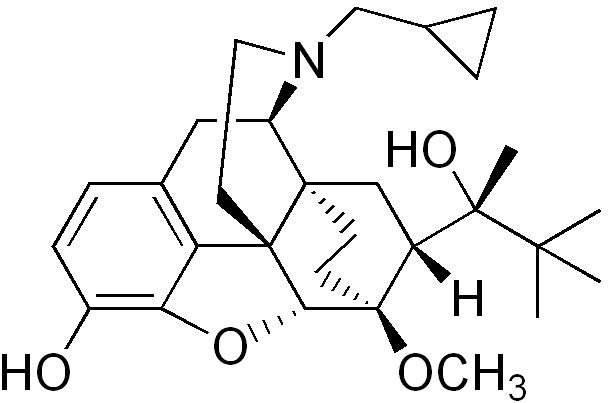
Buprenorphine (mucous membrane)
 | |
| Clinical data | |
|---|---|
| Pregnancy category |
|
| Routes of administration | sublingual, IM, IV,transdermal |
| ATC code | |
| Legal status | |
| Legal status |
|
| Pharmacokinetic data | |
| Bioavailability | 31% (sublingual, from ethanolic solution) ~50-60% (sublingual, high-dose tablet) ~50% (transdermal) |
| Protein binding | 96% |
| Metabolism | hepatic |
| Elimination half-life | 20-70, mean 37 hours |
| Excretion | biliary and renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C29H41NO4 |
| Molar mass | 467.64 g/mol |
Street Names/slangs: Box or Boxes, Bupe, Oranges, Saboxin, Sobos, Stop signs, Stops, Sub or plural Subs
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Buprenorphine, is an opioid drug with partial agonist and antagonist actions. Buprenorphine hydrochloride was first marketed in the 1980s by Reckitt & Colman (now Reckitt Benckiser) as an analgesic, available generally as Buprenex in a 0.3 mg/ml injectable formulation in the United States. In October 2002, the FDA additionally approved Suboxone and Subutex, buprenorphine's high-dose sublingual pill preparations for opioid addiction, and as such the drug is now also used for this purpose. It has been a Schedule III drug under the Convention on Psychotropic Substances[1] since it was rescheduled from Schedule V (the schedule with the lowest restrictions and penalties) just before FDA approval of Suboxone and Subutex. In the recent years, buprenorphine has been introduced in most European countries as transdermal formulation ("patch") for the treatment of chronic pain.
Commercial preparations
British firm Reckitt & Colman (now Reckitt Benckiser) first marketed buprenorphine under the trade names Temgesic (sublingual/parenteral preparations, no active additives) and Buprenex (parenteral, no active additives). Two more recent formulations from Reckitt Benckiser have been approved for opioid addiction treatment in the U.S.: Subutex (bitter sublingual, no active additives; in 2 mg and 8 mg dosages) and Suboxone (Lemon-lime flavored sublingual, one part naloxone for every four parts buprenorphine; hexagon shaped tablet in 2 mg and 8 mg dosages). Suboxone contains opiate agonist as well as the opioid antagonist naloxone to deter illicit intravenous preparation of the tablet. This is intended to attenuate the effects of buprenorphine on opioid-naive users should this formulation be injected - however no human studies have been done demonstrating the efficacy of this approach with buprenorphine and a growing number of street reports indicate that the naloxone is ineffective. The small amount of naloxone has no effect on the buprenorphine only opioids with lower affinities. [2]
TIP-40, a publication dealing with clincal use of Suboxone/Subutex, concede that the naloxone contained in the tablets would not effect someone stabilized on suboxone, even if injected. It mainly serves to deter diversion of the drug, as injecting it is not a viable alternative to injecting other short acting opioids. The naloxone would cause 15-45 minutes of extreme withdrawal (PWS, precipitated withdrawal syndrome) and even after that, the partial agonist nature of buprenorphine could cause continued withdrawal symptoms. Injecting a crushed tablet in an opioid naive person would cause a high, as the naloxone has nothing to displace in such people, and it is of insufficient binding strength to compete with buprenorphine for receptors.
