Mannitol: Difference between revisions
m (Robot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
m (Protected "Mannitol": Bot: Protecting all pages from category Drug ([Edit=Allow only administrators] (indefinite) [Move=Allow only administrators] (indefinite))) |
||
| (40 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{DrugProjectFormSinglePage | ||
|IUPAC_name = ( | |authorTag=Gerald Chi<!--Overview--> | ||
| image=Mannitol structure.png | |genericName=Mannitol | ||
| | |aOrAn=a | ||
| ATC_prefix=A06 | |drugClass=[[osmotic]] [[diuretic]] | ||
| ATC_suffix=AD16 | |indicationType=treatment | ||
| ATC_supplemental={{ATC|B05|BC01}} {{ATC|B05|CX04}} | |indication=[[oliguria|oliguric phase]] of [[acute renal failure]], [[cerebral edema]], and [[IICP|elevated intraocular pressure]]. Mannitol is also indicated for promoting the urinary excretion of toxic substances | ||
| PubChem= | |adverseReactions=[[nausea]], [[vomiting]], [[rhinitis]], [[skin]] [[necrosis]], [[thrombophlebitis]], [[chills]], [[dizziness]], [[urticaria]], [[hypotension]], [[hypertension]], [[tachycardia]], [[fever]], and [[angina]]-like [[chest pain]]s | ||
| DrugBank= | |||
<!--Black Box Warning--> | |||
|blackBoxWarningTitle=Title | |||
|blackBoxWarningBody=<i><span style="color:#FF0000;">ConditionName: </span></i> | |||
* Content | |||
<!--Adult Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Adult)--> | |||
|fdaLIADAdult=* Mannitol should be administered only by [[intravenous]] infusion. The total dosage, concentration, and rate of administration should be governed by the nature and severity of the condition being treated, fluid requirement, and urinary output. | |||
* The usual adult dosage ranges from '''20 to 100 g in a 24 hour period''', but in most instances an adequate response will be achieved at a dosage of approximately '''50 to 100 g in a 24 hour period'''. The rate of administration is usually adjusted to maintain a urine flow of at least 30 to 50 mL/hour. This outline of administration and dosage is only a general guide to therapy. | |||
* Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions, where possible. | |||
* Test Dose: A test dose of mannitol should be given prior to instituting Mannitol therapy for patients with marked [[oliguria]], or those believed to have inadequate [[renal function]]. Such a test dose may be approximately '''0.2 g/kg body weight''' (about 75 mL of a 20% solution or 100 mL of a 15% solution) infused in a period of three to five minutes to produce a urine flow of at least 30 to 50 mL/hour. If urine flow does not increase, a second test dose may be given; if there is an inadequate response, the patient should be reevaluated. | |||
* Measurement of [[glomerular filtration rate]] by [[creatinine clearance]] may be useful for determination of dosage. | |||
=====Prevention of Acute Renal Failure (Oliguria)===== | |||
* Dosing Information | |||
:* When used during cardiovascular and other types of surgery, '''50 to 100 g''' of mannitol as a 5, 10, or 15% solution may be given. The concentration will depend upon the fluid requirements of the patient. | |||
=====Treatment of Oliguria===== | |||
* Dosing Information | |||
:* The usual dose for treatment of [[oliguria]] is '''100 g''' administered as a 15 or 20% solution. | |||
=====Reduction of Intraocular Pressure===== | |||
* Dosing Information | |||
:* A dose of '''1.5 to 2.0 g/kg''' as a 20% solution (7.5 to 10 mL/kg) or as a 15% solution (10 to 13 mL/kg) may be given over a period as short as 30 minutes in order to obtain a prompt and maximal effect. When used preoperatively the dose should be given one to one and one-half hours before surgery to achieve maximal reduction of intraocular pressure before operation. | |||
=====Reduction of Intracranial Pressure===== | |||
* Dosing Information | |||
:* Usually a maximum reduction in [[intracranial pressure]] in adults can be achieved with a dose of '''0.25 g/kg''' given not more frequently than every six to eight hours. An osmotic gradient between the blood and [[cerebrospinal fluid]] of approximately 10 mOsmol will yield a satisfactory reduction in intracranial pressure. | |||
=====Adjunctive Therapy for Intoxications===== | |||
* Dosing Information | |||
:* As an agent to promote [[diuresis]] in intoxications, 5%, 10%, 15% or 20% mannitol is indicated. The concentration will depend upon the fluid requirement and urinary output of the patient. | |||
<!--Off-Label Use and Dosage (Adult)--> | |||
<!--Guideline-Supported Use (Adult)--> | |||
|offLabelAdultGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
<!--Non–Guideline-Supported Use (Adult)--> | |||
|offLabelAdultNoGuideSupport======Dysequilibrium Syndrome===== | |||
* Dosing Information | |||
:* '''9 mosm/kg'''<ref>{{Cite journal | issn = 0028-2766 | volume = 27 | issue = 3 | pages = 134–141 | last = Rosa | first = A. A. | coauthors = J. Shideman, R. McHugh, D. Duncan, C. M. Kjellstrand | title = The importance of osmolality fall and ultrafiltration rate on hemodialysis side effects. Influence of intravenous mannitol | journal = Nephron | date = 1981 | pmid = 6783970 }}</ref><ref>{{Cite journal | issn = 0003-9926 | volume = 141 | issue = 4 | pages = 493–497 | last = Warren | first = S. E. | coauthors = R. C. Blantz | title = Mannitol | journal = Archives of Internal Medicine | date = 1981-03 | pmid = 6782963 }}</ref> | |||
=====Intracranial Tumor===== | |||
* Dosing Information | |||
:* '''10 mL (2.5 g)''' as a 25% solution over 30 to 60 seconds<ref>{{Cite journal | issn = 0195-6108 | volume = 21 | issue = 5 | pages = 968–970 | last = Fortin | first = D. | coauthors = E. Osztie, E. A. Neuwelt | title = Iatrogenic arterial spasm relieved by intraarterial mannitol infusion | journal = AJNR. American journal of neuroradiology | date = 2000-05 | pmid = 10815679 }}</ref> | |||
=====Subdural Hematoma===== | |||
* Dosing Information | |||
:* '''0.6 to 0.7 g/kg'''<ref>{{Cite journal | issn = 0148-396X | volume = 49 | issue = 4 | pages = 864–871 | last = Cruz | first = J. | coauthors = G. Minoja, K. Okuchi | title = Improving clinical outcomes from acute subdural hematomas with the emergency preoperative administration of high doses of mannitol: a randomized trial | journal = Neurosurgery | date = 2001-10 | pmid = 11564247 }}</ref> | |||
<!--Pediatric Indications and Dosage--> | |||
<!--FDA-Labeled Indications and Dosage (Pediatric)--> | |||
|fdaLIADPed=* Safety and effectiveness in children below the age of 12 have not been established. | |||
<!--Off-Label Use and Dosage (Pediatric)--> | |||
<!--Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
<!--Non–Guideline-Supported Use (Pediatric)--> | |||
|offLabelPedNoGuideSupport======Dysequilibrium Syndrome===== | |||
* Dosing Information | |||
:* '''40 g/m<sup>2</sup> over 1 hour orally as a 5.5% or a 20% solution'''<ref>{{Cite journal | issn = 1468-2044 | volume = 62 | issue = 7 | pages = 729–731 | last = Poulton | first = A. | coauthors = M. H. Winterborn | title = Oral mannitol in control of fluid balance | journal = Archives of Disease in Childhood | date = 1987-07 | pmid = 3115192 | pmc = PMC1779249 }}</ref> | |||
<!--Contraindications--> | |||
|contraindications=* Well established [[anuria]] due to severe [[renal disease]] | |||
* Severe [[pulmonary congestion]] or frank [[pulmonary edema]] | |||
* Active intracranial [[bleeding]] except during [[craniotomy]] | |||
* Severe [[dehydration]] | |||
* Progressive renal damage or dysfunction after institution of mannitol therapy, including increasing [[oliguria]] and [[azotemia]] | |||
* Progressive [[heart failure]] or [[pulmonary congestion]] after institution of mannitol therapy | |||
<!--Warnings--> | |||
|warnings=* In patients with severe impairment of [[renal function]], a test dose should be utilized (see Dosage and Administration). A second test dose may be tried if there is an inadequate response, but no more than two test doses should be attempted. | |||
* The obligatory [[diuretic]] response following rapid infusion of 15% or 20% mannitol injection may further aggravate preexisting [[hemoconcentration]]. Excessive loss of water and [[electrolyte]]s may lead to serious imbalances. Serum [[sodium]] and [[potassium]] should be carefully monitored during mannitol administration. | |||
* If urine output continues to decline during mannitol infusion, the patient’s clinical status should be closely reviewed and mannitol infusion suspended if necessary. Accumulation of mannitol may result in overexpansion of the extracellular fluid which may intensify existing or latent [[congestive heart failure]]. | |||
* Excessive loss of water and [[electrolyte]]s may lead to serious imbalances. With continued administration of mannitol, loss of water in excess of [[electrolyte]]s can cause [[hypernatremia]]. [[Electrolyte]] measurements, including [[sodium]] and [[potassium]], are therefore, of vital importance in monitoring the infusion of mannitol. | |||
