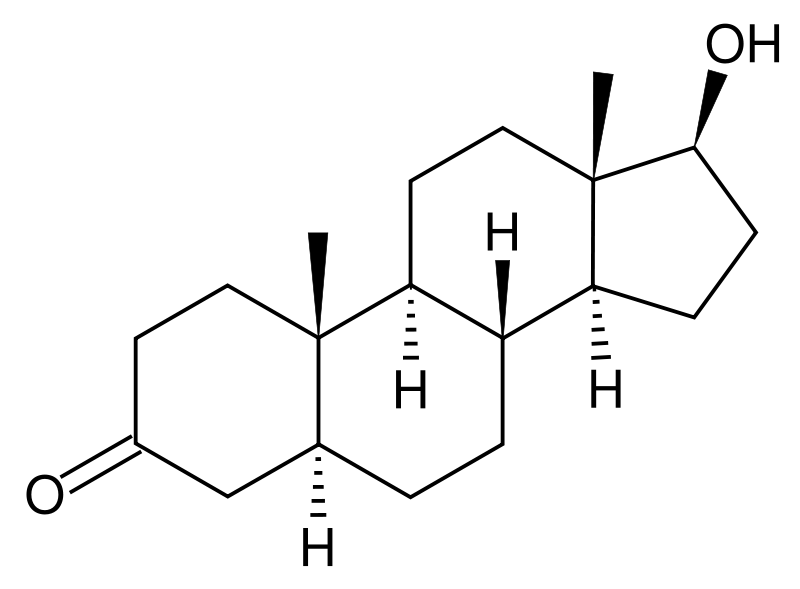
5α-Reductase
| 3-Oxo-5α-steroid 4-dehydrogenase | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Identifiers | |||||||||
| EC number | 1.3.99.5 | ||||||||
| CAS number | 9036-43-5 | ||||||||
| Databases | |||||||||
| IntEnz | IntEnz view | ||||||||
| BRENDA | BRENDA entry | ||||||||
| ExPASy | NiceZyme view | ||||||||
| KEGG | KEGG entry | ||||||||
| MetaCyc | metabolic pathway | ||||||||
| PRIAM | profile | ||||||||
| PDB structures | RCSB PDB PDBe PDBsum | ||||||||
| Gene Ontology | AmiGO / QuickGO | ||||||||
| |||||||||
| Steroid-5α-reductase, alpha polypeptide 1 | |
|---|---|
| Identifiers | |
| Symbol | SRD5A1 |
| Entrez | 6715 |
| HUGO | 11284 |
| OMIM | 184753 |
| RefSeq | NM_001047 |
| UniProt | P18405 |
| Other data | |
| EC number | 1.3.99.5 |
| Locus | Chr. 5 p15 |
| Steroid-5α-reductase, alpha polypeptide 2 | |
|---|---|
| Identifiers | |
| Symbol | SRD5A2 |
| Entrez | 6716 |
| HUGO | 11285 |
| OMIM | 607306 |
| RefSeq | NM_000348 |
| UniProt | P31213 |
| Other data | |
| EC number | 1.3.99.5 |
| Locus | Chr. 2 p23 |
5α-reductases, also known as 3-oxo-5α-steroid 4-dehydrogenases, are enzymes involved in steroid metabolism. They participate in 3 metabolic pathways: bile acid biosynthesis, androgen and estrogen metabolism. There are three isoenzymes of 5α-reductase, which vary in different tissues with age.
5α-Reductases catalyze the following chemical reaction:
- a 3-oxo-5α-steroid + acceptor ⇌ a 3-oxo-Δ4-steroid + reduced acceptor
Thus, the two substrates of these enzymes are a 3-oxo-5α-steroid and acceptor, whereas its two products are 3-oxo-Δ4-steroid and a reduced acceptor.
