Clonidine: Difference between revisions
Gerald Chi- (talk | contribs) mNo edit summary |
Gerald Chi- (talk | contribs) mNo edit summary |
||
| Line 397: | Line 397: | ||
6) Remove the clear plastic protective backing from the peach colored, rectangular PATCH by gently peeling off one half of the backing at a time as shown in Figure 4. Avoid touching the sticky side of the Clonidine Transdermal System PATCH. | 6) Remove the clear plastic protective backing from the peach colored, rectangular PATCH by gently peeling off one half of the backing at a time as shown in Figure 4. Avoid touching the sticky side of the Clonidine Transdermal System PATCH. | ||
7) Place the Clonidine Transdermal System PATCH on the prepared skin site (sticky side down) by applying firm pressure over the PATCH to ensure good contact with the skin, especially around the edges (Figure 5). Discard the clear plastic protective backing and wash your hands with soap and water to remove any drug from your hands. | 7) Place the Clonidine Transdermal System PATCH on the prepared skin site (sticky side down) by applying firm pressure over the PATCH to ensure good contact with the skin, especially around the edges (Figure 5). Discard the clear plastic protective backing and wash your hands with soap and water to remove any drug from your hands. | ||
Revision as of 00:29, 19 May 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING
See full prescribing information for complete Boxed Warning.
Obstetrical, postpartum, or perioperative pain management: Clonidine hydrochloride injection (epidural clonidine) is not recommended for obstetrical, postpartum, or perioperative pain management. The risk of hemodynamic instability, especially hypotension and bradycardia, from epidural clonidine may be unacceptable in these patients. However, in a rare obstetrical, postpartum or perioperative patient, potential benefits may outweigh the possible risks.
|
Overview
Clonidine is a central alpha-2 adrenergic agonist that is FDA approved for the treatment of hypertension. There is a Black Box Warning for this drug as shown here. Common adverse reactions include contact dermatitis, erythema, pruritus, xerostomia, dizziness, headache, sedation, somnolence, and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Hypertension: Oral Administration
Dosing information
- Hypertension treatment with clonidine can be administered orally (tablets) or by transdermal patches.
- Clonidine hydrochloride tablets must be adjusted according to the patient's individual blood pressure response. The following is a general guide to its administration:
- Initial dose: 0.1 mg tablet twice daily (morning and bedtime). Elderly patients may benefit from a lower initial dose.
- Maintenance: Further increments of 0.1 mg per day may be made at weekly intervals if necessary until the desired response is achieved. Taking the larger portion of the oral daily dose at bedtime may minimize transient adjustment effects of dry mouth and drowsiness. The therapeutic doses most commonly employed have ranged from 0.2 mg to 0.6 mg per day given in divided doses. Studies have indicated that 2.4 mg is the maximum effective daily dose, but doses as high as this have rarely been employed.
- Renal Impairment: Patients with renal impairment may benefit from a lower initial dose. Patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
Severe Cancer Pain: Epidural Infusion
Dosing information
- The recommended starting dose of clonidine hydrochloride for continuous epidural infusion is 30 mcg/hr. Although dosage may be titrated up or down depending on pain relief and occurrence of adverse events, experience with dosage rates above 40 mcg/hr is limited.
- Familiarization with the continuous epidural infusion device is essential. Patients receiving epidural clonidine from a continuous infusion device should be closely monitored for the first few days to assess their response.
- The 500 mcg/mL (0.5 mg/mL) strength product must be diluted prior to use in 0.9% Sodium Chloride for Injection, U.S.P., to a final concentration of 100 mcg/mL:
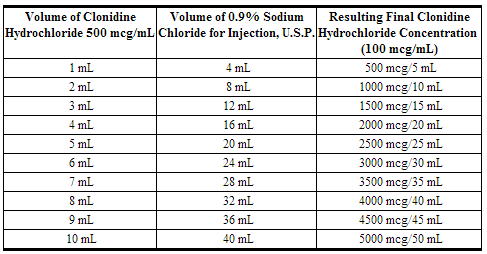
Renal Impairment
- Dosage should be adjusted according to the degree of renal impairment, and patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
- Clonidine hydrochloride mustnotbe used with a preservative.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clonidine in adult patients.
Non–Guideline-Supported Use
Postmenopausal Hot Sweats
- Dosing Information
Ischemic Foot Ulcer
- Dosing Information
- 0.1 mg/day.[3]
Nicotine Dependance
- Dosing Information
Opioid Withdrawal
- Dosing information
- Initial dose: 0.1 mg PO q8h, titrate every day according to needs:[5]
- Second day: 0.4-0.6 mg/day.
- Third day: 0.4-0.7 mg/day.
- Fourth day: 0.5-0.8 mg/day.
- Sixth day: 0.6-1 mg/day.
- Do not exceed 1mg/day.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Severe Cancer Pain: Epidural Infusion
Dosing Information
- The safety and effectiveness of clonidine hydrochloride in this limited indication and clinical population have been established in patients old enough to tolerate placement and management of an epidural catheter, based on evidence from adequate and well controlled studies in adults and experience with the use of clonidine in the pediatric age group for other indications. The use of clonidine hydrochloride should be restricted to pediatric patients with severe intractable pain from malignancy that is unresponsive to epidural or spinal opiates or other more conventional analgesic techniques. The starting dose of clonidine hydrochloride should be selected on per kilogram basis (0.5 mcg per kg per hour) and cautiously adjusted based on the clinical response
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Clonidine in pediatric patients.