A solution for injection (usually by the intramuscular route) is marketed for the UK veterinary market by Alstoe Animal Health as Vetergesic, licenced for analgesia and sedation in dogs.
Since 2001 buprenorphine is also available transdermally in 35, 52.5 and 70 mcg/hour transdermal patch, that delivers the dose over 96 hours. This application form is marketed as Transtec in most European countries by Grunenthal [3] (Napp Pharmaceuticals.in the UK [4] Norpharma in Denmark) for the treatment of moderate to severe cancer pain and severe non-cancer pain not responding to non-opioids. Moreover, a new 5, 10 and 20 mcg/hour patch is marketed as Butrans or Norspan, a once weekly patch for severe chronic pain not responding to non-opioids, marketed by Napp Pharmaceuticals Ltd., and Mundipharma and Grunenthal respectively.
Pharmacology and pharmacokinetics
Buprenorphine is a thebaine derivative with powerful analgesia approximately 25 to 40 times as potent as morphine[5], and its analgesic effect is due to partial agonist activity at μ-opioid receptors, i.e., when the molecule binds to a receptor, it is only partially activated in contrast to a full agonist such as morphine. Buprenorphine also has very high binding affinity for the μ receptor such that opioid receptor antagonists (e.g. naloxone) only partially reverse its effects. These two properties must be carefully considered by the practitioner, as an overdose cannot be easily reversed (although overdose is unlikely in addicted patients or people with tolerance to opioids who use the drug sublingually as meant in the case of Subutex/Suboxone, especially if there are no benzodiazepines involved), and use in persons physically dependent on full-agonist opioids may trigger opioid withdrawal that also cannot be easily reversed and can last over 24 hours, as the drug's mean half-life is 37 hours.
Buprenorphine is also a κ-opioid receptor antagonist, and partial/full agonist at the recombinant human ORL1 nociceptin receptor.[6]
Buprenorphine hydrochloride is administered by intramuscular injection, intravenous infusion, via a transdermal patch, or as a sublingual tablet. It is not administered orally, due to very high first-pass metabolism. Buprenorphine is metabolised by the liver, via the CYP3A4 isozyme of the cytochrome P450 enzyme system, into norbuprenorphine (by N-dealkylation) and other metabolites. The metabolites are further conjugated with glucuronic acid and eliminated mainly through excretion into the bile. The elimination half-life of buprenorphine is 20–73 hours (mean 37). Due to the mainly hepatic elimination there is no risk of accumulation in patients with renal impairment and in the elderly.
The main active metabolite, norbuprenorphine, is a δ-opioid receptor and ORL1 receptor agonist, μ- and κ-opioid receptor partial agonist, but buprenorphine antagonizes its effects.[6]
Plasma concentrations after application of transdermal buprenorphine increase steadily and the minimum effective therapeutic dose (100 pg/ml) is reached at 11 hours and 21 hours for a single 35 and 70 μg/h patch, respectively. Peak plasma concentration (Cmax) is reached in about 60 hours (305 and 624 pg/ml for the 35 and 70 μg/h strength patch, respectively), and is markedly longer than with 0.3 mg intravenous buprenorphine (0.41 hours). Transdermal buprenorphine has a half-life of approx. 30 hours, and a bioavailability of approximately 50%, which is comparable to sublingual buprenorphine .
Clinical use
Indications
Pain indications
Depending on the application form, buprenorphine is indicated for the treatment of moderate to severe chronic pain or for peri-operative analgesia. For the treatment of chronic pain, the transdermal formulations are nowadays preferred, which can be used both for chronic cancer pain as well as chronic non-malignant pain e.g. musculosceletal and neuropathic pain. The intravenous formulation is mainly used in postoperative pain (e.g. as PCA- patient controlled analgesia) and the sublingual formulation is e.g. used as breakthrough medication for patients with basic transdermal treatment. Advantages of buprenorphine in the treatment of chronic pain are – from a clinical perspective- its relatively long half-life, the option of sublingual and transdermal application and the excellent safety profile (ceiling effect for respiratory depression, lack of immunosuppressive effect, low pharmacokinetic interaction potential, no accumulation in renal impairment).