Production and activity
The enzyme is produced in many tissues in both males and females, in the reproductive tract, testes and ovaries,[1] skin, seminal vesicles, prostate, epididymis and many organs,[2] including the Nervous System.[3][4] There are three isoenzymes of 5α-reductase: steroid 5α-reductase 1, 2, and 3 (SRD5A1, SRD5A2 and SRD5A3).[2][5][6][7]
5α-Reductases act on 3-oxo (3-keto), Δ4,5 C19/C21 steroids as its substrates. “3-keto” refers to the double bond of the third carbon to oxygen. Carbons 4 and 5 also have a double bond, represented by 'Δ4,5'. The reaction involves a stereospecific and permanent break of the Δ4,5 with the help of NADPH as a cofactor. A hydride anion (H−) is also placed on the α face at the fifth carbon, and a proton on the β face at carbon 4.[8]
Distribution with age
This article needs attention from an expert on the subject. (September 2016) |
5α-R1 is expressed in fetal scalp and nongenital skin of the back, anywhere from 5 to 50 times less than in the adult. 5α-R2 is expressed in fetal prostates similar to adults. 5α-R1 is expressed mainly in the epithelium and 5α-R2 the stroma of the fetal prostate. Scientists looked for 5α-R2 expression in fetal liver, adrenal, testis, ovary, brain, scalp, chest, and genital skin, using immunoblotting, and were only able to find it in genital skin.[8]
After birth, the 5α-R1 is expressed in more locations, including the liver, skin, scalp and prostate. 5α-R2 is expressed in prostate, seminal vesicles, epididymis, liver, and to a lesser extent the scalp and skin. Hepatic expression of both 5α-R1 and 2 is immediate, but disappears in the skin and scalp at month 18. Then, at puberty, only 5α-R1 is reexpressed in the skin and scalp.
5α-R1 and 5α-R2 appear to be expressed in the prostate in male fetuses and throughout postnatal life. In adulthood, 5α-R1-3 is ubiquitously expressed. 5α-R1 and 5α-R2 are also expressed, although to different degrees in liver, genital and nongenital skin, prostate, epididymis, seminal vesicle, testis, ovary, uterus, kidney, exocrine pancreas, and the brain.[3][8]
Substrates
Specific substrates include testosterone, progesterone, androstenedione,[9] epitestosterone, cortisol, aldosterone, and deoxycorticosterone. Outside of dihydrotestosterone, much of the physiological role of 5α-reduced steroids is unknown.[8] Beyond reducing testosterone to dihydrotestosterone, 5alpha-reductase enzyme isoforms I and II reduce progesterone to dihydroprogesterone (DHP) and deoxycorticosterone to dihydrodeoxycorticosterone (DHDOC). In vitro and animal models suggest subsequent 3alpha-reduction of DHT, DHP and DHDOC lead to steroid metabolites with effects on cerebral function achieved by enhancing GABAergic inhibition. These neuroactive steroid derivatives enhance GABA via allosteric modulation at GABA(A) receptors and have anticonvulsant, antidepressant and anxiolytic effects, and also alter sexual and alcohol related behavior.[10] 5α-dihydrocortisol is present in the aqueous humor of the eye, is synthesized in the lens, and might help make the aqueous humor itself.[11] Allopregnanolone and THDOC are neurosteroids, with the latter having effects on the susceptibility of animals to seizures. In socially isolated mice, 5α-R1 is specifically down-regulated in glutamatergic pyramidal neurons that converge on the amygdala from cortical and hippocampal regions. This down-regulation may account for the appearance of behavioral disorders such as anxiety, aggression, and cognitive dysfunction.[3][4] 5α-dihydroaldosterone is a potent antinatriuretic agent, although different from aldosterone. Its formation in the kidney is enhanced by restriction of dietary salt, suggesting it may help retain sodium as follows:[12]
- <ce>{Substrate} + {NADPH} + H+ -> {5\alpha-substrate} + NADP+</ce>
5α-DHP is a major hormone in circulation of normal cycling and pregnant women.[13]
Testosterone
5α-Reductase is most known for converting testosterone, the male sex hormone, into the more potent dihydrotestosterone:
The major difference is the Δ4,5 double-bond on the A (leftmost) ring. The other differences between the diagrams are unrelated to structure.