Non–Guideline-Supported Use
Opioid Withdrawal
- Dosing Information
- Transdermal patch: 0.05 to 0.1 mg/day.[6]
Tic Disorder
- Dosing Information
- Administer clonidine via a trnasdermar patch, the doses should be adjusted according the wight of the patient:[7]
- 20 to 40 kg : 0.1 mg/week.
- 41 to 60 kg: 1.5 mg/week.
- > 60 kg: 2 mg/week.
Contraindications
- Clonidine hydrochloride tablets should not be used in patients with known hypersensitivity to clonidine.
- Epidural administration is contraindicated in:
- The presence of an injection site infection.
- In patients on anticoagulant therapy, and in those with a bleeding diathesis.
- Administration of clonidine hydrochloride above the C4 dermatome is contraindicated since there are no adequate safety data to support such use.
Warnings
|
WARNING
See full prescribing information for complete Boxed Warning.
Obstetrical, postpartum, or perioperative pain management: Clonidine hydrochloride injection (epidural clonidine) is not recommended for obstetrical, postpartum, or perioperative pain management. The risk of hemodynamic instability, especially hypotension and bradycardia, from epidural clonidine may be unacceptable in these patients. However, in a rare obstetrical, postpartum or perioperative patient, potential benefits may outweigh the possible risks.
|
Oral/Transdermal Administration
Withdrawal
Patients should be instructed not to discontinue therapy without consulting their physician. Sudden cessation of clonidine treatment has, in some cases, resulted in symptoms such as nervousness, agitation, headache, and tremor accompanied or followed by a rapid rise in blood pressure and elevated catecholamine concentrations in the plasma. The likelihood of such reactions to discontinuation of clonidine therapy appears to be greater after administration of higher doses or continuation of concomitant beta-blocker treatment and special caution is therefore advised in these situations. Rare instances of hypertensive encephalopathy, cerebrovascular accidents and death have been reported after clonidine withdrawal. When discontinuing therapy with clonidine tablets, the physician should reduce the dose gradually over 2 to 4 days to avoid withdrawal symptomatology.
An excessive rise in blood pressure following discontinuation of clonidine tablets therapy can be reversed by administration of oral clonidine hydrochloride or by intravenous phentolamine. If therapy is to be discontinued in patients receiving a beta-blocker and clonidine concurrently, the beta-blocker should be withdrawn several days before the gradual discontinuation of clonidine tablets.
Because children commonly have gastrointestinal illnesses that lead to vomiting, they may be particularly susceptible to hypertensive episodes resulting from abrupt inability to take medication.
Precautions
General
In patients who have developed localized contact sensitization to clonidine, continuation of clonidine or substitution of oral clonidine hydrochloride therapy may be associated with the development of a generalized skin rash.
In patients who develop an allergic reaction to clonidine, substitution of oral clonidine hydrochloride may also elicit an allergic reaction (including generalized rash, urticaria, or angioedema).
The sympatholytic action of clonidine may worsen sinus node dysfunction and atrioventricular (AV) block, especially in patients taking other sympatholytic drugs. There are post-marketing reports of patients with conduction abnormalities and/or taking other sympatholytic drugs who developed severe bradycardia requiring IV atropine, IV isoproterenol and temporary cardiac pacing while taking clonidine.
In hypertension caused by pheochromocytoma, no therapeutic effect of clonidine tablets can be expected.
Perioperative Use
Administration of clonidine hydrochloride tablets should be continued to within 4 hours of surgery and resumed as soon as possible thereafter. Blood pressure should be carefully monitored during surgery and additional measures to control blood pressure should be available if required.
Epidural Administration
Use in Postoperative or Obstetrical Analgesia
Epidural clonidine is not recommended for obstetrical, postpartum, or perioperative pain management. The risk of hemodynamic instability, especially hypotension and bradycardia, from epidural clonidine may be unacceptable in these patients.
Hypotension
Because severe hypotension may follow the administration of clonidine, it should be used with caution in all patients. It is not recommended in most patients with severe cardiovascular disease or in those who are otherwise hemodynamically unstable. The benefit of its administration in these patients should be carefully balanced against the potential risks resulting from hypotension.
Vital signs should be monitored frequently, especially during the first few days of epidural clonidine therapy. When clonidine is infused into the upper thoracic spinal segments, more pronounced decreases in the blood pressure may be seen.
Clonidine decreases sympathetic outflow from the central nervous system resulting in decreases in peripheral resistance, renal vascular resistance, heart rate, and blood pressure. However, in the absence of profound hypotension, renal blood flow and glomerular filtration rate remain essentially unchanged.
In the pivotal double-blind, randomized study of cancer patients, where 38 subjects were administered epidural clonidine hydrochloride at 30 mcg/hr in addition to epidural morphine, hypotension occurred in 45% of subjects. Most episodes of hypotension occurred within the first four days after beginning epidural clonidine. However, hypotensive episodes occurred throughout the duration of the trial. There was a tendency for these episodes to occur more commonly in women, and in those with higher serum clonidine levels. Patients experiencing hypotension also tended to weigh less than those who did not experience hypotension. The hypotension is usually responsive to intravenous fluids and, if necessary, appropriate parenterally-administered pressor agents.
Published reports on the use of epidural clonidine for intraoperative or postoperative analgesia also show a consistent and marked hypotensive response to clonidine. Severe hypotension may occur even if intravenous fluid pretreatment is given.