Antidepressant features
A clinical trial conducted at Harvard Medical School in the mid-1990s demonstrated that a majority of unipolar non-psychotic patients with major depression refractory to conventional thymoleptic antidepressants could be successfully treated with buprenorphine.[7] See opioids for other (predominantly favorable) experiments with buprenorphine and other opioids for psychological relief. However, psychological distress is currently not an approved indication for the use of any opioid, and legally it falls in a "grey zone" that is technically legal but a doctor could still face charges regardless (but not for off-label scripting in itself, simply being singled out by the Drug Enforcement Administration, who prosecute doctors often for using controlled substances for approved uses ("too much"). [2][3] The doctor still needs the proper DEA licensing under the Drug Addiction Treatment Act of 2000 to prescribe Subutex or Suboxone for opioid addiction/dependence.
Contraindication
Like full agonist opiates, buprenorphine can cause drowsiness, vomiting and respiratory depression. Taking buprenorphine in conjunction with central nervous system (CNS) depressants such as sedatives, tranquilizers, alcohol, and especially benzodiazepines can be particularly dangerous [8]. Falling asleep while abusing this drug, especially while combining it with other central nervous system depressants, can be extremely dangerous and thus greatly increases the chance of serious complications or death.
Adverse effects
Common adverse drug reactions associated with the use of buprenorphine are similar to those of other opioids and include: nausea and vomiting, drowsiness, dizziness, headache, itch, dry mouth, miosis, orthostatic hypotension, male ejaculatory difficulty, decreased libido, urinary retention. Constipation and CNS effects are seen less frequently than with e.g. morphine [9] Hepatic necrosis and hepatitis with jaundice have been reported with the use of buprenorphine, especially after intravenous injection of crushed tablets.
The most severe and serious adverse reaction associated with opioid use in general is respiratory depression, the mechanism behind fatal overdose. Buprenorphine behaves differently than other opioids in this respect, as it shows a ceiling effect for respiratory depression [9].Moreover, former doubts on the antagonisation of the respiratory effects by Naloxone have been disproved: Buprenorphine effects can be antogonised with a continuous infusion of Naloxone [10]. Of course, concurrent use of buprenorphine and CNS depressants (such as alcohol or benzodiazepines) is contraindicated as it may lead to fatal respiratory depression.
In people on medium- to long-term maintenance with Suboxone or Subutex do not have a major risk of overdose, as long as the drug is used properly (as prescribed), and benzodiazepines are not prescribed to individuals without a tolerance to opioids.
As with other Opioids, dependence and tolerance are rarely a problem when used safely. There is little evidence that buprenorphine is less likely to cause such problems. Maintenance dosages can remain at the same moderate level indefinitely, and in many cases even lowered, without discomfort. Due to buprenorphine's pharmacological actions, raising the dosage will not result in a stronger analgesic effect after a certain point (around 16–32 mg), beyond which the drug will actually have a reduced analgesic effect.
The partial agonist activity of buprenorphine combined with its high affinity for μ-opioid receptor means that it may act clinically as an antagonist and thus precipitate opioid withdrawal symptoms when an opioid-dependent patient is commenced on the drug soon after the use of another opioid drug. Patients are advised to wait between 24 and 36 hours after their last use of short-acting opioids (such as heroin or oxycodone) before beginning treatment with buprenorphine. Those using long-acting opioids, such as methadone, should only commence treatment once withdrawal symptoms are present. Beginning any earlier may result in extreme cases of opioid withdrawal. Additionally, it is recommended that the patient be on no more than 30 mg of methadone per day when switching to buprenorphine.
