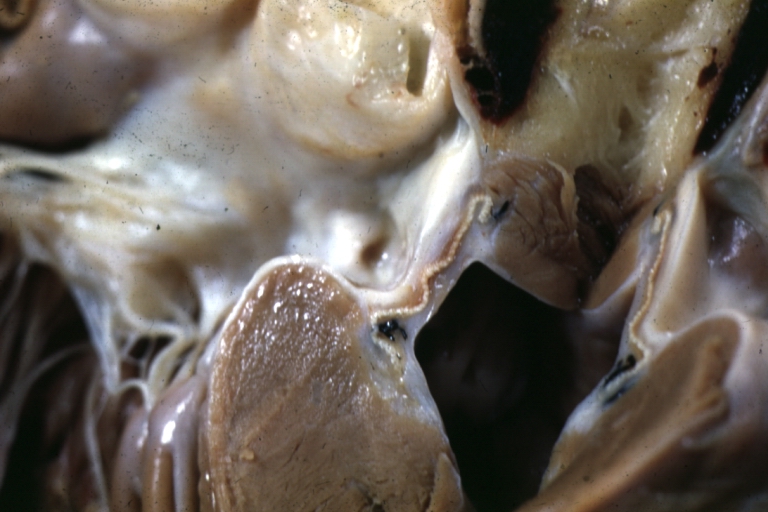
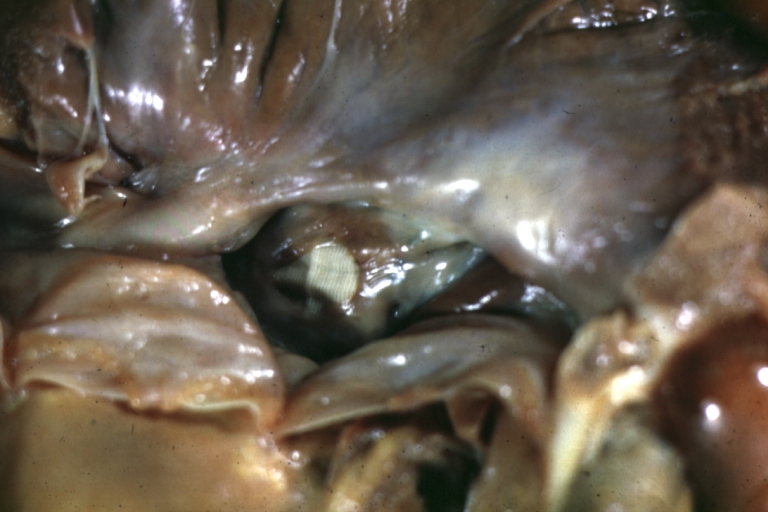
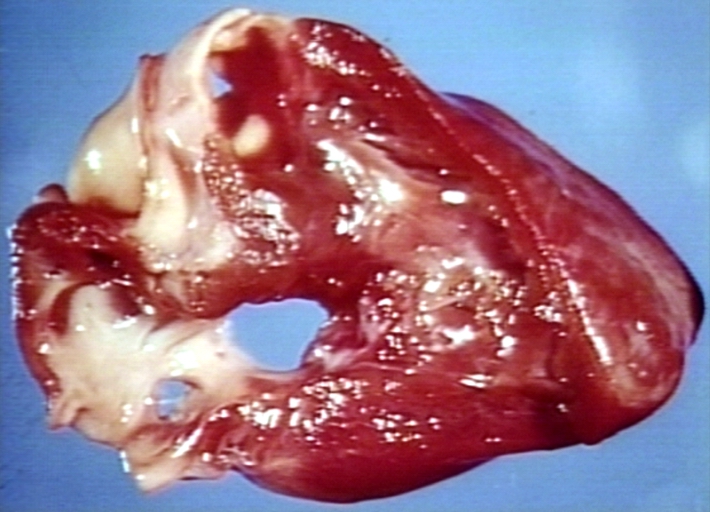
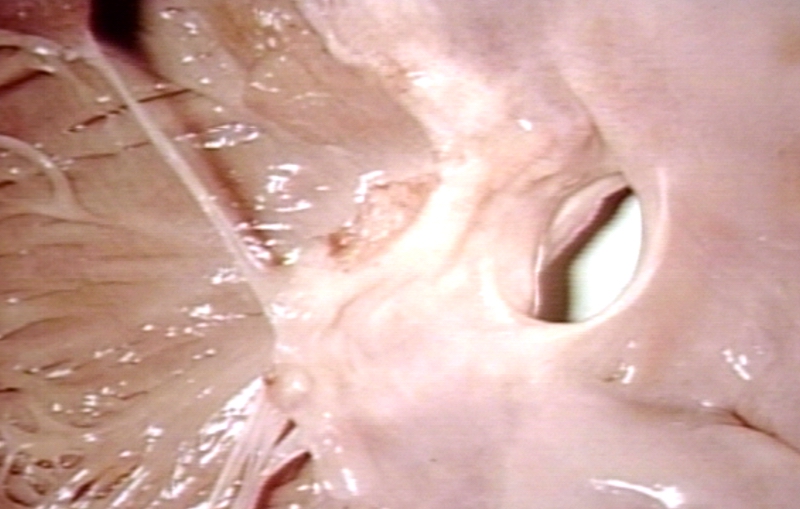
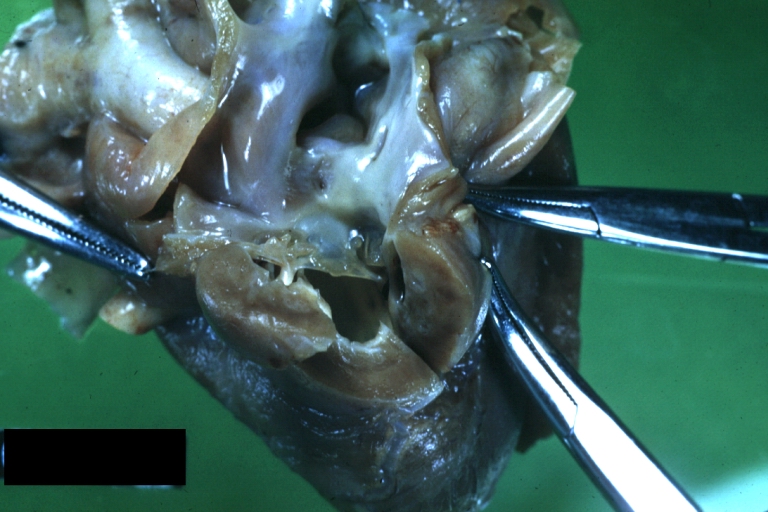
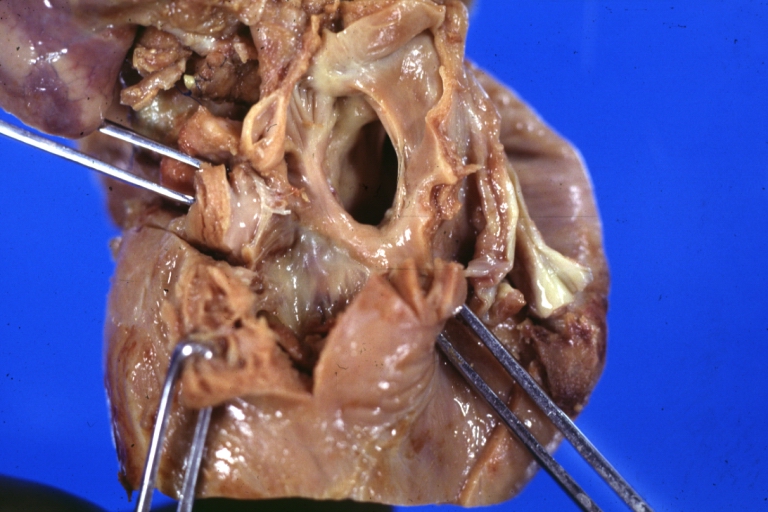
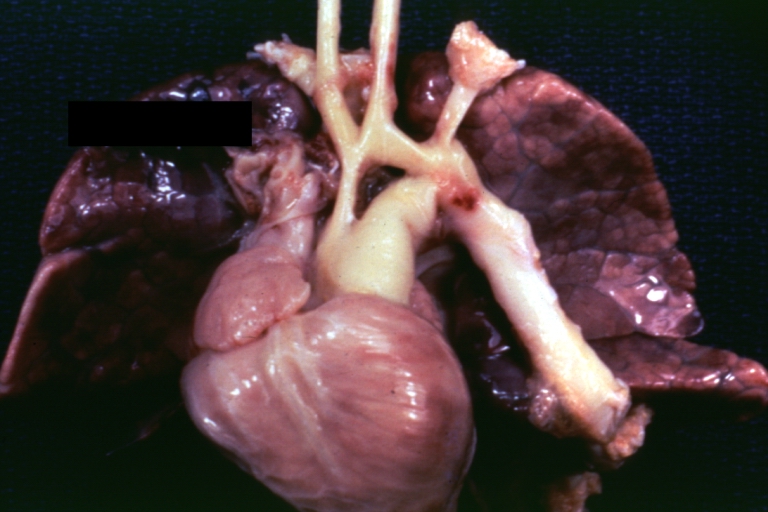
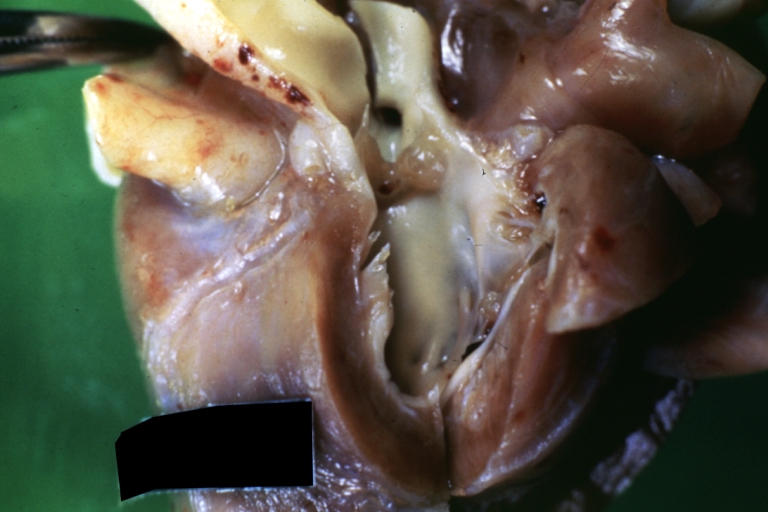
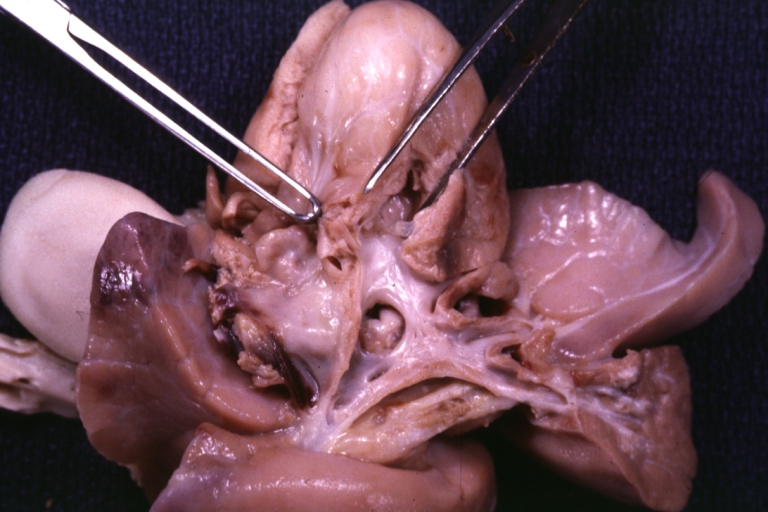
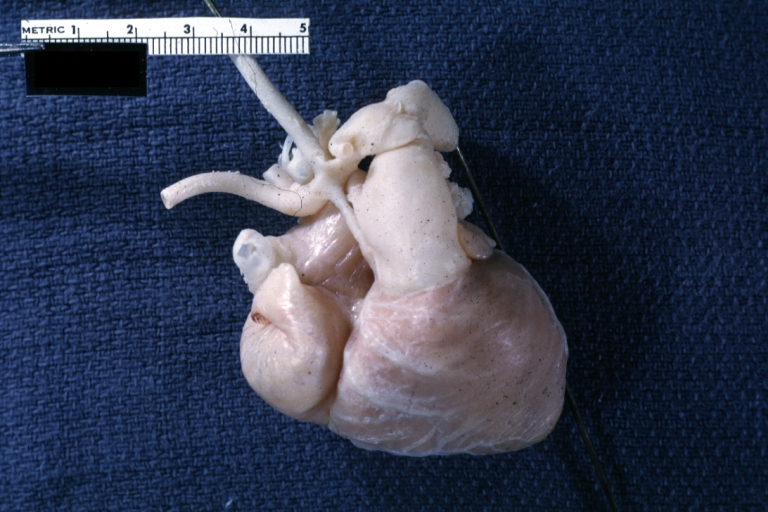
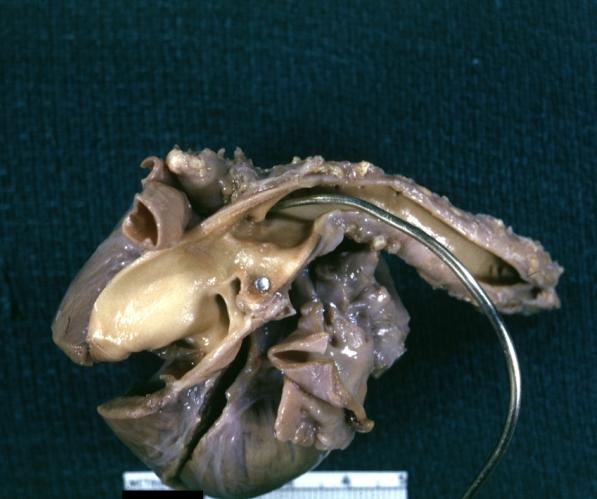
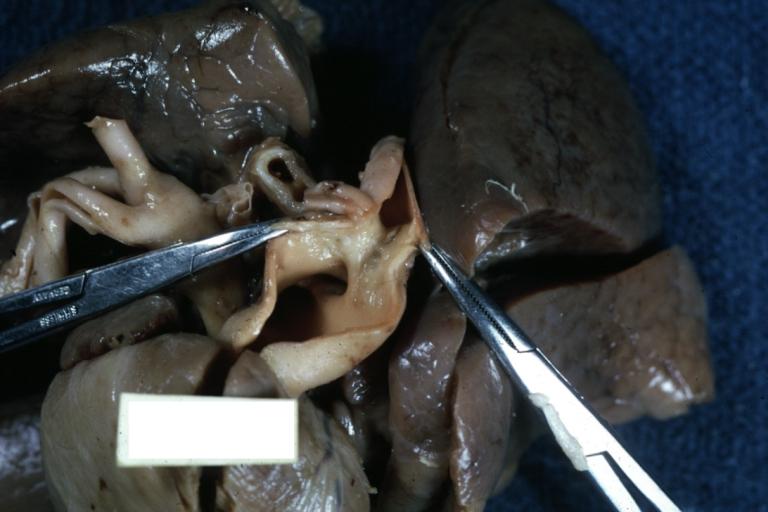
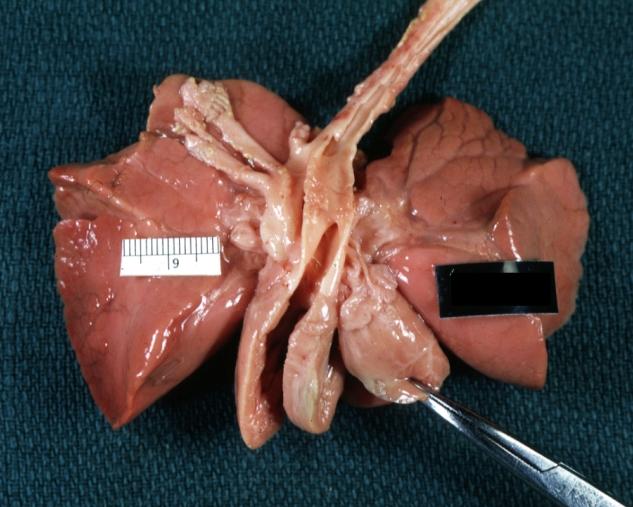