Withdrawal
Sudden cessation of clonidine treatment, regardless of the route of administration, has, in some cases, resulted in symptoms such as nervousness, agitation, headache, and tremor, accompanied or followed by a rapid rise in blood pressure. The likelihood of such reactions appears to be greater after administration of higher doses or with concomitant beta-blocker treatment. Special caution is therefore advised in these situations. Rare instances of hypertensive encephalopathy, cerebrovascular accidents and death have been reported after abrupt clonidine withdrawal. Patients with a history of hypertension and/or other underlying cardiovascular conditions may be at particular risk of the consequences of abrupt discontinuation of clonidine. In the pivotal double-blind, randomized cancer pain study, four of 38 subjects receiving 720 mcg of clonidine per day experienced rebound hypertension following abrupt withdrawal. One of these patients with rebound hypertension subsequently experienced a cerebrovascular accident.
Careful monitoring of infusion pump function and inspection of catheter tubing for obstruction or dislodgement can help reduce the risk of inadvertent abrupt withdrawal of epidural clonidine. Patients should notify their physician immediately if clonidine administration is inadvertently interrupted for any reason. Patients should also be instructed not to discontinue therapy without consulting their physician.
When discontinuing therapy with epidural clonidine, the physician should reduce the dose gradually over 2 to 4 days to avoid withdrawal symptoms.
An excessive rise in blood pressure following discontinuation of epidural clonidine can be treated by administration of clonidine or by intravenous phentolamine. If therapy is to be discontinued in patients receiving a beta-blocker and clonidine concurrently, the beta-blocker should be withdrawn several days before the gradual discontinuation of epidural clonidine.
Infections
Infections related to implantable epidural catheters pose a serious risk. Evaluation of fever in a patient receiving epidural clonidine should include the possibility of a catheter-related infection such as meningitis or epidural abscess.
Precautions
- Cardiac Effects: Epidural clonidine frequently causes decreases in heart rate. Symptomatic bradycardia can be treated with atropine. Rarely, atrioventricular block greater than first degree has been reported. Clonidine does not alter the hemodynamic response to exercise, but may mask the increase in heart rate associated with hypovolemia.
- Respiratory Depression and Sedation: Clonidine administration may result in sedation through the activation of alpha-adrenoceptors in the brainstem. High doses of clonidine cause sedation and ventilatory abnormalities that are usually mild. Tolerance to these effects can develop with chronic administration. These effects have been reported with bolus doses that are significantly larger than the infusion rate recommended for treating cancer pain.
- Depression: Depression has been seen in a small percentage of patients treated with oral or transdermal clonidine. Depression commonly occurs in cancer patients and may be exacerbated by treatment with clonidine. Patients, especially those with a known history of affective disorders, should be monitored for the signs and symptoms of depression.
- Pain of Visceral or Somatic Origin: In the clinical investigations, at doses tested, clonidine hydrochloride was most effective in well-localized, “neuropathic” pain that was characterized as electrical, burning, or shooting in nature, and which was localized to a dermatomal or peripheral nerve distribution. Clonidine hydrochloride may be less effective, or possibly ineffective in the treatment of pain that is diffuse, poorly localized, or visceral in origin.
Adverse Reactions
Clinical Trials Experience
Oral/Transdermal Administration
Most adverse effects are mild and tend to diminish with continued therapy. The most frequent (which appear to be dose-related) are dry mouth, occurring in about 40 of 100 patients; drowsiness, about 33 in 100; dizziness, about 16 in 100; constipation and sedation, each about 10 in 100.
The following less frequent adverse experiences have also been reported in patients receiving clonidine tablets, but in many cases patients were receiving concomitant medication and a causal relationship has not been established:
- Body as a Whole: Fatigue, fever, headache, pallor, weakness, and withdrawal syndrome. Also reported were a weakly positive Coombs’ test and increased sensitivity to alcohol.
- Cardiovascular: Bradycardia, congestive heart failure, electrocardiographic abnormalities (i.e., sinus node arrest, junctional bradycardia, high degree AV block and arrhythmias), orthostatic symptoms, palpitations, Raynaud’s phenomenon, syncope, and tachycardia. Cases of sinus bradycardia and atrioventricular block have been reported, both with and without the use of concomitant digitalis.
- Central Nervous System: Agitation, anxiety, delirium, delusional perception, hallucinations (including visual and auditory), insomnia, mental depression, nervousness, other behavioral changes, paresthesia, restlessness, sleep disorder, and vivid dreams or nightmares.
- Dermatological: Alopecia, angioneurotic edema, hives, pruritus, rash, and urticaria.
- Gastrointestinal: Abdominal pain, anorexia, constipation, hepatitis, malaise, mild transient abnormalities in liver function tests, nausea, parotitis, pseudo-obstruction (including colonic pseudo-obstruction), salivary gland pain, and vomiting.
- Genitourinary: Decreased sexual activity, difficulty in micturition, erectile dysfunction, loss of libido, nocturia, and urinary retention.
- Hematologic: Thrombocytopenia.
- Metabolic: Gynecomastia, transient elevation of blood glucose or serum creatine phosphokinase, and weight gain.
- Musculoskeletal: Leg cramps and muscle or joint pain.
- Oro-otolaryngeal: Dryness of the nasal mucosa.
- Ophthalmological: Accommodation disorder, blurred vision, burning of the eyes, decreased lacrimation, and dryness of eyes.