* Osmotic nephrosis, a reversible vacuolization of the tubules of unknown clinical significance, may proceed to severe irreversible [[nephrosis]], so that the [[renal function]] must be closely monitored during mannitol infusion. | |||
====Precautions==== | |||
* The cardiovascular status of the patient should be carefully evaluated before rapidly administering mannitol since sudden expansion of the extracellular fluid may lead to fulminating [[congestive heart failure]]. | |||
* Shift of [[sodium]] free intracellular fluid into the extracellular compartment following mannitol infusion may lower serum sodium concentration and aggravate preexisting [[hyponatremia]]. | |||
* By sustaining [[diuresis]], mannitol administration may obscure and intensify inadequate hydration or [[hypovolemia]]. | |||
* Electrolyte free mannitol injections should not be given conjointly with blood. If it is essential that blood be given simultaneously, at least 20 mEq of sodium [[chloride]] should be added to each liter of mannitol solution to avoid pseudoagglutination. | |||
* When exposed to low temperatures, solutions of mannitol may crystallize. Concentrations greater than 15% have a greater tendency to crystallization. Inspect for crystals prior to administration. If crystals are visible, redissolve by warming the solution up to 70°C, with agitation. Allow the solution to cool to room temperature before reinspection for crystals. Administer intravenously using sterile, filter-type administration set. | |||
=====Laboratory Tests===== | |||
* Although blood levels of mannitol can be measured, there is little if any clinical virtue in doing so. The appropriate monitoring of blood levels of [[sodium]] and [[potassium]]; degree of [[hemoconcentration]] or hemodilution, if any; indices of renal, cardiac and pulmonary function are paramount in avoiding excessive fluid and electrolyte shifts. The routine features of physical examination and clinical chemistries suffice in achieving an adequate degree of appropriate patient monitoring. | |||
<!--Adverse Reactions--> | |||
<!--Clinical Trials Experience--> | |||
|clinicalTrials=There is limited information regarding <i>Clinical Trial Experience</i> of {{PAGENAME}} in the drug label. | |||
<!--Postmarketing Experience--> | |||
|postmarketing=* Extensive use of mannitol over the last several decades has produced recorded adverse events, in a variety of clinical settings, that are isolated or idiosyncratic in nature. None of these adverse reactions have occurred with any great frequency nor with any security in attributing them to mannitol. | |||
* The inability to clearly exclude the drug related nature of such events in these isolated reports prompts the necessity to list the reactions that have been observed in patients during or following mannitol infusion. In this fashion, patients have exhibited [[nausea]], [[vomiting]], [[rhinitis]], local pain, skin [[necrosis]] and [[thrombophlebitis]] at the site of injection, [[chills]], [[dizziness]], [[urticaria]], [[hypotension]], [[hypertension]], [[tachycardia]], [[fever]] and [[angina]]-like [[chest pain]]s. | |||
* Of far greater clinical significance is a variety of events that are related to inappropriate recognition and monitoring of fluid shifts. These are not intrinsic adverse reactions to the drug but the consequence of manipulating [[osmolarity]] by any agency in a therapeutically inappropriate manner. Failure to recognize severe impairment of renal function with the high likelihood of nondiuretic response can lead to aggravated dehydration of tissues and increased vascular fluid load. Induced [[diuresis]] in the presence of preexisting [[hemoconcentration]] and preexisting deficiency of water and [[electrolytes]] can lead to serious imbalances. Expansion of the extracellular space can aggravate cardiac decompensation or induce it in the presence of latent [[heart failure]]. [[Pulmonary congestion]] or [[edema]] can be seriously aggravated with the expansion of the extracellular and therefore intravascular fluid load. Hemodilution and dilution of the extracellular fluid space by osmotic shift of water can induce or aggravate preexisting [[hyponatremia]]. | |||
* If unrecognized, such fluid and/or electrolyte shift can produce the reported adverse reactions of [[pulmonary congestion]], [[acidosis]], [[electrolyte]] loss, dryness of mouth, [[thirst]], [[edema]], [[headache]], [[blurred vision]], [[convulsions]] and [[congestive cardiac failure]]. | |||
