COVID-19-associated acute kidney injury: Difference between revisions
mNo edit summary |
No edit summary |
||
| Line 51: | Line 51: | ||
** [[Angiotensin-converting enzyme 2]] ([[ACE2]]), which is a primary [[receptor]] for [[SARS-CoV-2]] entry into cells, mostly presents in [[Kidneys]] as well as [[lungs]] and [[heart]].<ref name="MalhaMueller2020">{{cite journal|last1=Malha|first1=Line|last2=Mueller|first2=Franco B.|last3=Pecker|first3=Mark S.|last4=Mann|first4=Samuel J.|last5=August|first5=Phyllis|last6=Feig|first6=Peter U.|title=COVID-19 and the Renin-Angiotensin System|journal=Kidney International Reports|volume=5|issue=5|year=2020|pages=563–565|issn=24680249|doi=10.1016/j.ekir.2020.03.024}}</ref> | ** [[Angiotensin-converting enzyme 2]] ([[ACE2]]), which is a primary [[receptor]] for [[SARS-CoV-2]] entry into cells, mostly presents in [[Kidneys]] as well as [[lungs]] and [[heart]].<ref name="MalhaMueller2020">{{cite journal|last1=Malha|first1=Line|last2=Mueller|first2=Franco B.|last3=Pecker|first3=Mark S.|last4=Mann|first4=Samuel J.|last5=August|first5=Phyllis|last6=Feig|first6=Peter U.|title=COVID-19 and the Renin-Angiotensin System|journal=Kidney International Reports|volume=5|issue=5|year=2020|pages=563–565|issn=24680249|doi=10.1016/j.ekir.2020.03.024}}</ref> | ||
*** High expression of [[ACE2]] was found in the [[renal]] proximal [[tubular]] cells and rarely in [[podocytes]]. <ref name="pmid17021266">{{cite journal| author=Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D| title=Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. | journal=J Am Soc Nephrol | year= 2006 | volume= 17 | issue= 11 | pages= 3067-75 | pmid=17021266 | doi=10.1681/ASN.2006050423 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17021266 }} </ref> <ref name="pmid32203970">{{cite journal| author=Perico L, Benigni A, Remuzzi G| title=Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. | journal=Nephron | year= 2020 | volume= 144 | issue= 5 | pages= 213-221 | pmid=32203970 | doi=10.1159/000507305 | pmc=7179544 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32203970 }} </ref> | *** High expression of [[ACE2]] was found in the [[renal]] proximal [[tubular]] cells and rarely in [[podocytes]]. <ref name="pmid17021266">{{cite journal| author=Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D| title=Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes. | journal=J Am Soc Nephrol | year= 2006 | volume= 17 | issue= 11 | pages= 3067-75 | pmid=17021266 | doi=10.1681/ASN.2006050423 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17021266 }} </ref> <ref name="pmid32203970">{{cite journal| author=Perico L, Benigni A, Remuzzi G| title=Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade. | journal=Nephron | year= 2020 | volume= 144 | issue= 5 | pages= 213-221 | pmid=32203970 | doi=10.1159/000507305 | pmc=7179544 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=32203970 }} </ref> | ||
== Differentiating COVID-19-associated acute kidney injury from other Diseases == | |||
* [[COVID-19]]-associated [[AKI]] must be differentiated from other diseases that cause [[AKI]], such as [[congestive heart failure]], [[hemorrhage]], [[dehydration]], [[liver failure]], [[urinary tract obstruction]], [[Interstitial nephritis]], [[glomerulonephritis]] and [[nephrotoxic]] [[medications]]. | |||
To review the differential diagnosis of [[AKI]], click [[Acute kidney injury differential diagnosis#Acute kidney injury differential diagnosis|here]]. | |||
== Epidemiology and Demographics == | == Epidemiology and Demographics == | ||
Revision as of 05:19, 16 July 2020
|
COVID-19 Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
COVID-19-associated acute kidney injury On the Web |
|
American Roentgen Ray Society Images of COVID-19-associated acute kidney injury |
|
Risk calculators and risk factors for COVID-19-associated acute kidney injury |
For COVID-19 frequently asked inpatient questions, click here
For COVID-19 frequently asked outpatient questions, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Nasrin Nikravangolsefid, MD-MPH [2],Sogand Goudarzi, MD [3]
Synonyms and keywords: COVID-19-associated AKI
Overview
COVID-19 can involve many organs leading to organ failure, one of which is kidneys that manifest with mild proteinuria to advanced acute kidney injury (AKI).