Epidural Administration
Adverse reactions seen during continuous epidural clonidine infusion are dose-dependent and typical for a compound of this pharmacologic class. The adverse events most frequently reported in the pivotal controlled clinical trial of continuous epidural clonidine administration consisted of hypotension, postural hypotension, decreased heart rate, rebound hypertension, dry mouth, nausea, confusion, dizziness, somnolence, and fever. Hypotension is the adverse event that most frequently requires treatment. The hypotension is usually responsive to intravenous fluids and, if necessary, appropriate parenterally-administered pressor agents. Hypotension was observed more frequently in women and in lower weight patients, but no dose-related response was established.
Implantable epidural catheters are associated with a risk of catheter-related infections, including meningitis and/or epidural abscess. The risk depends on the clinical situation and the type of catheter used, but catheter related infections occur in 5% to 20% of patients, depending on the kind of catheter used, catheter placement technique, quality of catheter care, and length of catheter placement. The inadvertent intrathecal administration of clonidine has not been associated with a significantly increased risk of adverse events, but there are inadequate safety and efficacy data to support the use of intrathecal clonidine.
Epidural clonidine was compared to placebo in a two week double-blind study of 85 terminal cancer patients with intractable pain receiving epidural morphine. The following adverse events were reported in two or more patients and may be related to administration of either clonidine hydrochloride or morphine.
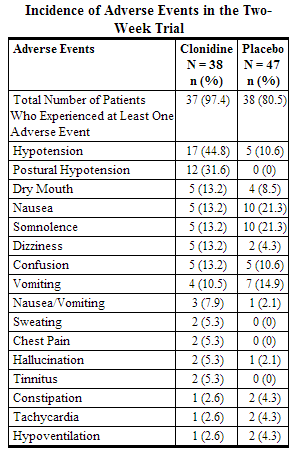
An open label long-term extension of the above trial was performed. Thirty-two subjects received epidural clonidine and morphine for up to 94 weeks with a median dosing period of 10 weeks. The following adverse events (and percent incidence) were reported: hypotension/postural hypotension (47%); nausea (13%); anxiety/confusion (38%); somnolence (25%); urinary tract infection (22%); constipation, dyspnea, fever, infection (6% each); asthenia, hyperaesthesia, pain, skin ulcer, and vomiting (5% each). Eighteen percent of subjects discontinued this study as a result of catheter-related problems (infections, accidental dislodging, etc.), and one subject developed meningitis, possibly as a result of a catheter-related infection. In this study, rebound hypertension was not assessed, and ECG and laboratory data were not systematically sought.
The following adverse reactions have also been reported with the use of any dosage form of clonidine. In many cases patients were receiving concomitant medication and a causal relationship has not been established:
- Body as a Whole: Weakness, 10%; fatigue, 4%; headache and withdrawal syndrome, each 1%. Also reported were pallor, a weakly positive Coomb's test, and increased sensitivity to alcohol.
- Cardiovascular: Palpitations and tachycardia, and bradycardia, each 0.5%. Syncope, Raynaud's phenomenon, congestive heart failure, and electrocardiographic abnormalities (i.e., sinus node arrest, functional bradycardia, high degree AV block) have been reported rarely. Rare cases of sinus bradycardia and atrioventricular block have been reported, both with and without the use of concomitant digitalis.
- Central Nervous System: Nervousness and agitation, 3%; mental depression, 1%; insomnia, 0.5%. Cerebrovascular accidents, other behavioral changes, vivid dreams or nightmares, restlessness, and delirium have been reported rarely.
- Dermatological: Rash, 1%; pruritus, 0.7%; hives, angioneurotic edema and urticaria, 0.5%; alopecia, 0.2%.
- Gastrointestinal: Anorexia and malaise, each 1%; mild transient abnormalities in liver function tests, 1%; hepatitis, parotitis, ileus and pseudo obstruction, and abdominal pain, rarely.
- Genitourinary: Decreased sexual activity and libido, impotence, 3%; nocturia, about 1%; difficulty in micturition, about 0.2%; urinary retention, about 0.1%.
- Hematologic: Thrombocytopenia, rarely.
- Metabolic: Weight gain, 0.1%; gynecomastia, 1%; transient elevation of glucose or serum phosphatase, rarely.
- Musculoskeletal: Muscle or joint pain, about 0.6%; leg cramps, 0.3%.
- Oro-otolaryngeal: Dryness of the nasal mucosa was rarely reported.
- Ophthalmological: Dryness of the eyes, burning of the eyes and blurred vision were rarely reported.
Postmarketing Experience
There is limited information regarding Clonidine Postmarketing Experience in the drug label.
Drug Interactions
- Clonidine may potentiate the CNS-depressive effects of alcohol, barbiturates or other sedating drugs.
- If a patient receiving clonidine hydrochloride is also taking tricyclic antidepressants, the hypotensive effect of clonidine may be reduced, necessitating an increase in the clonidine dose.
- If a patient receiving clonidine is also taking neuroleptics, orthostatic regulation disturbances (e.g., orthostatic hypotension, dizziness, fatigue) may be induced or exacerbated.
- Monitor heart rate in patients receiving clonidine concomitantly with agents known to affect sinus node function or AV nodal conduction, e.g., digitalis, calcium channel blockers and beta-blockers.
- Sinus bradycardia resulting in hospitalization and pacemaker insertion has been reported in association with the use of clonidine concomitantly with diltiazem or verapamil.
- Amitriptyline in combination with clonidine enhances the manifestation of corneal lesions in rats.