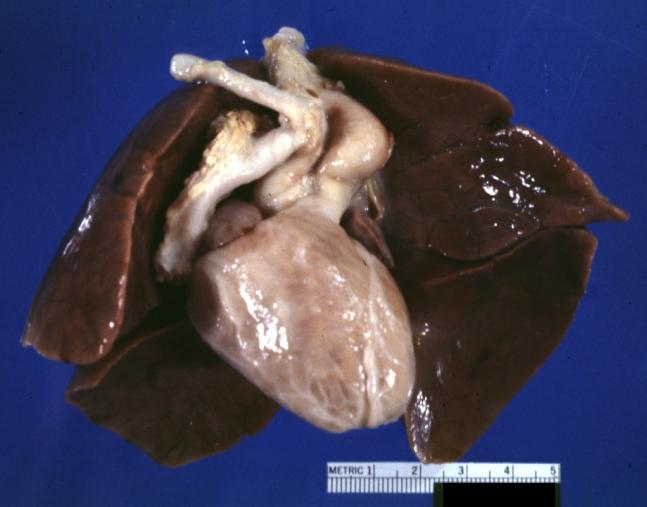
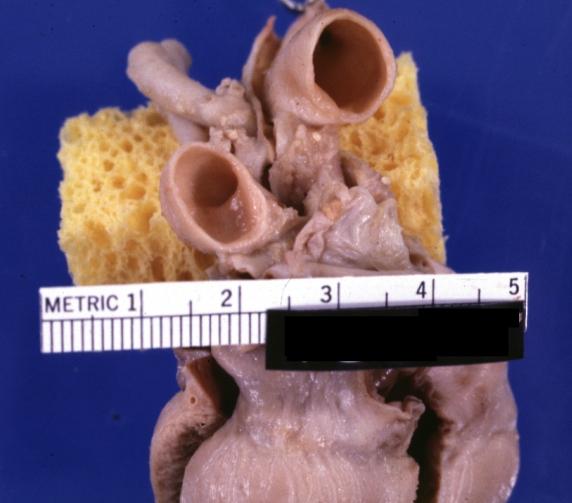
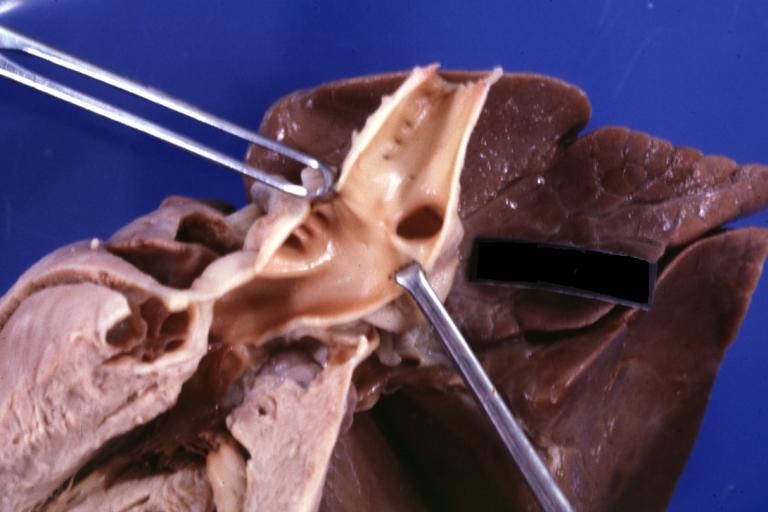
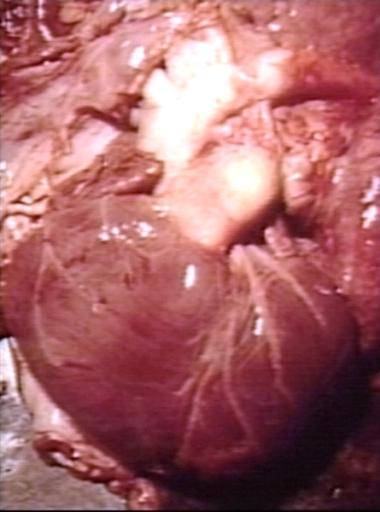
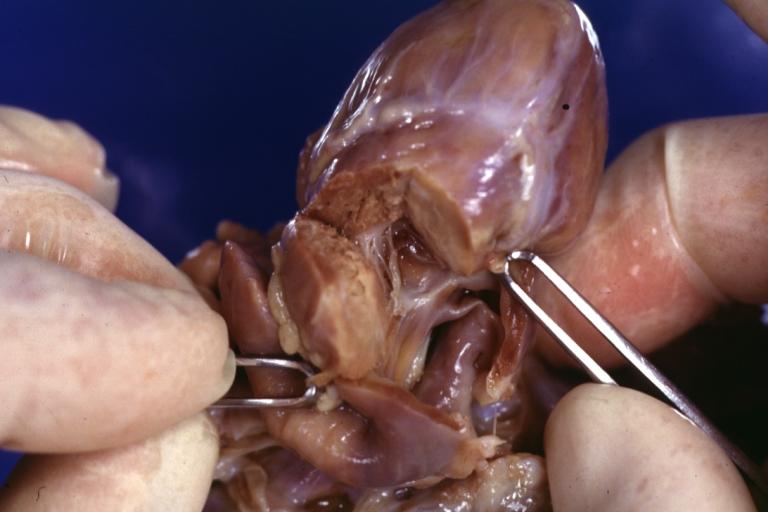
Pathological Findings
-
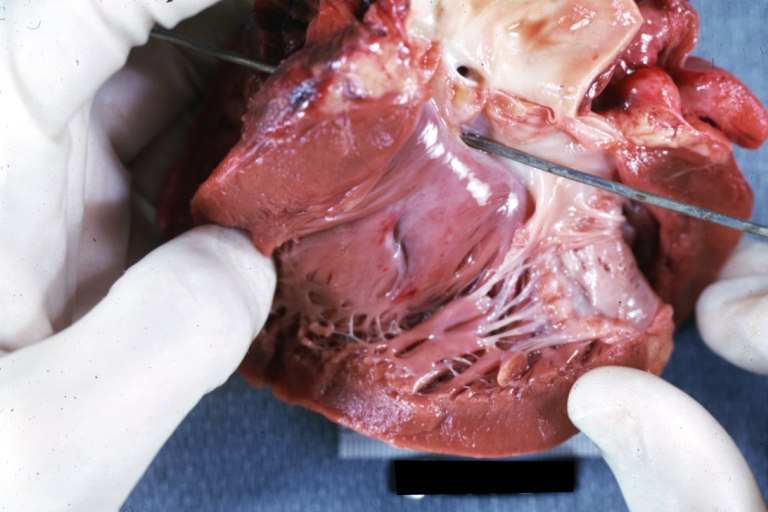
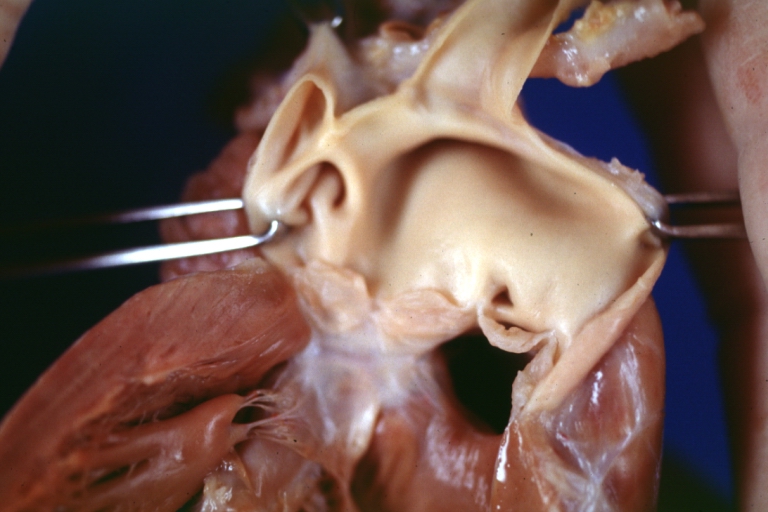
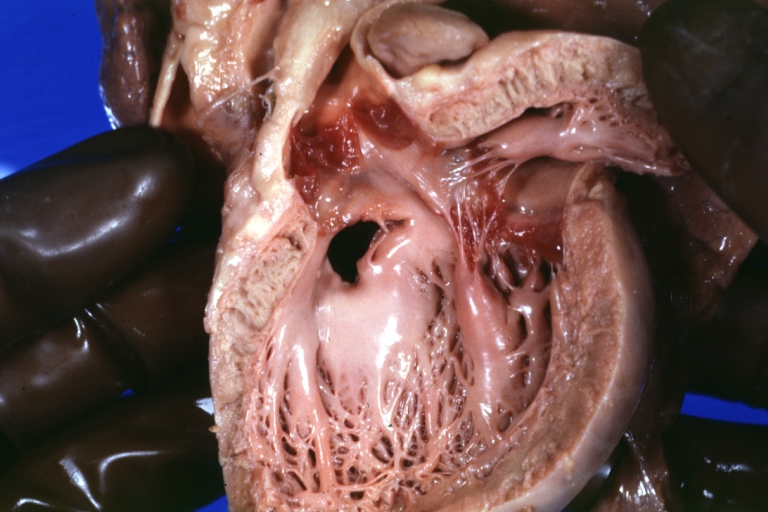
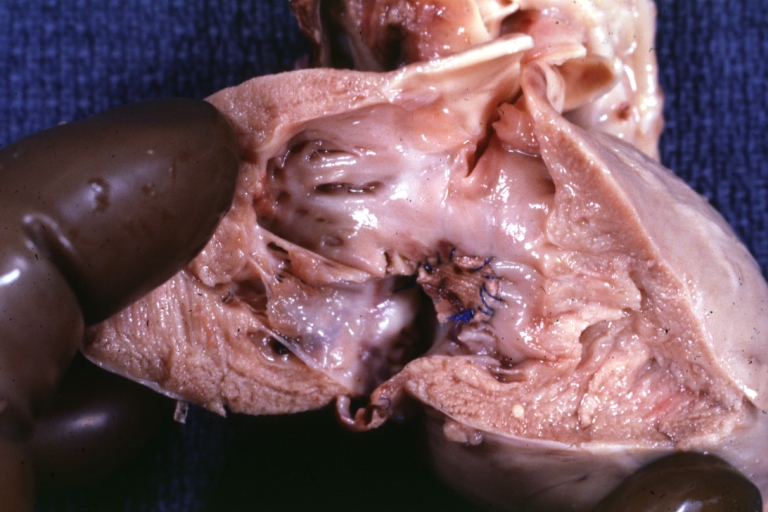
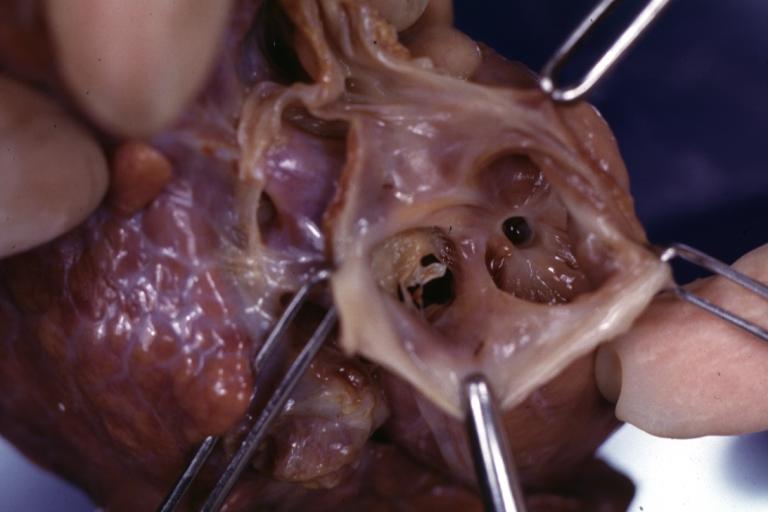
Tetralogy of Fallot: Gross, a good example of repaired perimembranous septal defect
-
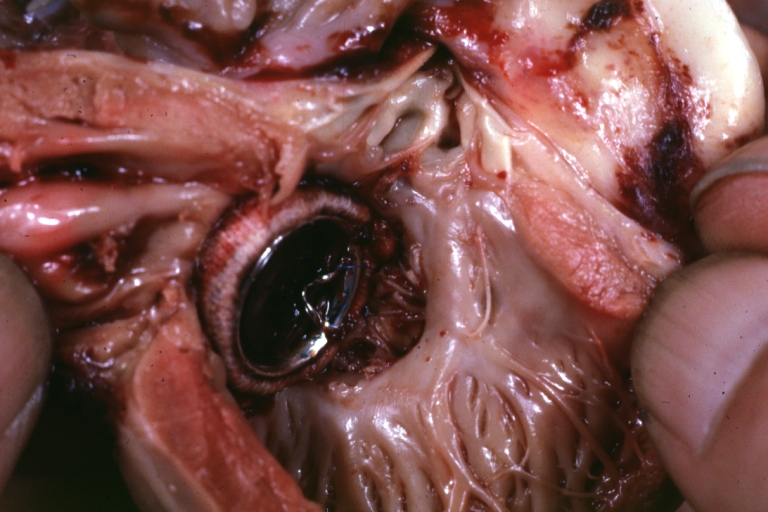