Dependence treatment
Buprenorphine sublingual preparations are often used in the management of opioid dependence (that is, dependence on heroin, oxycodone, hydrocodone, morphine, oxymorphone, fentanyl or other opioids). The Suboxone and Subutex preparations were approved for this indication by the United States FDA in October 2002. This was only possible due to the Drug Addiction Treatment Act of 2000 that for the first time since 1914-1920 (conflicting Supreme Court rulings - rulings that would not stand to today's Supreme Court as they ruled that maintenance or detox treatment is not medical treatment, and likely was not what was intended by Congress) made it legal for doctors to prescribe opioids themselves to manage addiction ("maintenance") or for short-term detox (special doctors in registered clinics are excluded from these blanket restrictions). This law is limited to Schedules III through V only - thus excluding methadone and stronger opioids.
The use of Medication assisted treatment in the management of opioid dependence is highly regulated, owing to the sometimes controversial nature of this aspect of harm reduction policy. In the United States, a special federal waiver is required to prescribe Subutex and Suboxone for opioid addiction treatment on an outpatient basis. However, if the doctor meets none of the other clarifications, an 8-hour course is all that is required). Each approved prescriber is allowed to manage only 30 patients on buprenorphine for opioid addiction as outpatients;[11] the U.S. Senate has passed a bill relaxing this restriction for group practices only as of May 25, 2006[citation needed]. Legislation was passed by Congress in the last few hours prior to Holiday recess in December, 2006 allowing physicians with one year of clinical experience Physicians are only required to have had their original waiver for 1 year, NO clinical expirience is required to request an additional exemption within DATA 2000 allowing 100 patient limit effective 12/29/2006 (public law 109-469). Similar restrictions are placed on prescribers in many other jurisdictions. Buprenorphine is heavily regulated in Australia relatively, and while the number of patients isn't limited generally daily visits for supervised dosing at a pharmacy is required, such as methadone, and methadone where used is used in lower relative doses.
Buprenorphine vs Methadone
Buprenorphine and methadone are both used for short-term and long-term opioid maintenance therapy. Each agent has its relative advantages and disadvantages.
In terms of efficacy (i.e. treatment retention, negative urine samples), high-dose buprenorphine (such as that commonly found with Subutex/Suboxone treatment; 8-16 mg typically) has been found to be superior to 20-40 mg of methadone/day (low dose) and equatable anywhere between 50 mg-70 mg (moderate dose)[12] to up to 100 mg (high dose)[13] methadone/day. (Methadone, however, can continue to increase in effectiveness over 100 mg, although it is a debatable topic, but this would consistute "very high dose" in this measurement commonly used by studies, including those quoted). In all cases, high-dose buprenorphine has been found to be far superior to placebo and an effective treatment for opioid addiction, with retention rates of 50% as a minimum.[12][13][14][15]
Buprenorphine sublingual tablets (Suboxone and Subutex for opioid addiction) have a long duration of action which may allow for dosing every two or three days, as tolerated by the patient, compared with the daily dosing required to prevent withdrawals with methadone. In the United States, following initial management, a patient is typically prescribed up to a one month supply for self-administration. It is often misunderstood that the patient has to receive other therapy in this situation, but the law simply states that the prescribing physician needs to be capable of referring the patient to other addiction treatment (i.e. psychotherapy or support groups,) and many (but not all) physicians are aware of this and simply recommend therapy, or as they deem fit have therapy required.