- Based on observations in patients in a state of alcoholic delirium it has been suggested that high intravenous doses of clonidine may increase the arrhythmogenic potential (QT-prolongation, ventricular fibrillation) of high intravenous doses of haloperidol. Causal relationship and relevance for clonidine oral tablets have not been established.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Reproduction studies performed in rabbits at doses up to approximately 3 times the oral maximum recommended daily human dose (MRDHD) of clonidine hydrochloride tablets, USP produced no evidence of a teratogenic or embryotoxic potential in rabbits. In rats, however, doses as low as 1/3 the oral MRDHD (1/15 the MRDHD on a mg/m2 basis) of clonidine were associated with increased resorptions in a study in which dams were treated continuously from 2 months prior to mating. Increased resorptions were not associated with treatment at the same time or at higher dose levels (up to 3 times the oral MRDHD) when the dams were treated on gestation days 6 to 15. Increases in resorption were observed at much higher dose levels (40 times the oral MRDHD on a mg/kg basis; 4 to 8 times the MRDHD on a mg/m2 basis) in mice and rats treated on gestation days 1 to14 (lowest dose employed in the study was 500 mcg/kg).
No adequate, well-controlled studies have been conducted in pregnant women. Clonidine crosses the placental barrier. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Clonidine in women who are pregnant.
Labor and Delivery
There are no adequate controlled clinical trials evaluating the safety, efficacy, and dosing of clonidine hydrochloride in obstetrical settings. Because maternal perfusion of the placenta is critically dependent on blood pressure, use of clonidine hydrochloride as an analgesic during labor and delivery is not indicated
Nursing Mothers
As clonidine hydrochloride tablets, USP are excreted in human milk, caution should be exercised when clonidine hydrochloride is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established in adequate and well-controlled trials.
Geriatic Use
There is no FDA guidance on the use of Clonidine in geriatric settings.
Gender
There is no FDA guidance on the use of Clonidine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Clonidine with respect to specific racial populations.
Renal Impairment
Patients with renal impairment may benefit from a lower initial dose. Patients should be carefully monitored. Since only a minimal amount of clonidine is removed during routine hemodialysis, there is no need to give supplemental clonidine following dialysis.
Hepatic Impairment
There is no FDA guidance on the use of Clonidine in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Clonidine in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Clonidine in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral/Transdermal/Epidural
Monitoring
Hypotension
Vital signs should be monitored frequently, especially during the first few days of epidural clonidine therapy. When clonidine is infused into the upper thoracic spinal segments, more pronounced decreases in the blood pressure may be seen.
Withdrawal
Careful monitoring of infusion pump function and inspection of catheter tubing for obstruction or dislodgement can help reduce the risk of inadvertent abrupt withdrawal of epidural clonidine. Patients should notify their physician immediately if clonidine administration is inadvertently interrupted for any reason. Patients should also be instructed not to discontinue therapy without consulting their physician.
IV Compatibility
There is limited information regarding the compatibility of Clonidine and IV administrations.
Overdosage
Hypertension may develop early and may be followed by hypotension, bradycardia, respiratory depression, hypothermia, drowsiness, decreased or absent reflexes, weakness, irritability and miosis. The frequency of CNS depression may be higher in children than adults. Large overdoses may result in reversible cardiac conduction defects or dysrhythmias, apnea, coma and seizures. Signs and symptoms of overdose generally occur within 30 minutes to two hours after exposure. As little as 0.1 mg of clonidine has produced signs of toxicity in children.
There is no specific antidote for clonidine overdosage. Clonidine overdosage may result in the rapid development of CNS depression; therefore, induction of vomiting with ipecac syrup is not recommended. Gastric lavage may be indicated following recent and/or large ingestions. Administration of activated charcoal and/or a cathartic may be beneficial. Supportive care may include atropine sulfate for bradycardia, intravenous fluids and/or vasopressor agents for hypotension and vasodilators for hypertension. Naloxone may be a useful adjunct for the management of clonidine-induced respiratory depression, hypotension and/or coma; blood pressure should be monitored since the administration of naloxone has occasionally resulted in paradoxical hypertension. Dialysis is not likely to significantly enhance the elimination of clonidine.
The largest overdose reported to date involved a 28-year old male who ingested 100 mg of clonidine hydrochloride powder. This patient developed hypertension followed by hypotension, bradycardia, apnea, hallucinations, semicoma, and premature ventricular contractions. The patient fully recovered after intensive treatment. Plasma clonidine levels were 60 ng/ml after 1 hour, 190 ng/ml after 1.5 hours, 370 ng/ml after 2 hours, and 120 ng/ml after 5.5 and 6.5 hours. In mice and rats, the oral LD50 of clonidine is 206 and 465 mg/kg, respectively.
Pharmacology
Mechanism of Action
Clonidine stimulates alpha-adrenoreceptors in the brain stem. This action results in reduced sympathetic outflow from the central nervous system and in decreases in peripheral resistance, renal vascular resistance, heart rate, and blood pressure.
Structure
(clonidine hydrochloride, USP) is a centrally acting alpha-agonist hypotensive agent available as tablets for oral administration in three dosage strengths: 0.1 mg, 0.2 mg and 0.3 mg. The 0.1 mg tablet is equivalent to 0.087 mg of the free base.
The inactive ingredients are colloidal silicon dioxide, corn starch, dibasic calcium phosphate, FD&C Yellow No. 6, gelatin, glycerin, lactose, and magnesium stearate. The Catapres 0.1 mg tablet also contains FD&C Blue No.1 and FD&C Red No.3.