List of conversions
The following reactions are known to be catalyzed by 5α-reductase:[9]
- Cholestenone → 5α-Cholestanone
- Progesterone → 5α-Dihydroprogesterone
- 3α-Dihydroprogesterone → Allopregnanolone
- 3β-Dihydroprogesterone → Isopregnanolone
- Deoxycorticosterone → 5α-Dihydrodeoxycorticosterone
- Corticosterone → 5α-Dihydrocorticosterone
- Cortisol → 5α-Dihydrocortisol
- Aldosterone → 5α-Dihydroaldosterone
- Androstenedione → 5α-Androstanedione
- Testosterone → 5α-Dihydrotestosterone
Inhibition
The mechanism of 5α reductase inhibition is complex, but involves the binding of NADPH to the enzyme followed by the substrate. 5α-reductase inhibitor drugs are used in benign prostatic hyperplasia, prostate cancer, pattern hair loss (androgenetic alopecia), and hormone replacement therapy for transgender women.
Inhibition of the enzyme can be classified into two categories: steroidal, which are irreversible, and nonsteroidal. There are more steroidal inhibitors, with examples including finasteride (MK-906), dutasteride (GG745), 4-MA, turosteride, MK-386, MK-434, and MK-963. Researchers have pursued synthesis of nonsteroidals to inhibit 5α-reductase due to the undesired side effects of steroidals. The most potent and selective inhibitors of 5α-R1 are found in this class, and include benzoquinolones, nonsteroidal aryl acids, butanoid acid derivatives, and more recognizably, polyunsaturated fatty acids (especially linolenic acid), zinc, and green tea.[8] Riboflavin was also identified as a 5α-reductase inhibitor .[14]
Additionally, it has been claimed that alfatradiol works through this mechanism of activity (5α-reductase), as well as the Ganoderic acids in Reishi, and the Saw Palmetto.
Inhibition of 5α-reductase results in decreased conversion of testosterone to DHT, leading to increased testosterone and estradiol. Other enzymes compensate to a degree for the absent conversion, specifically with local expression at the skin of reductive 17β-hydroxysteroid dehydrogenase, oxidative 3α-hydroxysteroid dehydrogenase, and 3β-hydroxysteroid dehydrogenase enzymes.[15]
Gynecomastia, erectile dysfunction, impaired cognitive function, fatigue, hypoglycemia, impaired liver function, constipation, and depression, are only a few of the possible side-effects of 5α-reductase inhibition. Long term side effects, that continued even after discontinuation of the drug have been reported.[16]
Finasteride
Finasteride inhibits two 5α-reductase isoenzymes (II and III), while dutasteride inhibits all three.[2] Finasteride potently inhibits 5α-R2 at a mean inhibitory concentration IC50 of 69 nM, but is less effective with 5α-R1 till an IC50 of 360 nM.[17] Finasteride decreases mean serum level of DHT by 71% after 6 months,[18] and was shown in vitro to inhibit 5α-R3 at a similar potency to 5α-R2 in transfected cell lines.[2]
Dutasteride
Dutasteride inhibits 5α-reductase isoenzymes type 1 and 2 better than finasteride, leading to a more complete reduction in DHT at 24 weeks (94.7% versus 70.8%).[19] It also reduces intraprostatic DHT 97% in men with prostate cancer at 5 mg/day over three months.[20] A second study with 3.5 mg/day for 4 months decreased intraprostatic DHT even further by 99%.[21] The suppression of DHT in vivo, and the report that dutasteride inhibits 5α-R3 in vitro[22] suggest that dutasteride may be a triple 5α reductase inhibitor.[8]
Congenital deficiencies
5α-Reductase 1
5α-Reductase type 1 inactivated male mice have reduced bone mass and forelimb muscle grip strength, which has been proposed to be due to lack of 5α-reductase type 1 expression in bone and muscle.[23] In 5 alpha reductase type 2 deficient males, the type 1 isoenzyme is thought to be responsible for their virilization at puberty.[6]
5α-Reductase 2
The second isoenzyme of 5α reductase is deficient in the classic intersex condition (pseudovaginal perineoscrotal hypospadias), or 5α-reductase deficiency. It was first discovered in indigenous cultures of Papua, New Guinea, where children were born with feminine genitalia in the absence of endogenous DHT during pregnancy, but with the surge of testosterone during adolescence, changed to males at puberty. Because of this change at puberty, the condition is also sometimes called "guevedoche."[24] There is a range of external appearance that has been described of external genitalia at birth, with varying degrees of virilization.