Interventricular Septal Defect (Muscular Septum): Gross, natural color, muscular septal defect in newborn
-
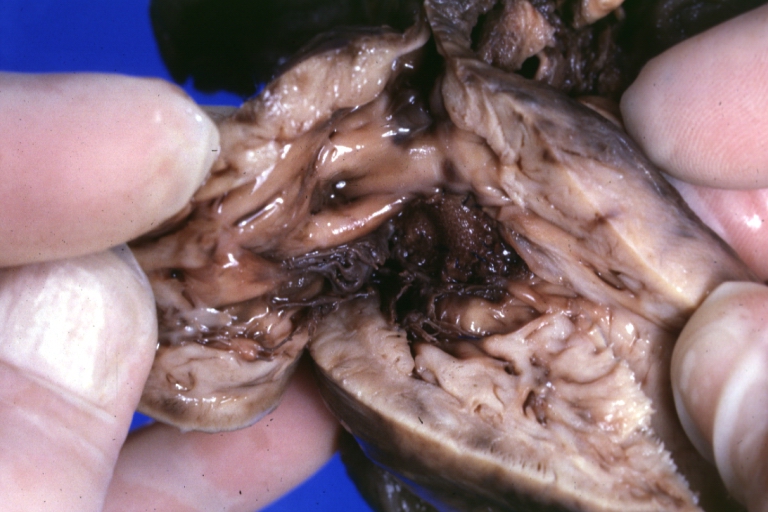
Subvalvular Ventricular Septal Defect: Gross, good view of defect with overriding aorta
-
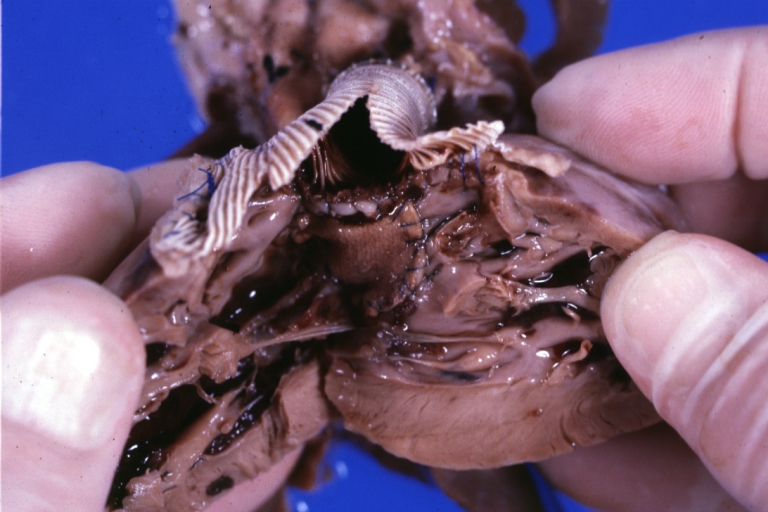
Ventricular Septal Defect: Gross, infant heart, pulmonary outlet, muscular septal defect
-
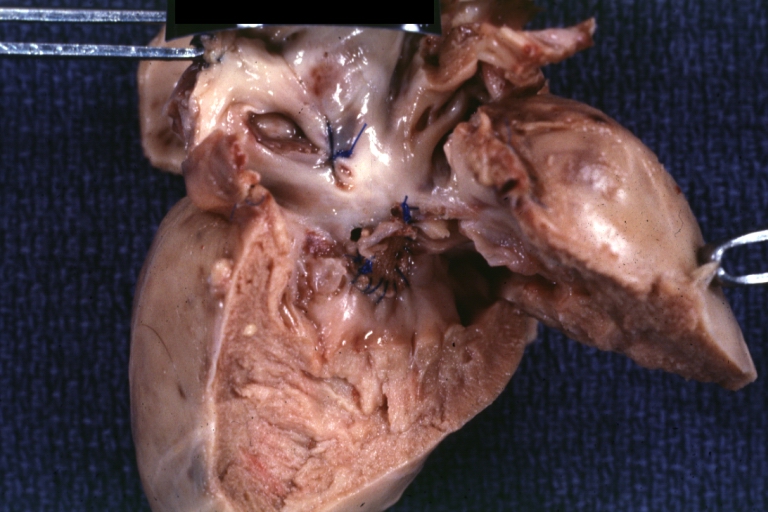
Atrioventricular Canal: Gross, right ventricular view of canal defect
-
Atrioventricular Canal: Gross, left ventricle view of canal defect (very good example)