Clonidine hydrochloride is an imidazoline derivative and exists as a mesomeric compound. The chemical name is 2-(2,6-dichlorophenylamino)-2-imidazoline hydrochloride. The following is the structural formula:
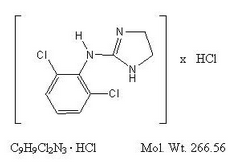
Clonidine hydrochloride is an odorless, bitter, white, crystalline substance soluble in water and alcohol.
Pharmacodynamics
The patient’s blood pressure declines within 30 to 60 minutes after an oral dose, the maximum decrease occurring within 2 to 4 hours. Renal blood flow and glomerular filtration rate remain essentially unchanged. Normal postural reflexes are intact; therefore, orthostatic symptoms are mild and infrequent.
Acute studies with clonidine hydrochloride in humans have demonstrated a moderate reduction (15% to 20%) of cardiac output in the supine position with no change in the peripheral resistance: at a 45° tilt there is a smaller reduction in cardiac output and a decrease of peripheral resistance. During long term therapy, cardiac output tends to return to control values, while peripheral resistance remains decreased. Slowing of the pulse rate has been observed in most patients given clonidine, but the drug does not alter normal hemodynamic response to exercise.
Tolerance to the antihypertensive effect may develop in some patients, necessitating a reevaluation of therapy.
Other studies in patients have provided evidence of a reduction in plasma renin activity and in the excretion of aldosterone and catecholamines. The exact relationship of these pharmacologic actions to the antihypertensive effect of clonidine has not been fully elucidated.
Clonidine acutely stimulates growth hormone release in both children and adults, but does not produce a chronic elevation of growth hormone with long-term use.
Pharmacokinetics
The pharmacokinetics of clonidine is dose-proportional in the range of 100 to 600 µg. The absolute bioavailability of clonidine on oral administration is 70% to 80%. Peak plasma clonidine levels are attained in approximately 1 to 3 hours.
Following intravenous administration, clonidine displays biphasic disposition with a distribution half-life of about 20 minutes and an elimination half-life ranging from 12 to 16 hours. The half-life increases up to 41 hours in patients with severe impairment of renal function. Clonidine crosses the placental barrier. It has been shown to cross the blood-brain barrier in rats.
Following oral administration about 40% to 60% of the absorbed dose is recovered in the urine as unchanged drug in 24 hours. About 50% of the absorbed dose is metabolized in the liver. Neither food nor the race of the patient influences the pharmacokinetics of clonidine.
The antihypertensive effect is reached at plasma concentrations between about 0.2 and 2.0 ng/mL in patients with normal excretory function. A further rise in the plasma levels will not enhance the antihypertensive effect.
Nonclinical Toxicology
In several studies with oral clonidine hydrochloride, a dose-dependent increase in the incidence and severity of spontaneous retinal degeneration was seen in albino rats treated for six months or longer. Tissue distribution studies in dogs and monkeys showed a concentration of clonidine in the choroid.
In view of the retinal degeneration seen in rats, eye examinations were performed during clinical trials in 908 patients before, and periodically after, the start of clonidine therapy. In 353 of these 908 patients, the eye examinations were carried out over periods of 24 months or longer. Except for some dryness of the eyes, no drug-related abnormal ophthalmological findings were recorded and, according to specialized tests such as electroretinography and macular dazzle, retinal function was unchanged.
In combination with amitriptyline, clonidine hydrochloride administration led to the development of corneal lesions in rats within 5 days.
Carcinogenesis, Mutagenesis, Impairment of Fertility
Chronic dietary administration of clonidine was not carcinogenic to rats (132 weeks) or mice (78 weeks) dosed, respectively, at up to 46 or 70 times the maximum recommended daily human dose as mg/kg (9 or 6 times the MRDHD on a mg/m2 basis). There was no evidence of genotoxicity in the Ames test for mutagenicity or mouse micronucleus test for clastogenicity.
Fertility of male or female rats was unaffected by clonidine doses as high as 150 µg/kg (approximately 3 times MRDHD). In a separate experiment, fertility of female rats appeared to be affected at dose levels of 500 to 2000 µg/kg (10 to 40 times the oral MRDHD on a mg/kg basis; 2 to 8 times the MRDHD on a mg/m2 basis).
Clinical Studies
In a double-blind, randomized study of cancer patients with severe intractable pain below the C4 dermatome not controlled by morphine, 38 patients were randomized to an epidural infusion of clonidine hydrochloride plus epidural morphine, whereas 47 subjects received epidural placebo plus epidural morphine. Both groups were allowed rescue doses of epidural morphine. Successful analgesia, defined as a decrease in either morphine use or Visual Analog Score (VAS) pain, was significantly more common with epidural clonidine than placebo (45% vs 21%, p=0.016). Only the subgroup of 36 patients with “neuropathic” pain, characterized by the investigator as well-localized, burning, shooting, or electric-like pain in a dermatomal or peripheral nerve distribution had significant analgesic effects relative to placebo in this study.
The most frequent adverse events with clonidine were hypotension (45% vs 11% for placebo, p< 0.001), postural hypotension (32% vs 0%, p< 0.001), dizziness (13% vs 4%, p=0.234), anxiety (11% vs 2%, p=0.168) and dry mouth (13% vs 9%, p=0.505). Both mean blood pressure and heart rate were reduced in the clonidine group. At the conclusion of the two week study period in the clinical trial, all patients were abruptly withdrawn from study drug or placebo. Four patients of the clonidine group suffered rebound hypertension upon withdrawal of clonidine; one of these patients suffered a cerebrovascular accident. Asymptomatic bradycardia was noted in one clonidine patient.