Buprenorphine may be more convenient for some users because patients can be given a 30 day take home dose relatively soon after starting treatment, hence making them more compliant relative to those who need to visit a methadone dispensing facility daily to receive their methadone. The facilities, which are regulated at the state and federal level, initially only allow patients to take home weekend and holiday doses and after months of compliance without missing a day, they are given a week or more worth of methadone to take home. However, at many clinics, patients are given enough methadone to last for 2 weeks or 1 month, after significant time in treatment. At some US clinics, patients may get enough methadone to last 3 months. Therefore, buprenorphine does not have an advantage in terms of convenience for patients who have access to relatively liberal clinics, stay free of other drugs, and who stay on methadone long enough to earn the take-homes. In fact, users who are taking home a supply of methadone which lasts 3 months, 1 month, or even 2 weeks at a time may have difficulty finding a buprenorphine doctor who would offer equally convenient accommodations in some areas. There are also many professionals who are advocating for office-based methadone treatment, like office-based buprenorphine treatment, in the US and elsewhere. Such treatment with full opiate agonists is already available in the UK, and has been ever since heroin was made illegal, with an interruption of a few decades which occurred, likely under pressure from the United States, during the worldwide escalation of the War on Drugs which occurred during the 1960's and 1970's. In fact, in the UK a doctor may prescribe any opiate to a person, regardless of their complaint. In practice, methadone is most often used, although morphine and heroin are also frequently prescribed. The UK has a smaller number of opiate users, per capita, than the United States, which many attribute to the availability of full opiate agonist prescriptions to users, which reduces the amount of opiates sold illicitly and, in turn, the number of users of other drugs who encounter and begin using the opiates. Therefore, buprenorphine holds no advantage in convenience over methadone for users in the UK, and elsewhere in the world where prescriptions of full opiate agonists to opiate users are not discouraged.
Buprenorphine may and is generally viewed to have a lower dependence-liability than methadone. In other words, withdrawal from buprenorphine is less difficult. Buprenorphine treatment can last anywhere from several days (for detoxification purposes) to several months (sometimes for only a few weeks or up to two or three years) or longer. While not the general goal, and often not intentional with buprenorphine, it can sometimes but rarely be used in an indefinite, often life-long regimen just as methadone can be. The choice of buprenorphine vs. methadone in the mentioned situation (by the patient) is usually due to the benefits of the less-restrictive outpatient treatment; prescriptions for take-home doses for up to a month vs. the heavy restrictions for take-home methadone doses and frequent visits to the clinic, as well as the stigma of going to a methadone clinic.
The usually less-severe withdrawal effects make it usually much easier to discontinue use as opposed to methadone, but no evidence thus far exists that sustaining abstinence post-buprenorphine maintenance is any more likely than post-methadone maintenance, or post-heroin withdrawal. On the other side, going from heroin/other potent opioids to buprenorphine is generally harder than going from the same to methadone. For patients making decisions about whether to use buprenorphine or methadone, avoiding withdrawal symptoms is very important -- the discomfort which is more likely to occur while switching to buprenorphine from illicit opiates may interfere with daily life, whereas withdrawal symptoms while getting on methadone are less likely, and more easily remedied by increasing dosage. Many doctors believe buprenorphine has a ceiling, and further increases in buprenorphine dose will not ease the discomfort of a person switching from illicit opiates to buprenorphine, whereas methadone has no ceiling, and even the heaviest users' withdrawal symptoms can be stopped by an appropriate methadone dose.
Buprenorphine, as a partial μ-opioid receptor agonist, has been claimed and is generally viewed to have a less euphoric effect compared to the full agonist methadone, and was therefore predicted less likely to be diverted to the black market (as reflected by its CIII status vs. methadone's more restrictive CII status), as well as that buprenorphine is generally accepted as unable to be abused (for euphoria) by those with a heroin or other potent opioid habit (however neither drug is supposed to have a euphoric effect when used long-term). However, in at least one study in which opiate users who were currently not using were given buprenorphine, several other opioids, and placebo intramusuclarly, subjects identified the drug they were injected with as heroin when it was actually buprenorphine.[16] This evidence tends to support the contentions of those who reject the notion that buprenorphine, when injected, is only marginally euphoric, or significantly less euphoric than other opiates. It should be noted that, in an effort to prevent injection of the drug, the Suboxone formulation includes naloxone in addition to the buprenorphine. When naloxone is injected, it precipitates opiate withdrawal and blocks the effects of any opiate, thus making "getting high" on Suboxone an impossibility. (The naloxone does not precipitate withdrawal or block the effect of the buprenorphine when taken sublingually.) However, the Subutex formulation does not include naloxone and may thus be injected by users to achieve the effect which was sufficiently heroin-like as to fool experienced users. Methadone, on the other hand, is given to patients at clinics in solution, with large amounts of water. This makes injection difficult without evaporating the liquid and taking other measures. Therefore, injection of buprenorphine as found in the preparations provided to opiate users simpler than injection of methadone, although data on the relative incidence is not currently available. Thus far in the United States buprenorphine is far less often found on the black market vs. methadone, but most street heroin addicts don't even know it exists, and its (legitimate) usage is far less than methadone (and according to many newspapers "underused"). It should also be noted that the vast majority of the black market methadone does not come from prescriptions to opiate users, but rather from prescriptions of methadone for pain. Since buprenorphine is used rarely for this purpose in the United States due to its comparatively poor efficacy in pain management, this is not surprising. In France where it is used more often than methadone there is more black market availability, although this and the apparent attraction is possibly due to a heroin dry-spell. Evidence indicates buprenorphine is often combined with benzodiazepines for more of an effect.