Historical Perspective
- Early reports from China revealed that COVID-19 rarely involves the kidneys, as acute renal failure was not seen among COVID-19 hospitalized patients and mild BUN or creatinine rise [10.8%] and mild proteinuria [7.2%] occurred. [1]
- However, recent study found 75.4% of hospitalized patients with COVID-19 pneumonia developed hematuria, proteinuria, and AKI. But, these findings are not significantly different from other critical diseases.[2]
Classification
Pathophysiology
- Angiotensin-converting enzyme 2 (ACE2), which is a primary receptor for SARS-CoV-2 entry into cells, mostly presents in renal tubular epithelial cells as well as lungs and heart.[4]
- Despite kidney injury following COVID-19 infection is less frequent than severe lung injury, ACE2: ACE ratio is higher in the kidneys compared to the respiratory system. (1:1 in the kidneys VS 1:20 in the respiratory system)[4]
- After SARS-CoV-2 enters through the nasal cavity, it may travel to the kidneys and enters the bloodstream leading to severe inflammatory response activation and cytokine storm.
- Cytokine induced AKI may occur due to intrarenal inflammation, hyperpermeability of vessels, hypovolemia and cardiomyopathy, leading to cardiorenal syndrome type 1 that is characterized by third space volume overload such as pleural effusion, edema and intravascular volume loss (hypovolemia) and hypotension.[5]
- cardiomyopathy and COVID-19-associated myocarditis can lead to hypotension and reduction in renal perfusion.
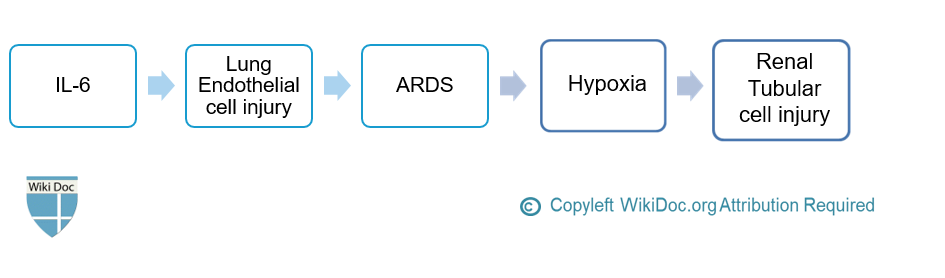
- The major cytokine is IL-6, which induces inflammation and lung endothelial cell injury, leading to ARDS and hypoxia that subsequently cause renal tubular cell injury and AKI. [6][5]