-
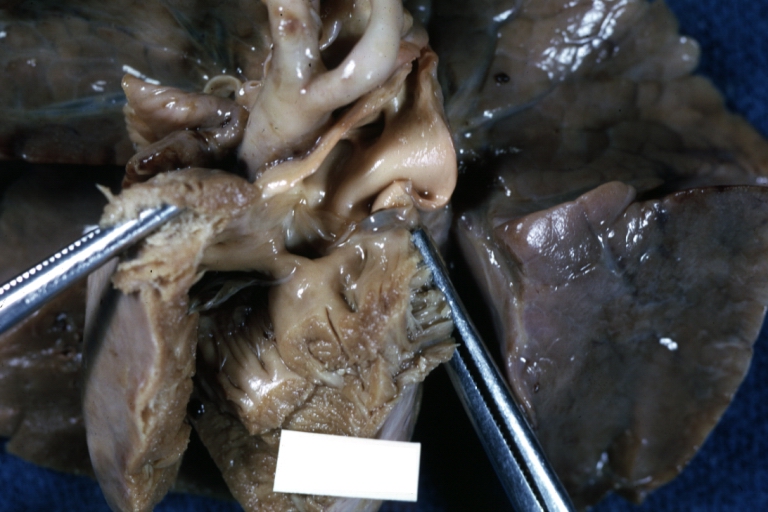
Perimembranous Ventricular Septal Defect: Gross, an excellent example
-
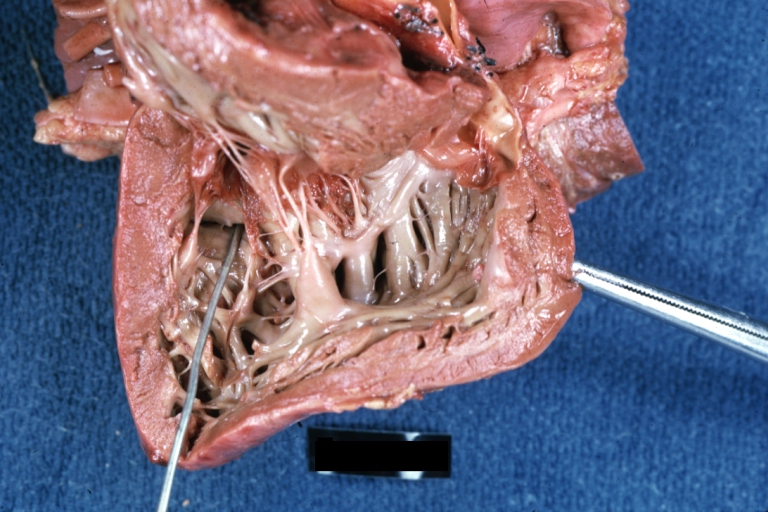
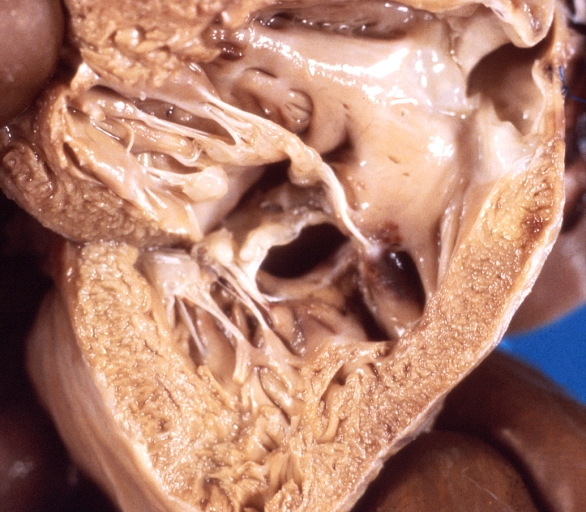
Ventricular Septal Defect: Gross, subvalvular defect, left ventricle view of tetralogy of Fallot (very good example)
-
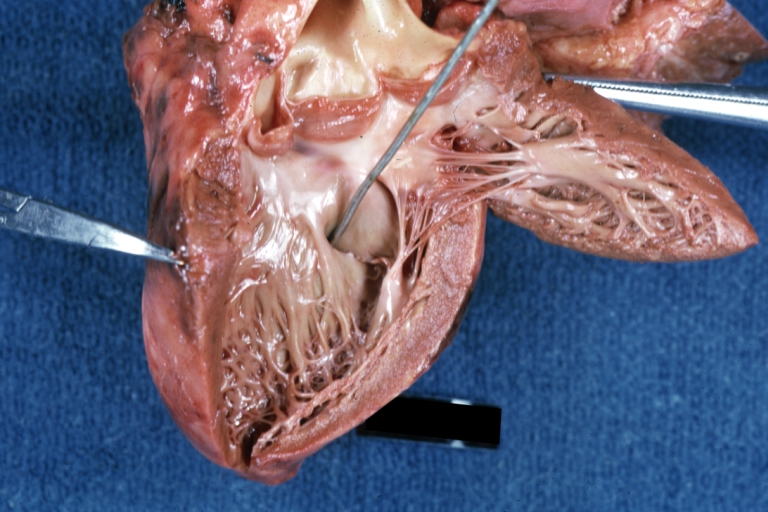
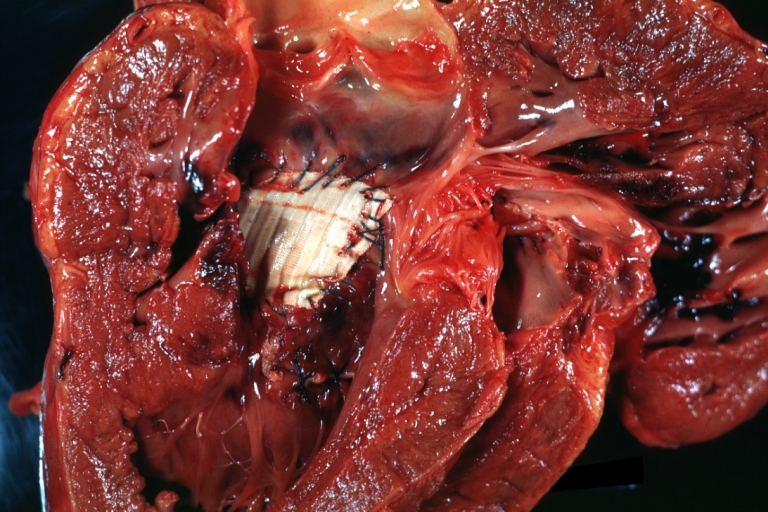
Tetralogy of Fallot: Gross, close-up of aortic valve with subvalvular septal defect with Dacron patch (very good example)
-