How Supplied
Clonidine hydrochloride tablets are supplied as follows:
- 0.1 mg: Bottle of 100 (NDC 0597-0006-01)
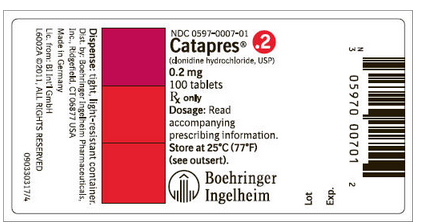
- 0.2 mg: Bottle of 100 (NDC 0597-0007-01)
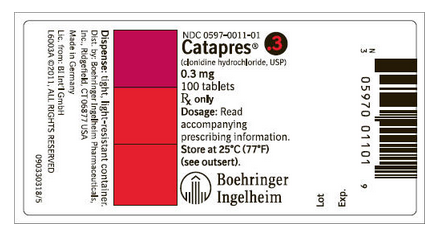
- 0.3 mg: Bottle of 100 (NDC 0597-0011-01)
Clonidine transdermal patch:
- Principal display panel - 0.1 mg/day
- 4 Systems + 4 Adhesive Covers (NDC 0378-0871-99).
Clonidine hydrochloride injection is available as:
- 100 mcg/mL solution in 10 mL vials, packaged individually (NDC 0517-0730-01)
- 500 mcg/mL solution in 10 mL vials, packaged individually (NDC 0517-0731-01)
Storage
Tablets
- Store at 25°C (77°F); excursions permitted to 15°-30°C (59°-86°F).
- Dispense in tight, light-resistant container.
Injection
- Store at 20°to 25°C (68°to 77°F); excursions permitted to 15°to 30°C (59°to 86°F).
Images
Drug Images
{{#ask: Page Name::Clonidine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Clonidine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Clonidine Tablets
- Patients should be cautioned against interruption of clonidine tablets therapy without their physician's advice.
- Since patients may experience a possible sedative effect, dizziness, or accommodation disorder with use of clonidine, caution patients about engaging in activities such as driving a vehicle or operating appliances or machinery. Also, inform patients that this sedative effect may be increased by concomitant use of alcohol, barbiturates, or other sedating drugs.
- Patients who wear contact lenses should be cautioned that treatment with clonidine tablets may cause dryness of eyes.
Clonidine Patch
Patients should be cautioned against interruption of Clonidine Transdermal System therapy without their physician’s advice.
Since patients may experience a possible sedative effect, dizziness, or accommodation disorder with use of clonidine, caution patients about engaging in activities such as driving a vehicle or operating appliances or machinery. Also, inform patients that this sedative effect may be increased by concomitant use of alcohol, barbiturates, or other sedating drugs.
Patients who wear contact lenses should be cautioned that treatment with Clonidine Transdermal System may cause dryness of eyes.
Patients should be instructed to consult their physicians promptly about the possible need to remove the patch if they observe moderate to severe localized erythema and/or vesicle formation at the site of application or generalized skin rash.
If a patient experiences isolated, mild localized skin irritation before completing 7 days of use, the system may be removed and replaced with a new system applied to a fresh skin site.
If the system should begin to loosen from the skin after application, the patient should be instructed to place the adhesive cover directly over the system to ensure adhesion during its 7-day use.
Used Clonidine Transdermal System patches contain a substantial amount of their initial drug content which may be harmful to infants and children if accidentally applied or ingested. THEREFORE, PATIENTS SHOULD BE CAUTIONED TO KEEP BOTH USED AND UNUSED CLONIDINE TRANSDERMAL SYSTEM PATCHES OUT OF THE REACH OF CHILDREN. After use, Clonidine Transdermal System should be folded in half with the adhesive sides together and discarded away from children’s reach.
Instructions for use, storage and disposal of the system are provided at the end of this monograph. These instructions are also included in each box of Clonidine Transdermal System.
Clonidine Transdermal System, USP
(Read the following instructions carefully before using this medication. If you have any questions, please consult with your doctor.)
General Information
Clonidine Transdermal System is a peach colored, rectangular PATCH with rounded corners, containing an active blood-pressure-lowering medication. It is designed to deliver the drug into the body through the skin smoothly and consistently for one full week. Normal exposure to water, as in showering, bathing, and swimming, should not affect the PATCH.
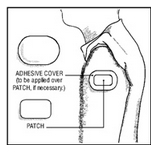
The optional ivory ADHESIVE COVER should be applied directly over the PATCH, should the PATCH begin to separate from the skin. The ADHESIVE COVER ensures that the PATCH sticks to the skin. The Clonidine Transdermal System PATCH must be replaced with a new one on a fresh skin site if the one in use significantly loosens or falls off.
How to Apply the Clonidine Transdermal System PATCH
1) Apply the peach colored, rectangular PATCH with rounded corners, once a week, preferably at a convenient time on the same day of the week (i.e., prior to bedtime on Tuesday of week one; prior to bedtime on Tuesday of week two, etc.).
Each box of Clonidine Transdermal System contains two types of pouches:

2) Select a hairless area such as on the upper, outer arm or upper chest. The area chosen should be free of cuts, abrasions, irritation, scars or calluses and should not be shaved before applying the Clonidine Transdermal System PATCH. Do not place the Clonidine Transdermal System PATCH on skin folds or under tight undergarments, since premature loosening may occur.
3) Wash hands with soap and water and thoroughly dry them.