- Cytokine induced AKI may occur due to intrarenal inflammation, hyperpermeability of vessels, hypovolemia and cardiomyopathy, leading to cardiorenal syndrome type 1 that is characterized by third space volume overload such as pleural effusion, edema and intravascular volume loss (hypovolemia) and hypotension.[5]
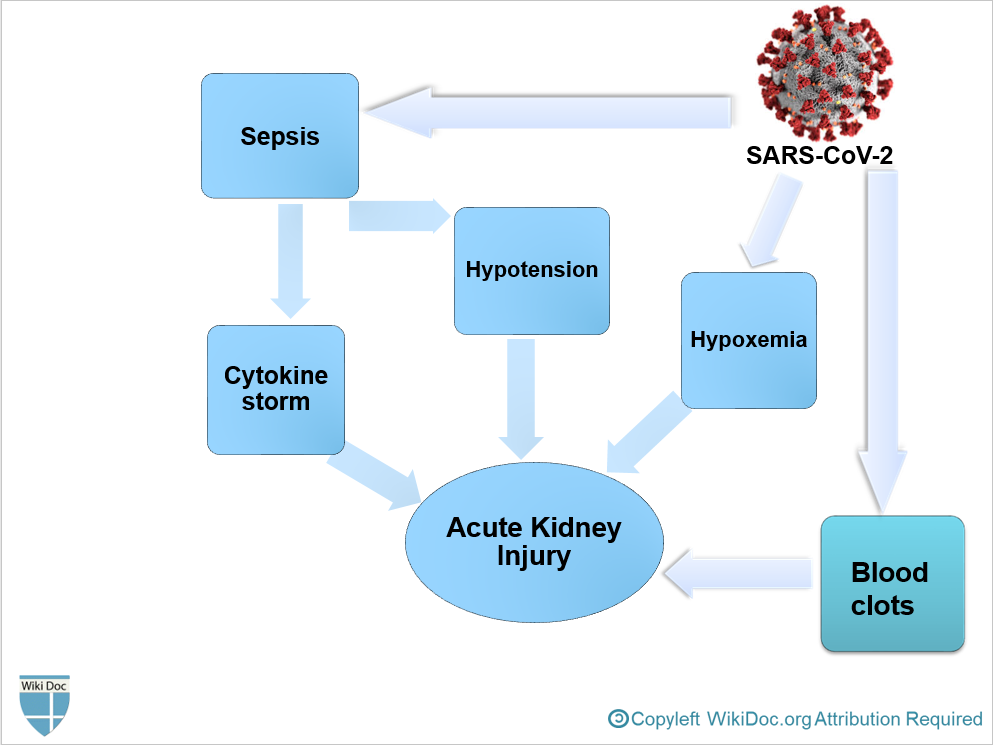
- To conclude, COVID-19-associated AKI can occur as a result of[4]:
- Sepsis and cytokine storm
- Hypovolemia and Hypotension
- Hypoxemia
- Blood clots formation due to hypercoagulable state, leading to impaired blood flow in the renal arterioles.
Causes
- SARS-CoV-2 may have a Kidney tropism. As a recent study found SARS-CoV-2 antigens in renal tubules which suggests the direct damage of SARS-CoV-2 on the kidneys. https://www.medrxiv.org/content/10.1101/2020.03.04.20031120v4. Missing or empty
|title=(help)
Differentiating COVID-19-associated acute kidney injury from other Diseases
- COVID-19-associated AKI must be differentiated from other diseases that cause AKI, such as congestive heart failure, hemorrhage, dehydration, liver failure, urinary tract obstruction, Interstitial nephritis, glomerulonephritis and nephrotoxic medications.
To review the differential diagnosis of AKI, click here.
Epidemiology and Demographics
- AKI is frequently seen among patients with COVID-19 hospitalized in ICU, with prevalence of 0.6-29% in China "Acute Kidney Injury in COVID-19 Patients | COVID-19". and 22.2% in the USA.[9]
- Approximately 43% of critically ill patients with COVID-19 developed AKI during the admission period. [3]
- In a cohort study on 99 patients with severe COVID-19, AKI was reported in 42 patients (42.9%) and among them 32 (74.4%) patients had severe AKI (stage III based on KDIGO definition). [10]
- The actual incidence of AKI in critcally ill patients with COVID-19 is uncertain but estimated between 27-85%. "Acute Kidney Injury in COVID-19 Patients | COVID-19".