Subpulmonic Ventricular Septal Defect: Gross, a well shown lesion.
-
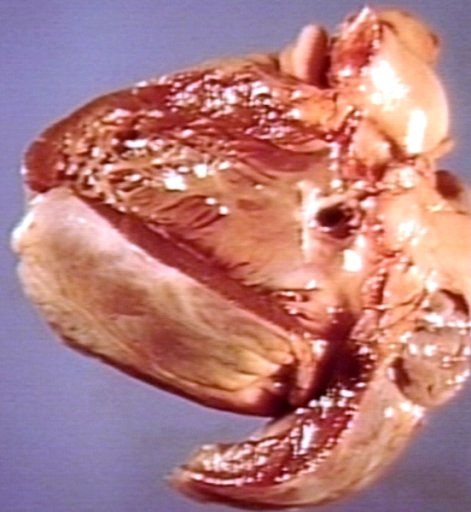
Subvalvular Ventricular Septal Defect
-
Subvalvular Ventricular Septal Defect
-
Ventricular Septal Defect: Gross, natural color, view of opened heart with lungs attached shows rather well a subvalvular VSD
-
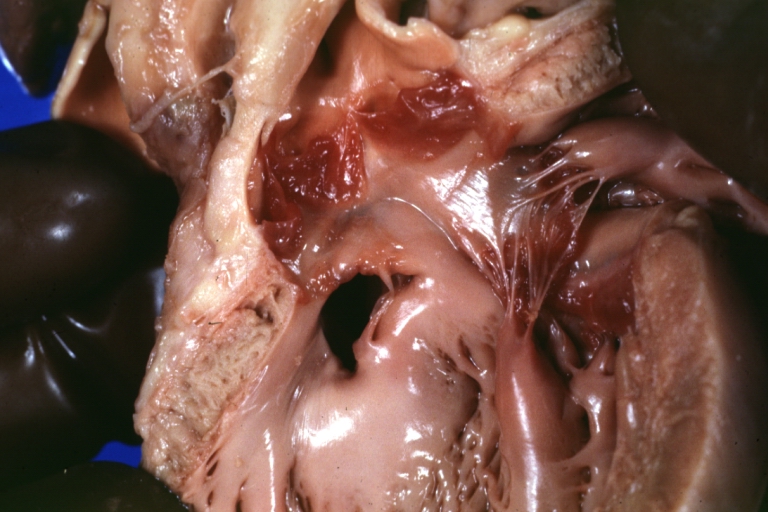
Atrioventricular Canal: Gross, patch repair of defect seen from left side showing left atrial portion extending into a cleft mitral valve
-
Atrioventricular Canal: Gross, corrected defect with patch viewed from left side atrium and cleft mitral valve