5α-Reductase 3
When small interfering RNA is used to knock down the expression of 5α-R3 isozyme in cell lines, there is decreased cell growth, viability, and a decrease in DHT/T ratios.[25] It has also shown the ability to reduce testosterone, androstenedione, and progesterone in androgen stimulated prostate cell lines by adenovirus vectors.[8]
Congenital deficiency of 5α-R3 at the gene SRD53A has been linked to a rare, autosomal recessive condition in which patients are born with severe intellectual dysfunction and cerebellar and ocular defects. The presumed deficiency is reduction of the terminal bond of polyprenol to dolichol, an important step in N-glycosylation of proteins, which in turn is important for proper folding of asparagine residues on nascent protein in the endoplasmic reticulum.[26]
Nomenclature
This enzyme belongs to the family of oxidoreductases, to be specific, those acting on the CH-CH group of donor with other acceptors. The systematic name of this enzyme class is 3-oxo-5α-steroid:acceptor Δ4-oxidoreductase. Other names in common use include:
- 5α-Reductase
- 3-Oxosteroid Δ4-dehydrogenase
- 3-Oxo-5α-steroid Δ4-dehydrogenase
- Steroid Δ4-5α-reductase
- Δ4-3-Keto steroid 5α-reductase
- Δ4-3-Oxo steroid reductase
- Δ4-3-Ketosteroid-5α-oxidoreductase
- Δ4-3-Oxosteroid-5α-reductase
- 3-Keto-Δ4-steroid-5α-reductase
- Testosterone 5α-reductase
- 4-Ene-3-ketosteroid-5α-oxidoreductase
- Δ4-5α-Dehydrogenase
- 3-Oxo-5α-steroid:(acceptor) Δ4-oxidoreductase
See also
- Steroidogenic enzyme
- Acne vulgaris
- Cholestenone 5α-reductase
- Hirsutism
- Lower urinary tract symptoms
- Polycystic ovarian syndrome
- List of steroid metabolism modulators
References
- ↑ Pinna G, Agis-Balboa RC, Pibiri F, Nelson M, Guidotti A, Costa E (October 2008). "Neurosteroid biosynthesis regulates sexually dimorphic fear and aggressive behavior in mice". Neurochem. Res. 33 (10): 1990–2007. doi:10.1007/s11064-008-9718-5. PMID 18473173.
- ↑ 2.0 2.1 2.2 2.3 Yamana K, Labrie F, Luu-The V (January 2010). "Human type 3 5α-reductase is expressed in peripheral tissues at higher levels than types 1 and 2 and its activity is potently inhibited by finasteride and dutasteride". Hormone Molecular Biology and Clinical Investigation. 2 (3). doi:10.1515/hmbci.2010.035.
- ↑ 3.0 3.1 3.2 Agís-Balboa RC, Pinna G, Zhubi A, Maloku E, Veldic M, Costa E, Guidotti A (September 2006). "Characterization of brain neurons that express enzymes mediating neurosteroid biosynthesis". Proc Natl Acad Sci U S A. 103 (39): 14602–7. doi:10.1073/pnas.0606544103. PMC 1600006. PMID 16984997.
- ↑ 4.0 4.1 Agís-Balboa RC, Pinna G, Pibiri F, Kadriu B, Costa E, Guidotti A (November 2007). "Down-regulation of neurosteroid biosynthesis in corticolimbic circuits mediates social isolation-induced behavior in mice". Proc Natl Acad Sci U S A. 104 (47): 18736–41. doi:10.1073/pnas.0709419104. PMC 2141846. PMID 18003893.
- ↑ Killian J, Pratis K, Clifton RJ, Stanton PG, Robertson DM, O'Donnell L (May 2003). "5alpha-reductase isoenzymes 1 and 2 in the rat testis during postnatal development". Biol. Reprod. 68 (5): 1711–8. doi:10.1095/biolreprod.102.009142. PMID 12606426.