- Differences of AKI prevalence among studies could be related to several factors, including[10]:
- Patient characteristics
- Comorbidities
- smoking history
- Medications history
- Race
- Severity of COVID-19
- Differences of COVID-19 Management among countries
- Patient characteristics
Age
Gender
- Men are more likely to be affected and have higher risk of COVID-19 complications. [11]
- 57.1% of AKI cases following COVID-19 were male.[3]
Race
Risk Factors
- The most potent risk factors in the development of COVID-19 associated AKI include[12] [13]:
- Elderly
- Age>60 years
- Comorbidities
- Elderly
Screening
Natural History, Complications, and Prognosis
Natural History
- AKI is more likely to develop in the late stages of COVID-19 in critically ill patients.[14]
- Severe COVID-19 pneumonia and severe acute respiratory distress syndrome are associated with developing AKI.[2]
Diagnosis
Diagnostic Study of Choice
- The diagnosis of AKI is based on the KDIGO criteria, which includes[15]:
- Elevated serum Creatinine by ≥0.3 mg/dl (≥26.5 μmol/l) within 48 hours; or
- Elevated serum Creatinine to ≥1.5 times baseline within the previous 7 days; or
- Urine volume < 0.5 ml/kg/h for >6 hours
Symptoms
- COVID-19-associated AKI commonly occurs between 5 to 9 days after hospitalization. [10]
- Patients in the early stages of kidney failure may be asymptomatic.
- If left untreated, patients may progress to develop Azotemia and Uremia, which occur due to the buildup of waste materials in the blood.
- Symptoms of kidney injury include [16]:
Physical Examination
- Physical examination of patients with AKI is usually remarkable for:
- Signs of dehydration, such as tachycardia, tachypnea, hypotension, and dry mucosa
- Fluid retention, leading to edema and swelling of periorbital and extremities
- Confusion due to severe dehydration and electrolyte imbalances
- Decrease in urine output:Oliguria or Anuria
- cardiac arrhythmia due to electrolyte imbalances such as high level of Potassium
Laboratory Findings
- Laboratory findings of COVID-19-associated AKI include:
- Elevated serum creatinine
- Elevated BUN level
- Plasma BUN-creatinine ratio> 20 in prerenal AKI
- Plasma BUN-creatinine ratio< 15 in intrinsic AKI or acute tubular necrosis
- Fractional excretion of sodium (FENa)
- Urinary sediment
- Hyaline casts in prerenal AKI
- Granular or Muddy brown casts in intrinsic AKI or acute tubular necrosis
- Several biomarkers have been found to diagnose and predict AKI that include[17] [18] [19]:
Electrocardiogram
- There are no specific ECG findings associated with AKI. However, electrolyte disturbances such as hyperkalemia might lead to various ECG abnormalities.
X-ray
- There are no x-ray specific findings associated with AKI. However, X-ray may be used in the diagnosis of AKI associated volume overload.
Echocardiography or Ultrasound
- Ultrasound may be helpful in the diagnosis of AKI. Findings on ultrasound suggestive of AKI include hydrenephrosis and reduced renal blood flow.
CT scan
MRI
Other Imaging Findings
- 99m Technetium (Tc) scan may be helpful in the diagnosis of AKI, which shows reduced renal blood flow and tubular function.
Other Diagnostic Studies
- There are no other diagnostic studies associated with AKI.
Treatment
Medical Therapy
- Management of AKI following COVID-19 includes antiviral therapies, identifying electrolyte disorders, and intravenous fluid resuscitation.
- Treatment of AKI following COVID-19 includes:[14] [13]
- Correction of hypovolemia and hypotension by the administration of adequate intravenous fluid
- Isotonic crystalloid is recommended among all patients who develop AKI. [15]
- Correction of electrolyte disorders
- antiviral therapy:
- Recently, Remdesivir has been found effective against COVID-19. [20]
- Anticoagulants in hypercoagulable conditions
- Loop diuretics
- In volume overload conditions
- Diuretics should not be used regularly as they predispose patients to volume depletion.