-
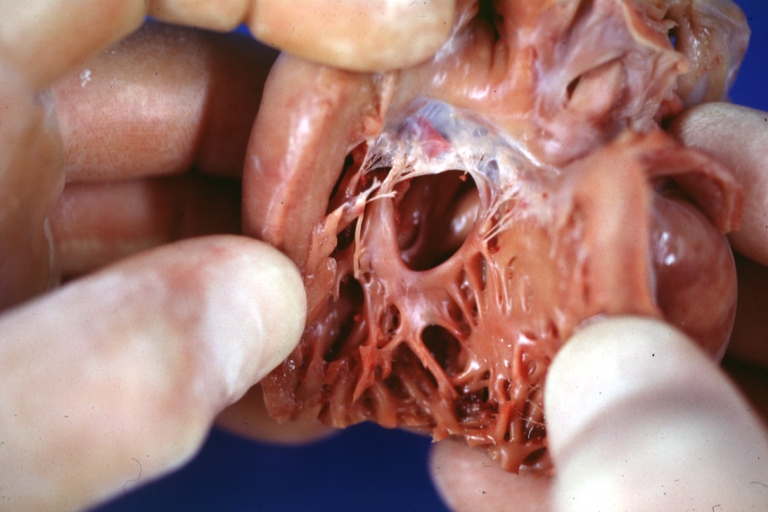
Atrial Septal Defect: Gross, (an excellent example) foramen ovale defect with right ventricular hypertrophy and fatty infiltration of the right ventricular wall, enlarged right atrium
-
Ventricular Septal Defect: Gross close-up adult heart, small perimembranous septal defect (very good example)
-
Interventricular Septal Defect (Muscular Septum): Gross, natural color, low septal defect shown from aortic outlet. The same defect (with a probe in hole) shown from right ventricle.