- ↑ 6.0 6.1 Thiele S, Hoppe U, Holterhus PM, Hiort O (June 2005). "Isoenzyme type 1 of 5alpha-reductase is abundantly transcribed in normal human genital skin fibroblasts and may play an important role in masculinization of 5alpha-reductase type 2 deficient males". Eur. J. Endocrinol. 152 (6): 875–80. doi:10.1530/eje.1.01927. PMID 15941927.
- ↑ Godoy A, Kawinski E, Li Y, Oka D, Alexiev B, Azzouni F, Titus MA, Mohler JL (July 2011). "5α-reductase type 3 expression in human benign and malignant tissues: a comparative analysis during prostate cancer progression". Prostate. 71 (10): 1033–46. doi:10.1002/pros.21318. PMID 21557268.
- ↑ 8.0 8.1 8.2 8.3 8.4 8.5 8.6 Azzouni F, Godoy A, Li Y, Mohler J (2012). "The 5 alpha-reductase isozyme family: a review of basic biology and their role in human diseases". Adv Urol. 2012: 530121. doi:10.1155/2012/530121. PMC 3253436. PMID 22235201.
- ↑ 9.0 9.1 Paba S, Frau R, Godar SC, Devoto P, Marrosu F, Bortolato M (2011). "Steroid 5α-reductase as a novel therapeutic target for schizophrenia and other neuropsychiatric disorders". Curr. Pharm. Des. 17 (2): 151–67. doi:10.2174/138161211795049589. PMID 21361868.
- ↑ Finn, D. A.; Beadles-Bohling, A. S.; Beckley, E. H.; Ford, M. M.; Gililland, K. R.; Gorin-Meyer, R. E.; Wiren, K. M. (2006). "A New Look at the 5?-Reductase Inhibitor Finasteride". CNS Drug Reviews. 12 (1): 53–76. doi:10.1111/j.1527-3458.2006.00053.x. PMID 16834758.
- ↑ Weinstein BI, Kandalaft N, Ritch R, Camras CB, Morris DJ, Latif SA, Vecsei P, Vittek J, Gordon GG, Southren AL (June 1991). "5 alpha-dihydrocortisol in human aqueous humor and metabolism of cortisol by human lenses in vitro". Invest. Ophthalmol. Vis. Sci. 32 (7): 2130–5. PMID 2055703.
- ↑ Kenyon, CJ; Brem, AS; McDermott, MJ; Deconti, GA; Latif, SA; Morris, DJ (1 May 1983). "Antinatriuretic and kaliuretic activities of the reduced derivatives of aldosterone". Endocrinology. 112 (5): 1852–6. doi:10.1210/endo-112-5-1852. PMID 6403339.
- ↑ Milewich L, Gomez-Sanchez C, Crowley G, Porter JC, Madden JD, MacDonald PC (October 1977). "Progesterone and 5alpha-pregnane-3,20-dione in peripheral blood of normal young women: Daily measurements throughout the menstrual cycle". J. Clin. Endocrinol. Metab. 45 (4): 617–22. doi:10.1210/jcem-45-4-617. PMID 914969.
- ↑ Nakayama O, Yagi M, Kiyoto S, Okuhara M, Kohsaka M (December 1990). "Riboflavin, a testosterone 5 alpha-reductase inhibitor". J. Antibiot. 43 (12): 1615–6. doi:10.7164/antibiotics.43.1615. PMID 2276981.
- ↑ Andersson S (2001). "Steroidogenic enzymes in skin". Eur J Dermatol. 11 (4): 293–5. PMID 11399532.
- ↑ Irwig MS, Kolukula S (June 2011). "Persistent sexual side effects of finasteride for male pattern hair loss". J Sex Med. 8 (6): 1747–53. doi:10.1111/j.1743-6109.2011.02255.x. PMID 21418145.
- ↑ Tian G, Stuart JD, Moss ML, Domanico PL, Bramson HN, Patel IR, Kadwell SH, Overton LK, Kost TA, Mook RA (March 1994). "17 beta-(N-tert-butylcarbamoyl)-4-aza-5 alpha-androstan-1-en-3-one is an active site-directed slow time-dependent inhibitor of human steroid 5 alpha-reductase 1". Biochemistry. 33 (8): 2291–6. doi:10.1021/bi00174a041. PMID 8117686.