- Correction of hypovolemia and hypotension by the administration of adequate intravenous fluid
Interventions
- renal replacement therapy
- If AKI is unresponsive to conservative therapy
- In volume overload conditions
- Modality of choice in unstable hemodynamic status and ESRD, severe metabolic acidosis, severe hyperkalemia
- renal replacement therapy is associated with hypercoagulation.[13]
- Sequential extracorporeal therapy
- It removes cytokines, which reduces systemic inflammation and subsequent organ failure.
Prevention
- Patients with COVID-19 should be evaluated for intravascular volume status based on physical examination and fluid balance.[13]
- Serial monitoring of BUN, serum creatinine, and electrolytes such as sodium, potassium and bicarbonate should be considered frequently every 48 hours or more in high risk patients.[13]
- Isotonic saline is recommended as a prevention strategy for patients who are at increased risk for AKI by expanding intravascular volume. [15]
References
- ↑ Wang, Luwen; Li, Xun; Chen, Hui; Yan, Shaonan; Li, Dong; Li, Yan; Gong, Zuojiong (2020). "Coronavirus Disease 19 Infection Does Not Result in Acute Kidney Injury: An Analysis of 116 Hospitalized Patients from Wuhan, China". American Journal of Nephrology. 51 (5): 343–348. doi:10.1159/000507471. ISSN 0250-8095.
- ↑ 2.0 2.1 2.2 Pei, Guangchang; Zhang, Zhiguo; Peng, Jing; Liu, Liu; Zhang, Chunxiu; Yu, Chong; Ma, Zufu; Huang, Yi; Liu, Wei; Yao, Ying; Zeng, Rui; Xu, Gang (2020). "Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia". Journal of the American Society of Nephrology. 31 (6): 1157–1165. doi:10.1681/ASN.2020030276. ISSN 1046-6673.
- ↑ 3.0 3.1 3.2 3.3 Pei G, Zhang Z, Peng J, Liu L, Zhang C, Yu C; et al. (2020). "Renal Involvement and Early Prognosis in Patients with COVID-19 Pneumonia". J Am Soc Nephrol. 31 (6): 1157–1165. doi:10.1681/ASN.2020030276. PMC 7269350 Check
|pmc=value (help). PMID 32345702 Check|pmid=value (help). - ↑ 4.0 4.1 4.2 4.3 Malha, Line; Mueller, Franco B.; Pecker, Mark S.; Mann, Samuel J.; August, Phyllis; Feig, Peter U. (2020). "COVID-19 and the Renin-Angiotensin System". Kidney International Reports. 5 (5): 563–565. doi:10.1016/j.ekir.2020.03.024. ISSN 2468-0249.
- ↑ 5.0 5.1 5.2 Ronco C, Reis T (2020). "Kidney involvement in COVID-19 and rationale for extracorporeal therapies". Nat Rev Nephrol. 16 (6): 308–310. doi:10.1038/s41581-020-0284-7. PMC 7144544 Check
|pmc=value (help). PMID 32273593 Check|pmid=value (help). - ↑ Husain-Syed F, Slutsky AS, Ronco C (2016). "Lung-Kidney Cross-Talk in the Critically Ill Patient". Am J Respir Crit Care Med. 194 (4): 402–14. doi:10.1164/rccm.201602-0420CP. PMID 27337068.
- ↑ Ye M, Wysocki J, William J, Soler MJ, Cokic I, Batlle D (2006). "Glomerular localization and expression of Angiotensin-converting enzyme 2 and Angiotensin-converting enzyme: implications for albuminuria in diabetes". J Am Soc Nephrol. 17 (11): 3067–75. doi:10.1681/ASN.2006050423. PMID 17021266.