-
Interventricular Septal Defect (Muscular Septum): Gross natural color right ventricular outlet (probe in defect) view from left ventricular side
-
Atrial Septal Defect: Gross natural color infant heart foramen ovale defect, septum secundum
-
Aortic Subvalvular Ventricular Septal Defect: Gross, natural color, septal defect has patch repair. Aortic valve is myxomatous. A complex case of truncus with interrupted arch.
-
Interventricular Septal Defect Membranous Septum: Gross natural color close-up (an excellent demonstration)
-
Interventricular Septal Defect Membranous Septum: Gross natural color small defect well shown. Aortic cusps are scarred and one is perforated
-
Subvalvular Ventricular Septal Defect: Gross, natural color, close-up view of aortic outflow tract with a large subvalvular defect
-
Membranous Interventricular Septal Defect: Gross natural color subvalvular defect with probe immediately inferior to membranous septum
-
Subvalvular Ventricular Septal Defect: Gross, fixed tissue, large subpulmonic defect apparently represent left displacement of the pulmonary artery
-
Interventricular Septal Defect: Gross, fixed tissue, opened right ventricular outflow tract positioned to show perimembranous septal defect (as surgeon would see it during repair)
-
Ventricular Septal Defect Muscular: Gross, natural color, view from right ventricle with probe in defect right ventricular hypertrophy is evident
-
Ventricular Septal Defect Muscular: Gross, natural color, view from left ventricle with probe in defect
-
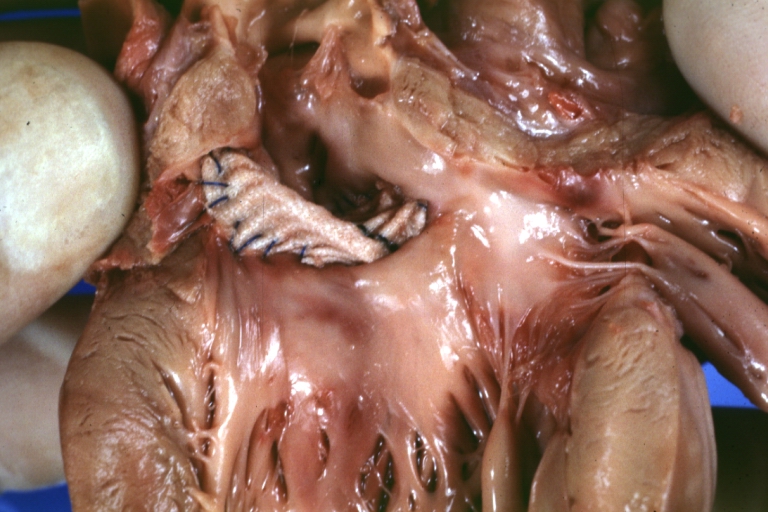
Interventricular Septal Defect Subvalvular with Patch Repair: Gross natural color 19yo with Tetralogy of Fallot also shows overriding aorta
-
Interventricular Septal Defect Subvalvular with Patch Repair: Gross, natural color, close-up
-
Interventricular Septal Defect (Perimembranous) with Patch Repair: Gross, natural color, view from right ventricle. A case of inverted ventricles
-
Interventricular Septal Defect (Perimembranous) with Patch Repair: Gross, natural color, view from left ventricular outflow tract
-
Ventricular Septal Defect (Subvalvular): Gross, fixed tissue, small heart with opened aorta and subvalvular defect shown. A case of pulmonary artery atresia
-
Truncus Arteriosus with Subvalvular Ventricular Septal Defect: Gross, natural color, an excellent view of subvalvular defect. Quadricuspid truncus valve and type I origin of pulmonary arteries
-
runcus Arteriosus with Subvalvular Interventricular Septal Defect: Gross, natural color, defect is shown from the right side (view toward right ventricular outlet)
-
Truncus Arteriosus with Subvalvular Interventricular Septal Defect: Gross natural color excellent view of lesion looking at opened aortic ring with quadricuspid aortic valve. A large subvalvular defect (origin of pulmonary arteries is at forceps)
-
Av Canal with Left Side Bjork Shiley Prosthetic Valve: Gross, natural color, a close-up view of valve and the bridging defect
-
Interventricular Septal Defect (Perimembranous) with Patch Repair: Gross, fixed tissue, a close-up view of patch repair from right ventricle
-
Conduit Right Ventricle to Pulmonary Artery: Gross, fixed tissue, opened conduit showing sutures into ventricle and patch closed perimembranous interventricular septal defect
-
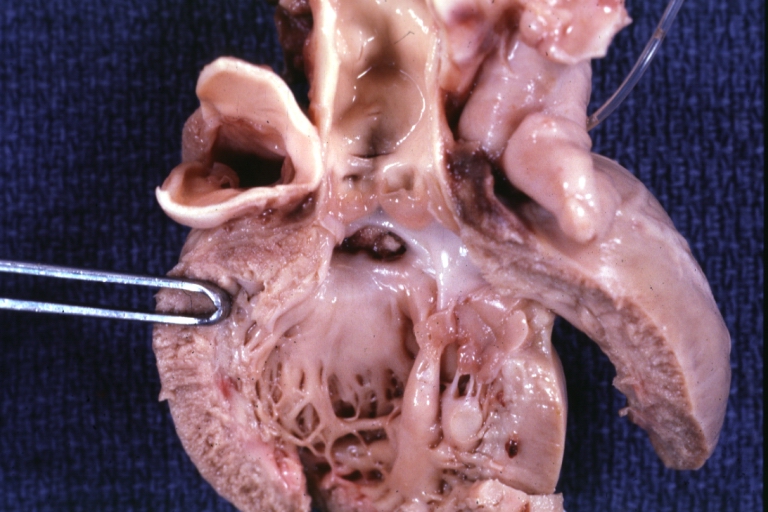
Ventricular Septal Defect (Perimembranous): Gross, natural color, (quite good photo - lesion before the operation)
-
Ventricular Septal Defect (Subvalvular) Repaired: Tetralogy of Fallot; Gross, fixed tissue, close-up view of a large subvalvular defect repaired with a Dacron patch (overgrown with fibrous tissue prominent subaortic shelf with endocardial thickening).
-
Ventricular Septal Defect (Subvalvular) Repaired: Tetralogy of Fallot; Gross, fixed tissue, close-up view of a large subvalvular defect repaired with a Dacron patch
-
Ventricular Septal Defect (Subvalvular) Repaired: Gross, fixed tissue, close-up view of Dacron patch. Nearly completely covered with fibrous tissue
-
Transposition Great Vessels with Interventricular Septal Defect: Gross, fixed tissue, opened left ventricular outflow tract into a pulmonary artery (perimembranous defect)
-
Transposition Great Vessels with Interventricular Septal Defect: Gross, fixed tissue, close-up of interventricular septal defect and pulmonary valve
-
Double Outlet Right Ventricle: Gross, fixed tissue, close-up view of left ventricular outflow tract and patched ventricular septal defect. The override is obvious in this (very good) close-up view
-
Perimembranous Ventricular Septal Defect: Gross, fixed tissue, opened left ventricular outflow tract into aorta. Defect was patched 3 days prior to death
-
Perimembranous Ventricular Septal Defect: Gross, fixed tissue, lesion seen from right ventricle (with patch)
-
Perimembranous Interventricular Septal Defect: Gross, fixed tissue, view from right atrium and ventricle with patch placed three days prior to death.