4) Clean the area chosen with soap and water. Rinse and wipe dry with a clean, dry tissue.
5) Select the pouch labeled Clonidine Transdermal System, USP and open it as illustrated in Figure 3. Remove the contents of the pouch and discard the additional pieces of clear protective film above and below the PATCH.
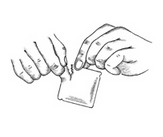
6) Remove the clear plastic protective backing from the peach colored, rectangular PATCH by gently peeling off one half of the backing at a time as shown in Figure 4. Avoid touching the sticky side of the Clonidine Transdermal System PATCH.
7) Place the Clonidine Transdermal System PATCH on the prepared skin site (sticky side down) by applying firm pressure over the PATCH to ensure good contact with the skin, especially around the edges (Figure 5). Discard the clear plastic protective backing and wash your hands with soap and water to remove any drug from your hands.
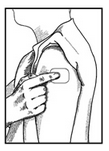
8) After one week, remove the old PATCH and discard it (refer to Instructions for Disposal). After choosing a different skin site, repeat instructions 2 through 7 for the application of your next Clonidine Transdermal System PATCH.
What to do if your Clonidine Transdermal System PATCH becomes loose while wearing:
How to Apply the ADHESIVE COVER
NOTE: The ivory ADHESIVE COVER does not contain any drug and should not be used alone. The COVER should be applied directly over the Clonidine Transdermal System PATCH only if the PATCH begins to separate from the skin, thereby ensuring that it sticks to the skin for 7 full days.
1) Wash hands with soap and water and thoroughly dry them.
2) Using a clean, dry tissue, make sure that the area around the rectangular, peach Clonidine Transdermal System PATCH is clean and dry. Press gently on the Clonidine Transdermal System PATCH to ensure that the edges are in good contact with the skin.
3) Take the ivory ADHESIVE COVER (Figure 6) from the plain white pouch and remove the paper liner backing from the COVER.

4) Carefully center the ivory ADHESIVE COVER over the rectangular, peach Clonidine Transdermal System PATCH and apply firm pressure, especially around the edges in contact with the skin.
Instructions for Disposal
KEEP OUT OF REACH OF CHILDREN
During or even after use, a PATCH contains active medication which may be harmful to infants and children if accidentally applied or ingested. After use, fold in half with the sticky sides together. Dispose of carefully out of reach of children.
Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
For more information, call Mylan Pharmaceuticals Inc. at 1-877-446-3679 (1-877-4-INFO-RX).
Precautions with Alcohol
Clonidine may potentiate the CNS-depressive effects of alcohol
Brand Names
- Catapres-TTS-1
- Catapres-TTS-2
- Catapres-TTS-3
- Nexiclon XR
Look-Alike Drug Names
- Clonidine - Clonazepam
- Clonidine - Clozapine
- Clonidine - Klonopin
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Hammar M, Berg G (1985). "Clonidine in the treatment of menopausal flushing. A review of clinical studies". Acta Obstet Gynecol Scand Suppl. 132: 29–31. PMID 3895809.
- ↑ Nagamani M, Kelver ME, Smith ER (1987). "Treatment of menopausal hot flashes with transdermal administration of clonidine". Am J Obstet Gynecol. 156 (3): 561–5. PMID 3826200.
- ↑ O'Connor KJ, Grady JF, Moore CJ, Licandro J, Brenner LA (2000). "Use of a clonidine patch in the treatment of ischemic ulcerations of the foot". J Am Podiatr Med Assoc. 90 (6): 324–7. doi:10.7547/87507315-90-6-324. PMID 10881467.
- ↑ 4.0 4.1 Covey LS, Glassman AH (1991). "A meta-analysis of double-blind placebo-controlled trials of clonidine for smoking cessation". Br J Addict. 86 (8): 991–8. PMID 1833003.
- ↑ Kleber HD, Riordan CE, Rounsaville B, Kosten T, Charney D, Gaspari J; et al. (1985). "Clonidine in outpatient detoxification from methadone maintenance". Arch Gen Psychiatry. 42 (4): 391–4. PMID 3977557.
- ↑ Deutsch ES, Nadkarni VM (1996). "Clonidine prophylaxis for narcotic and sedative withdrawal syndrome following laryngotracheal reconstruction". Arch Otolaryngol Head Neck Surg. 122 (11): 1234–8. PMID 8906060.
- ↑ Du YS, Li HF, Vance A, Zhong YQ, Jiao FY, Wang HM; et al. (2008). "Randomized double-blind multicentre placebo-controlled clinical trial of the clonidine adhesive patch for the treatment of tic disorders". Aust N Z J Psychiatry. 42 (9): 807–13. doi:10.1080/00048670802277222. PMID 18696285.
- ↑ 8.0 8.1 Lowenthal, DT; Matzek, KM; MacGregor, TR (May 1988). "Clinical pharmacokinetics of clonidine". Clinical Pharmacokinetics. 14 (5): 287–310. doi:10.2165/00003088-198814050-00002. PMID 3293868.
- ↑ 9.0 9.1 9.2
{{#subobject:
|Label Page=Clonidine |Label Name=ClonidineIVPackage1.png
}}
{{#subobject:
|Label Page=Clonidine |Label Name=ClonidineIVPackage2.png
}}
{{#subobject:
|Label Page=Clonidine |Label Name=ClonidineIVPackage3.png
}}
{{#subobject:
|Label Page=Clonidine |Label Name=ClonidineIVPackage4.png
}}