- ↑ Perico L, Benigni A, Remuzzi G (2020). "Should COVID-19 Concern Nephrologists? Why and to What Extent? The Emerging Impasse of Angiotensin Blockade". Nephron. 144 (5): 213–221. doi:10.1159/000507305. PMC 7179544 Check
|pmc=value (help). PMID 32203970 Check|pmid=value (help). - ↑ Richardson S, Hirsch JS, Narasimhan M, Crawford JM, McGinn T, Davidson KW; et al. (2020). "Presenting Characteristics, Comorbidities, and Outcomes Among 5700 Patients Hospitalized With COVID-19 in the New York City Area". JAMA. doi:10.1001/jama.2020.6775. PMC 7177629 Check
|pmc=value (help). PMID 32320003 Check|pmid=value (help). - ↑ 10.0 10.1 10.2 Gabarre P, Dumas G, Dupont T, Darmon M, Azoulay E, Zafrani L (2020). "Acute kidney injury in critically ill patients with COVID-19". Intensive Care Med. 46 (7): 1339–1348. doi:10.1007/s00134-020-06153-9. PMC 7290076 Check
|pmc=value (help). PMID 32533197 Check|pmid=value (help). - ↑ Sharma G, Volgman AS, Michos ED (2020). "Sex Differences in Mortality from COVID-19 Pandemic: Are Men Vulnerable and Women Protected?". JACC Case Rep. doi:10.1016/j.jaccas.2020.04.027. PMC 7198137 Check
|pmc=value (help). PMID 32373791 Check|pmid=value (help). - ↑ Rabb H (2020). "Kidney diseases in the time of COVID-19: major challenges to patient care". J Clin Invest. 130 (6): 2749–2751. doi:10.1172/JCI138871. PMC 7259985 Check
|pmc=value (help). PMID 32250968 Check|pmid=value (help). - ↑ 13.0 13.1 13.2 13.3 13.4 13.5 Selby NM, Forni LG, Laing CM, Horne KL, Evans RD, Lucas BJ; et al. (2020). "Covid-19 and acute kidney injury in hospital: summary of NICE guidelines". BMJ. 369: m1963. doi:10.1136/bmj.m1963. PMID 32457068 Check
|pmid=value (help). - ↑ 14.0 14.1 14.2 Ronco C, Reis T, Husain-Syed F (2020). "Management of acute kidney injury in patients with COVID-19". Lancet Respir Med. doi:10.1016/S2213-2600(20)30229-0. PMC 7255232 Check
|pmc=value (help). PMID 32416769 Check|pmid=value (help). - ↑ 15.0 15.1 15.2 Khwaja A (2012). "KDIGO clinical practice guidelines for acute kidney injury". Nephron Clin Pract. 120 (4): c179–84. doi:10.1159/000339789. PMID 22890468.
- ↑ Skorecki K, Green J, Brenner BM (2005). "Chronic renal failure". In Kasper DL, Braunwald E, Fauci AS, et al. Harrison's Principles of Internal Medicine (16th ed.). New York, NY: McGraw-Hill. pp. 1653–63. ISBN 978-0-07-139140-5.
- ↑ Kashani K, Cheungpasitporn W, Ronco C (2017). "Biomarkers of acute kidney injury: the pathway from discovery to clinical adoption". Clin Chem Lab Med. 55 (8): 1074–1089. doi:10.1515/cclm-2016-0973. PMID 28076311.
- ↑ Schrezenmeier EV, Barasch J, Budde K, Westhoff T, Schmidt-Ott KM (2017). "Biomarkers in acute kidney injury - pathophysiological basis and clinical performance". Acta Physiol (Oxf). 219 (3): 554–572. doi:10.1111/apha.12764. PMC 5575831. PMID 27474473.
- ↑ Oh DJ (2020). "A long journey for acute kidney injury biomarkers". Ren Fail. 42 (1): 154–165. doi:10.1080/0886022X.2020.1721300. PMC 7034110 Check
|pmc=value (help). PMID 32050834 Check|pmid=value (help). - ↑ Grein J, Ohmagari N, Shin D, Diaz G, Asperges E, Castagna A; et al. (2020). "Compassionate Use of Remdesivir for Patients with Severe Covid-19". N Engl J Med. 382 (24): 2327–2336. doi:10.1056/NEJMoa2007016. PMC 7169476 Check
|pmc=value (help). PMID 32275812 Check|pmid=value (help).