COVID-19 other diagnostic studies
For COVID-19 frequently asked inpatient questions, click here
For COVID-19 frequently asked outpatient questions, click here
|
COVID-19 Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
COVID-19 other diagnostic studies On the Web |
|
American Roentgen Ray Society Images of COVID-19 other diagnostic studies |
|
Risk calculators and risk factors for COVID-19 other diagnostic studies |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Syed Hassan A. Kazmi BSc, MD [2] Mandana Safakhah, MD[3]
Overview
Research laboratories have used isolation methods, electron microscopy, serology and PCR-based assays to diagnose coronavirus infections for surveillance studies.
Other Diagnostic Studies
Specific laboratory tests may include:
- Virus isolation in cell culture.
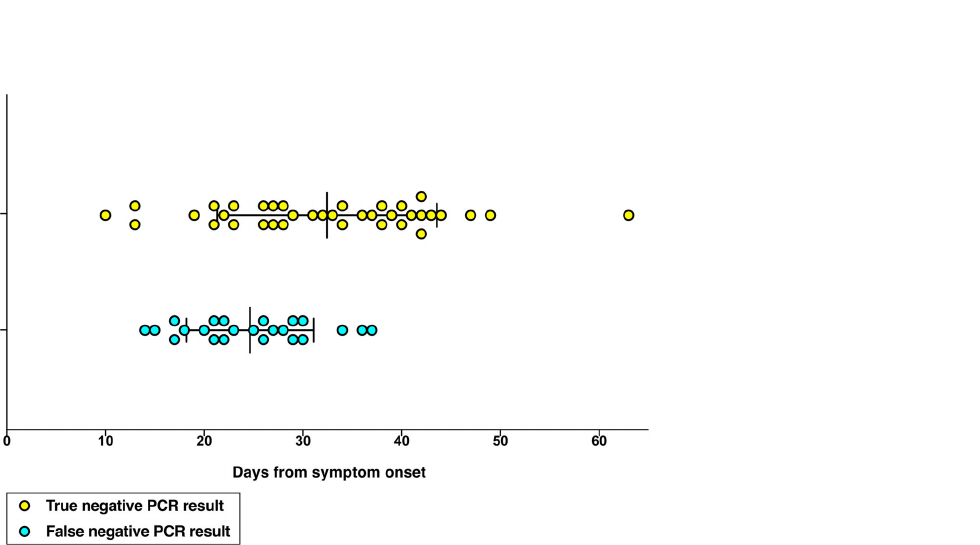
- Polymerase chain reaction (PCR) assays that are more practical and available commercially .However, false negative cases have been reported by several studies. hence it is suggested to obtain three negative consecutive r-RT-PCR tests before discharging patients with covid-19 .[1]
- Serological testing for antibodies to human coronaviruses. Serological assays play an essential role in contact tracing among populations and confirming covid-19 cases when RNA negative patients presents late in the illness.[2] [3]
Nose and throat swabs are the best specimens for detecting common human coronaviruses. Serological testing requires the collection of blood specimens.
Gallery
-
SEM reveals the “rosette-like” appearance of the matured coronavirus particles. From Public Health Image Library (PHIL). [4]
-
TEM revealed the presence of a number of infectious bronchitis virus (IBV) virions. From Public Health Image Library (PHIL). [4]
-
Scanning electron micrograph reveals the prolific exportation of virus particles at the pseudopodial and cell surfaces. From Public Health Image Library (PHIL). [4]
-
Scanning electron micrograph reveals the prolific exportation of virus particles at the pseudopodial and cell surfaces. From Public Health Image Library (PHIL). [4]
-
TEM from a tissue culture isolate, revealing numbers of severe acute respiratory virus (SARS) virions. From Public Health Image Library (PHIL). [4]
References
- ↑ 1.0 1.1 Wang, Guan; Yu, Na; Xiao, Weimin; Zhao, Chen; Wang, Zhenning (2020). "Consecutive false‐negative rRT‐PCR test results for SARS‐CoV‐2 in patients after clinical recovery from COVID‐19". Journal of Medical Virology. doi:10.1002/jmv.26192. ISSN 0146-6615.
- ↑ Udugama B, Kadhiresan P, Kozlowski HN, Malekjahani A, Osborne M, Li VYC; et al. (2020). "Diagnosing COVID-19: The Disease and Tools for Detection". ACS Nano. 14 (4): 3822–3835. doi:10.1021/acsnano.0c02624. PMC 7144809 Check
|pmc=value (help). PMID 32223179 Check|pmid=value (help). - ↑ Tang YW, Schmitz JE, Persing DH, Stratton CW (2020). "Laboratory Diagnosis of COVID-19: Current Issues and Challenges". J Clin Microbiol. 58 (6). doi:10.1128/JCM.00512-20. PMC 7269383 Check
|pmc=value (help). PMID 32245835 Check|pmid=value (help). - ↑ 4.0 4.1 4.2 4.3 4.4 "Public Health Image Library (PHIL)".
![SEM reveals the “rosette-like” appearance of the matured coronavirus particles. From Public Health Image Library (PHIL). [4]](/images/2/25/Coronavirus21.jpeg)
![TEM revealed the presence of a number of infectious bronchitis virus (IBV) virions. From Public Health Image Library (PHIL). [4]](/images/c/cc/Coronavirus20.jpeg)
![Scanning electron micrograph reveals the prolific exportation of virus particles at the pseudopodial and cell surfaces. From Public Health Image Library (PHIL). [4]](/images/2/2a/Coronavirus18.jpeg)
![Scanning electron micrograph reveals the prolific exportation of virus particles at the pseudopodial and cell surfaces. From Public Health Image Library (PHIL). [4]](/images/5/52/Coronavirus16.jpeg)
![TEM from a tissue culture isolate, revealing numbers of severe acute respiratory virus (SARS) virions. From Public Health Image Library (PHIL). [4]](/images/4/4a/Coronavirus15.jpeg)