- ↑ McConnell JD, Wilson JD, George FW, Geller J, Pappas F, Stoner E (March 1992). "Finasteride, an inhibitor of 5 alpha-reductase, suppresses prostatic dihydrotestosterone in men with benign prostatic hyperplasia". J. Clin. Endocrinol. Metab. 74 (3): 505–8. doi:10.1210/jc.74.3.505. PMID 1371291.
- ↑ Clark RV, Hermann DJ, Cunningham GR, Wilson TH, Morrill BB, Hobbs S (May 2004). "Marked suppression of dihydrotestosterone in men with benign prostatic hyperplasia by dutasteride, a dual 5alpha-reductase inhibitor". J. Clin. Endocrinol. Metab. 89 (5): 2179–84. doi:10.1210/jc.2003-030330. PMID 15126539.
- ↑ Andriole GL, Humphrey P, Ray P, Gleave ME, Trachtenberg J, Thomas LN, Lazier CB, Rittmaster RS (September 2004). "Effect of the dual 5alpha-reductase inhibitor dutasteride on markers of tumor regression in prostate cancer". J. Urol. 172 (3): 915–9. doi:10.1097/01.ju.0000136430.37245.b9. PMID 15310997.
- ↑ Gleave M, Qian J, Andreou C, Pommerville P, Chin J, Casey R, Steinhoff G, Fleshner N, Bostwick D, Thomas L, Rittmaster R (November 2006). "The effects of the dual 5alpha-reductase inhibitor dutasteride on localized prostate cancer--results from a 4-month pre-radical prostatectomy study". Prostate. 66 (15): 1674–85. doi:10.1002/pros.20499. PMID 16927304.
- ↑ Moss GP (December 1989). "IUPAC-IUB Joint Commission on Biochemical Nomenclature (JCBN). The nomenclature of steroids. Recommendations 1989". Eur. J. Biochem. 186 (3): 429–58. doi:10.1111/j.1432-1033.1989.tb15228.x. PMID 2606099.
- ↑ Windahl SH, Andersson N, Börjesson AE, Swanson C, Svensson J, Movérare-Skrtic S, Sjögren K, Shao R, Lagerquist MK, Ohlsson C (2011). Vanacker J, ed. "Reduced bone mass and muscle strength in male 5α-reductase type 1 inactivated mice". PLoS ONE. 6 (6): e21402. doi:10.1371/journal.pone.0021402. PMC 3120862. PMID 21731732.
- ↑ Flam, Faye (2008). The Score: How the Quest for Sex Has Shaped the Modern Man. Avery. p. 88.
- ↑ Uemura M, Tamura K, Chung S, Honma S, Okuyama A, Nakamura Y, Nakagawa H (January 2008). "Novel 5 alpha-steroid reductase (SRD5A3, type-3) is overexpressed in hormone-refractory prostate cancer". Cancer Sci. 99 (1): 81–6. doi:10.1111/j.1349-7006.2007.00656.x. PMID 17986282.
- ↑ Cantagrel V, Lefeber DJ, Ng BG, Guan Z, Silhavy JL, Bielas SL, Lehle L, Hombauer H, Adamowicz M, Swiezewska E, De Brouwer AP, Blümel P, Sykut-Cegielska J, Houliston S, Swistun D, Ali BR, Dobyns WB, Babovic-Vuksanovic D, van Bokhoven H, Wevers RA, Raetz CR, Freeze HH, Morava E, Al-Gazali L, Gleeson JG (July 2010). "SRD5A3 is required for converting polyprenol to dolichol and is mutated in a congenital glycosylation disorder". Cell. 142 (2): 203–17. doi:10.1016/j.cell.2010.06.001. PMC 2940322. PMID 20637498.
Further reading
External links
- Testosterone+5-alpha-Reductase at the US National Library of Medicine Medical Subject Headings (MeSH)
- Pages with broken file links
- Protein pages needing a picture
- Genes on human chromosome 5
- Genes on human chromosome 2
- Articles needing expert attention with no reason or talk parameter
- Articles needing unspecified expert attention
- Articles needing expert attention from September 2016
- Articles with invalid date parameter in template
- All articles needing expert attention
- Portal templates with all redlinked portals
- EC 1.3.99