Blockade effect
The Suboxone preparation contains the μ-opioid receptor antagonist naloxone which is intended only to prevent abuse (i.e. injection) of the buprenorphine, not, as is commonly misunderstood, to block the effects of other opiates. Buprenorphine itself is mixed agonist/antagonist, and, as such, buprenorphine blocks the activity of other opiates and induces withdrawal in opiate dependent individuals who are currently physically dependent on another opiate. This is why users must wait until they are in withdrawal before beginning treatment with buprenorphine.
Buprenorphine itself binds more strongly to receptors in the brain than do other opioids, making it more difficult to become intoxicated via other opioids when buprenorphine is in the system, regardless of the presence of the naloxone. (Measurable, but small, amounts of naloxone can be absorbed and detected via the sublingual route, and while this is insignificant and has no subjective effect, there are anecdotal reports of hypersensitivity to naloxone in rare cases. These reports are not fully substantiated.) If enough buprenorphine is in the system, however, it has the same type of effect as naloxone, i.e. it completely or nearly completely blocks or reverses opiate effects from other opioids. 0.3 mg of buprenorphine parenterally is equivalent in antagonistic effect to between 0.4 and 2.0 mg of naloxone parenterally, but with a much longer half-life. Methadone also blocks the effects of other opioids, and at commonly used methadone maintenance doses, the degree of blockade is similar. Unlike buprenorphine, however, this is not due to any opiate antagonist-like action of methadone. Instead, daily use of methadone, like daily use of all opiate agonists, results in tolerance to all opiates, called "cross-tolerance". However, it is still possible to abuse other opioids on either treatment regime, although many people find "getting high" to be impossible. In the case of preganancy, buprenorphine causes milder neo-natal withdrawals than methadone.[17] (and is one of the few cases where Subutex is likely to be used over Suboxone in the United States.) Thus, buprenorphine is preferable during pregnancy, although methadone and the common opiate agonists are safe.
Switching to buprenorphine from methadone is often difficult and withdrawals lasting several days or more are often encountered mostly when the methadone dose is any higher than 30 mg/day (the suggested and usual dose for switching to buprenorphine). A 30 mg dose of methadone is relatively low, and some patients have difficulty reaching that dose, for a variety of reasons.[citation needed] Healthy users of methadone who commit to a slow taper, however, frequently find success in tapering to 30 mg in order to switch to buprenorphine, as well as in tapering off of methadone completely without the use of buprenorphine. Switching to buprenorphine at higher doses of methadone may be uncomfortable for the user. One reason is that users must be in withdrawal before switching to buprenorphine, and users of opiates with long half-lives, like methadone, may need to wait several days after their last dose of methadone before they are fully in withdrawal and ready to begin buprenorphine. User of heroin, hydrocodone, oxycodone, and morphine, as well as most other common opiates, only need to wait a maximum of 24 hours before they are fully in withdrawal and ready to begin buprenorphine. For this reason, some doctors switch methadone users to a shorter acting opiate, such as morphine, before allowing withdrawal to occur and beginning buprenorphine. Unfortunately, due to the unique qualities of both methadone and buprenorphine, switching to and using buprenorphine during pregnancy instead of methadone is unlikely to be helpful, since the strain of withdrawal on the body is far more dangerous for a fetus than the use of an opiate such as methadone. Also, data regarding buprenorphine's safety during pregnancy is less available than data on methadone during pregnancy -- data which has established the safety of methadone during pregnancy and the lack of lasting effects on children of mothers on methadone during preganancy. On the other hand, switching from buprenorphine to methadone is relatively easy as methadone is a full opiate agonist which does not have a ceiling, and can stop the withdrawal symptoms of users at any dosage of other opiates, including buprenorphine.