-
Ventricular septal defect
-
Ventricular septal defect, view from left ventricle
-
Atrial Septal Defect, Septum Primum; View from Right Atrium (a 4 month old baby)
-
Atrial Septal Defect, Septum Primum; Also Cleft in Anterior Cusp of Mitral Valve
-
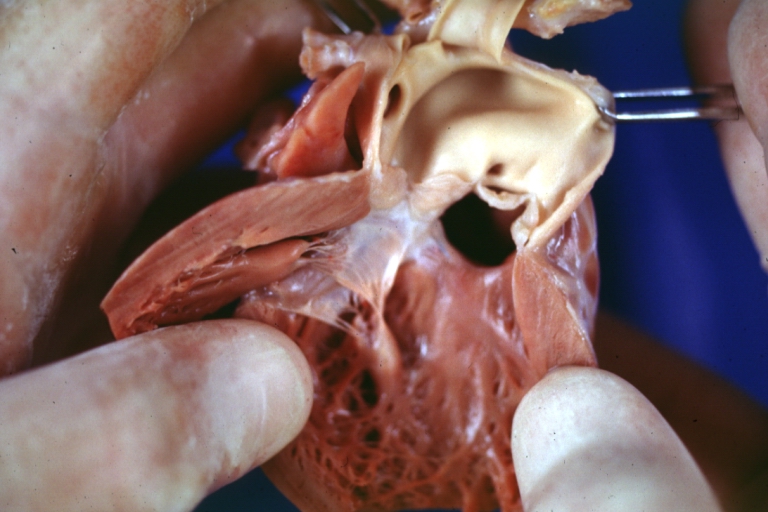
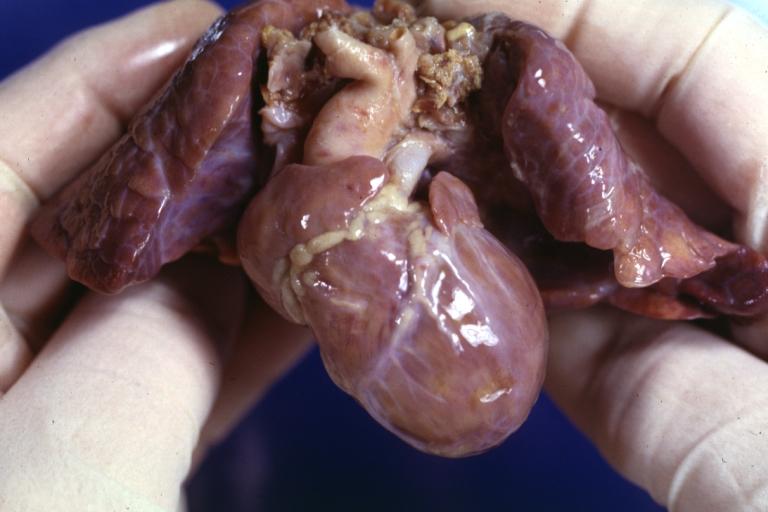
Patent Ductus Arteriosus: Gross example in an infant heart
-
Patent Ductus Arteriosus: Gross fixed tissue probe in ductus
-
Patent Ductus Arteriosus: Gross fixed tissue view of ductus opened from pulmonary artery into aorta with edematous appearing intimal surface
-
Patent Ductus Arteriosus: Gross natural color opened ductus in infant shows apparent intimal edema in ductus.
-
Patent Ductus Arteriosus with Aneurysmal Dilation: Gross fixed tissue external photo of heart shows the lesion
-
Patent Ductus Arteriosus with Aneurysmal Dilation: Gross fixed tissue aorta and ductus have been cross sectioned showing arch of aorta and huge ductus in a 5 day old infant
-
Patent Ductus Arteriosus with Aneurysmal Dilation: Gross fixed tissue opened aortic arch and descending thoracic showing very large opening of ductus into aorta
-
Patent Ductus Arteriosus
-
Right Ventricle Hypoplasia: Gross natural color good example showing tiny tricuspid inlet and very small but quite thick right ventricle
-
Right Ventricle Hypoplasia: Gross natural color view from right atrium showing patent foramen ovale and very small tricuspid valve
-
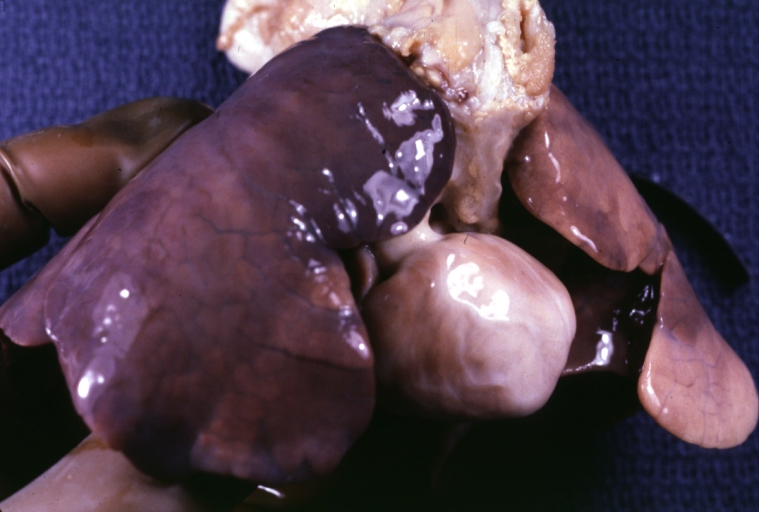
Right Ventricle Hypoplasia: Gross natural color external view of heart showing very large left ventricle and very small right ventricle delineated by anterior descending branch of left coronary artery
External links
- Cleveland Clinic Webchat - Adult Congenital Heart Disease Webchat with Dr. Richard Krasuski.
- Cleveland Clinic Webchat - Adult Congenital Heart Disease Surgery Webchat with Dr. Gosta Pettersson.
- It's My Heart Advocating for and Supporting those affected by Congenital Heart Defects - US Non-Profit under section 501(c)3.
- Saving Little Hearts
- Card-AG, The Cardiologycal Working Group of the University Pediatric Clinic Munster
- The Heart Chest
- American Heart Association
- Congenital Heart Defect
- Treating Congenital Heart Disease
- Coping with Congenital Heart Disease
- Fetal Treatment for Congenital Heart Disease (UCSF Fetal Treatment Center)
- EACTS Congenital Database ― European database of cardiothoracic surgeries with publicly available reports
- Adult Congenital Heart Association
- Congenital Heart Information Network
- Fixing Tiny Tickers - Fetal heart surgery
- Tiny Tickers - Antenatal congenital heart disease information
- Cardiacdiseases.org
Sources
- The ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities [1]
References
1. “The Heart Chest.” Non-profit Organization.
- ↑ 1.0 1.1 Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008; 117: 2820–2840. PMID 18483207
de:Herzfehler lv:Iedzimtās sirds slimības nn:Medfødd hjartefeil sr:Урођене срчане мане uk:Вроджені вади серця wa:Maladeye des bleus påpåds