Inpatient rehabilitation
The practice of using buprenorphine (Subutex or Suboxone) in an inpatient rehabilitation setting is increasing rapidly[citation needed], whereas methadone-based detox is the standard. It is also being used in social model treatment settings. These rehabilitation programs consist of "detox" and "treatment" phases. The detoxification ("detox") phase consists of medically-supervised withdrawal from the drug of dependency on to buprenorphine, sometimes aided by the use of medications such as benzodiazepines like oxazepam or diazepam (modern milder tranquilizers that assist with anxiety, sleep, and muscle relaxation), clonidine (a blood-pressure medication that may reduce some opioid withdrawal symptoms), and anti-inflammatory/pain relief drugs such as ibuprofen. Switching to buprenorphine from a short-acting drug including Heroin, morphine, fentanyl, hydromorphone and hydrocodone (Vicodin), or oxycodone (Oxycontin and Percocet) is not too difficult for most people, and as long as the patient waited until they were in full withdrawals or longer before starting the buprenorphine medication, little further acute symptoms are an issue; The patient needs to be stabilized on a proper dose and monitored regardless. Switching from methadone is much more difficult, and with all cases if the patient takes buprenorphine prematurely (before full withdrawal symptoms) it can precipitate worse withdrawals than would have been had if the person had waited properly, and they can be long-lasting. The treatment phase begins once the patient is stabilized and receives medical clearance. This portion of treatment is comprised of multiple therapy sessions, which include both group and individual counseling with various chemical dependency counselors, psychologists, psychiatrists, social workers, and other professionals. Additionally, many treatment centers strongly base their treatment models on 12-step principles, such as those practiced by Alcoholics Anonymous and Narcotics Anonymous. Narcotics Anonymous and other 12-step programs do not have any organizational position on the use of any medically prescribed medications, it is each individual members personal decision to determine if their use of prescribed medications has compromised their sobriety or recovery. There are systems similar to or modeled after 12-step programs that accept its use and even acknowledge its power as a tool.
Patients who enter rehabilitation voluntarily (as opposed to those who are court-ordered) can often choose a facility with the option of only staying for detox. Alternatively they can enter treatment facilities that provide the option to complete both detox and longer-term treatment. Completing both increases the probability of success.. Abstinence alone has a very low efficacy in rehabilitating patients. In contrast, buprenorphine maintenance has a high efficacy [13] [12]. Most rehabilitation programs do not have or do not allow scientific studies to be conducted to contrast to abstinence alone and buprenorphine or methadone maintenance, including Narcotics Anonymous. NA's 12 traditions and overriding principle of anonymity would make such research potentially contentious and internally problematic. Whilst the maintenance / abstinence debate is a hot topic and strong arguments in support of both Narcotics Anonymous and buprenorphine maintenance have been made, individuals tend to gravitate the alternative that works best for them. Furthermore, the two approaches need not necessarily be mutually exclusive. Rehabilitation programs typically average about 30 days for primary care, but some may extend anywhere from 90 days to 6 months in an extended care unit. It is considered essential by the programs that administer them that patients in abstinence-based treatment form networks with other addiction survivors and engage in mutual-help groups, aftercare and other related activities after treatment in order to improve their chances of achieving long-term abstinence from opioids. Statistically, long-term abstinence is not widely prevalent.
Buprenorphine is sometimes used only during the detox protocol with the purpose of reducing the patient's use of mood-altering substances. It considerably reduces acute opioid withdrawal symptoms that are normally experienced by opioid-dependent patients on cessation of those opioids, including diarrhea, vomiting, fever, chills, cold sweats, muscle and bone aches, muscle cramps and spasms, restless legs, agitation, gooseflesh, insomnia, nausea, watery eyes, runny nose and post-nasal drip, nightmares, etc. The buprenorphine detox protocol usually lasts about 7-10 days, provided that the patient does not need to be detoxed from any additional addictive substances, as previously mentioned.
During this time, Suboxone or Subutex will be administered or the patient will be monitored taking the medication. Generally, the patient takes a single dose each day (a single dose may keep the patient comfortable for up to 48-72 hours, but medical professionals in many treatment facilities prescribe one or more than one dose every 24 hours to ensure that a consistent, active level of the medication remains in the patient's central nervous system, a key element of maintenance; also the level of dosage is usually around the previously described plateau, after which there is no noticeable increase in the effects of the drug. Typically, the first day dosage is no more than 8 mg or it may precipitate withdrawals as antagonistic effects overwhelm agonistic effects, after which initial daily dose totals around 8-16 mg (of either Suboxone or Subutex). The dosage is slowly tapered each day and the medication is usually stopped 36-48 hours prior to the end of the detox program, with the patient's vitals monitored up until discharge from the detox program.
During the detox period of any situation, despite the evidence that suggests that the naloxone in Suboxone has no clinically significant effect (except for anecdotal reports of hypersensitivity in (if proven) rare cases), Subutex is urged over Suboxone by the manufacturer and you are likely to receive it during the first few days.
References
- ↑ List of psychotropic Substances under international control
- ↑ NIDA study
- ↑ Transtec Summary of Product Characteristics
- ↑ Napp Pharmaceuticals
- ↑ http://coretext.org/show_detail.asp?recno=6481 Reckitt Benckiser Buprenorphine Bibliography
- ↑ 6.0 6.1 Huang P. et al. (2001): "Comparison of pharmacological activities of buprenorphine and norbuprenorphine: norbuprenorphine is a potent opioid agonist", J. Pharmacol. Exp. Ther. 297(2):688-95. PMID 11303059
- ↑ Bodkin JA. et al. (1995): "Buprenorphine treatment of refractory depression", Journal of Clinical Psychopharmacology 15:49-57. PMID 7714228
- ↑ Suboxone FAQ
- ↑ 9.0 9.1 Budd K, Raffa RB. (edts.) Buprenorphine- The unique opioid analgesic. Thieme 2005 (ISBN 3-13-1342211-0)
- ↑ Van Dorp E. et al. (2006) Naloxone reversal of buprenorphine- induced respiratory depression. Anesthesiology 105 (1): 51-57
- ↑ naabt.org
- ↑ 12.0 12.1 12.2 R. S. Schottenfeld et. al (1997) Department of Psychiatry, Yale University School of Medicine
- ↑ 13.0 13.1 13.2 Rolley Johnson et al., NEJM, 343(18):1290-1297, 2000
- ↑ Strain et al. (1998)
- ↑ Ling et al. (1998)
- ↑ http://opioids.com/buprenorphine/buprenreward.html
- ↑ G Fischer, P Etzersdorfer, H Eder, R Jagsch, M Langer, M Weninger (1998). Buprenorphine Maintenance in Pregnant Opioid Addicts. European Addiction Research;4 (suppl 1):32-36
Template:Drugs used in addictive disorders
cs:Buprenorfin da:Buprenorfin de:Buprenorphin hu:Buprenorfin no:Buprenorfin fi:Buprenorfiini sv:Buprenorfin th:บิวพรีนอร์ฟีน
- Pages with script errors
- Drugs with non-standard legal status
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Articles containing unverified chemical infoboxes
- All articles with unsourced statements
- Articles with unsourced statements from February 2007
- Articles with invalid date parameter in template
- Articles with unsourced statements from April 2007
- Drugs
- Substance abuse
- Abuse
- Anesthesiology