* These are not truly adverse reactions to the drug and can be appropriately prevented by evaluation of degree of renal failure with a test dose response to mannitol when indicated; evaluation of [[hypervolemia]] and [[hypovolemia]]; [[sodium]] and [[potassium]] levels; hemodilution or [[hemoconcentration]]; and evaluation of renal, cardiac and pulmonary function at the onset of therapy. | |||
<!--Drug Interactions--> | |||
|drugInteractions=There is limited information regarding <i>Drug Interactions</i> of {{PAGENAME}} in the drug label. | |||
<!--Use in Specific Populations--> | |||
|useInPregnancyFDA=* '''Pregnancy Category C''' | |||
:* Animal reproduction studies have not been conducted with mannitol. It is also not known whether mannitol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Mannitol should be given to a pregnant woman only if clearly needed. | |||
|useInPregnancyAUS=* '''Australian Drug Evaluation Committee (ADEC) Pregnancy Category''' | |||
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of {{PAGENAME}} in women who are pregnant. | |||
|useInLaborDelivery=There is no FDA guidance on use of {{PAGENAME}} during labor and delivery. | |||
|useInNursing=* It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when mannitol is administered to a nursing woman. | |||
|useInPed=* Safety and effectiveness in children below the age of 12 have not been established. | |||
|useInGeri=* Clinical studies of Osmitrol Injection (Mannitol Injection, USP) did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. | |||
* This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* Oral | |||
* Intravenous | |||
|monitoring=* Electrolyte measurements, including [[sodium]] and [[potassium]] are of vital importance in monitoring the infusion of mannitol. | |||
* [[Osmotic nephrosis]], a reversible vacuolization of the tubules of unknown clinical significance, may proceed to severe irreversible [[nephrosis]], so that the [[renal function]] must be closely monitored during mannitol infusion. | |||
<!--IV Compatibility--> | |||
|IVCompat=There is limited information regarding <i>IV Compatibility</i> of {{PAGENAME}} in the drug label. | |||
<!--Overdosage--> | |||
|overdose====Acute Overdose=== | |||
There is limited information regarding <i>Acute Overdose</i> of {{PAGENAME}} in the drug label. | |||
===Chronic Overdose=== | |||
There is limited information regarding <i>Chronic Overdose</i> of {{PAGENAME}} in the drug label. | |||
<!--Pharmacology--> | |||
<!--Drugbox2--> | |||
|drugBox={{Drugbox2 | |||
| Watchedfields = changed | |||
| verifiedrevid = 456482551 | |||
| IUPAC_name = (2''R'',3''R'',4''R'',5''R'')-Hexan-1,2,3,4,5,6-hexol | |||
| image = Mannitol structure.png | |||
| image2 = D-Mannitol 3d space fill.png | |||
| drug_name = <small>D</small>-Mannitol | |||
<!--Clinical data--> | |||
| tradename = Osmitrol | |||
| Drugs.com = {{drugs.com|monograph|mannitol}} | |||
| pregnancy_category = C: ([[United States|USA]]) | |||
| routes_of_administration = [[Intravenous]]<br>[[Mouth|Oral]] | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = ~7% | |||
| metabolism = [[Liver|Hepatic]], negligible. | |||
| elimination_half-life = 100 minutes | |||
| excretion = [[Kidney|Renal]]: 90% | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 69–65–8 | |||
| ATC_prefix = A06 | |||
| ATC_suffix = AD16 | |||
| ATC_supplemental = {{ATC|B05|BC01}} {{ATC|B05|CX04}} {{ATC|R05|CB16}} | |||
| ChEBI_Ref = {{ebicite|correct|EBI}} | |||
| ChEBI = 16899 | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4-,5-,6-/m1/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = FBPFZTCFMRRESA-KVTDHHQDSA-N | |||
| PubChem = 6251 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = DB00742 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 6015 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = 3OWL53L36A | |||
| KEGG_Ref = {{keggcite|correct|kegg}} | |||
| KEGG = D00062 | |||
| ChEMBL_Ref = {{ebicite|correct|EBI}} | |||
| ChEMBL = 689 | |||
<!--Chemical data--> | |||
| C=6 | H=14 | O=6 | | C=6 | H=14 | O=6 | ||
| molecular_weight = 182.172 | | molecular_weight = 182.172 | ||
| | | smiles = O[C@H]([C@H](O)CO)[C@H](O)[C@H](O)CO | ||
| InChI = 1/C6H14O6/c7-1-3(9)5(11)6(12)4(10)2-8/h3-12H,1-2H2/t3-,4-,5-,6-/m1/s1 | |||
| InChIKey = FBPFZTCFMRRESA-KVTDHHQDBH | |||
| | |||
| | |||
}} | }} | ||
<!--Mechanism of Action--> | |||
|mechAction=* Osmitrol Injection (Mannitol Injection, USP) is one of the nonelectrolyte, obligatory, osmotic diuretics. It is freely filterable at the renal glomerulus, only poorly reabsorbed by the renal tubule, not secreted by the tubule, and is pharmacologically inert. | |||
<!--Structure--> | |||
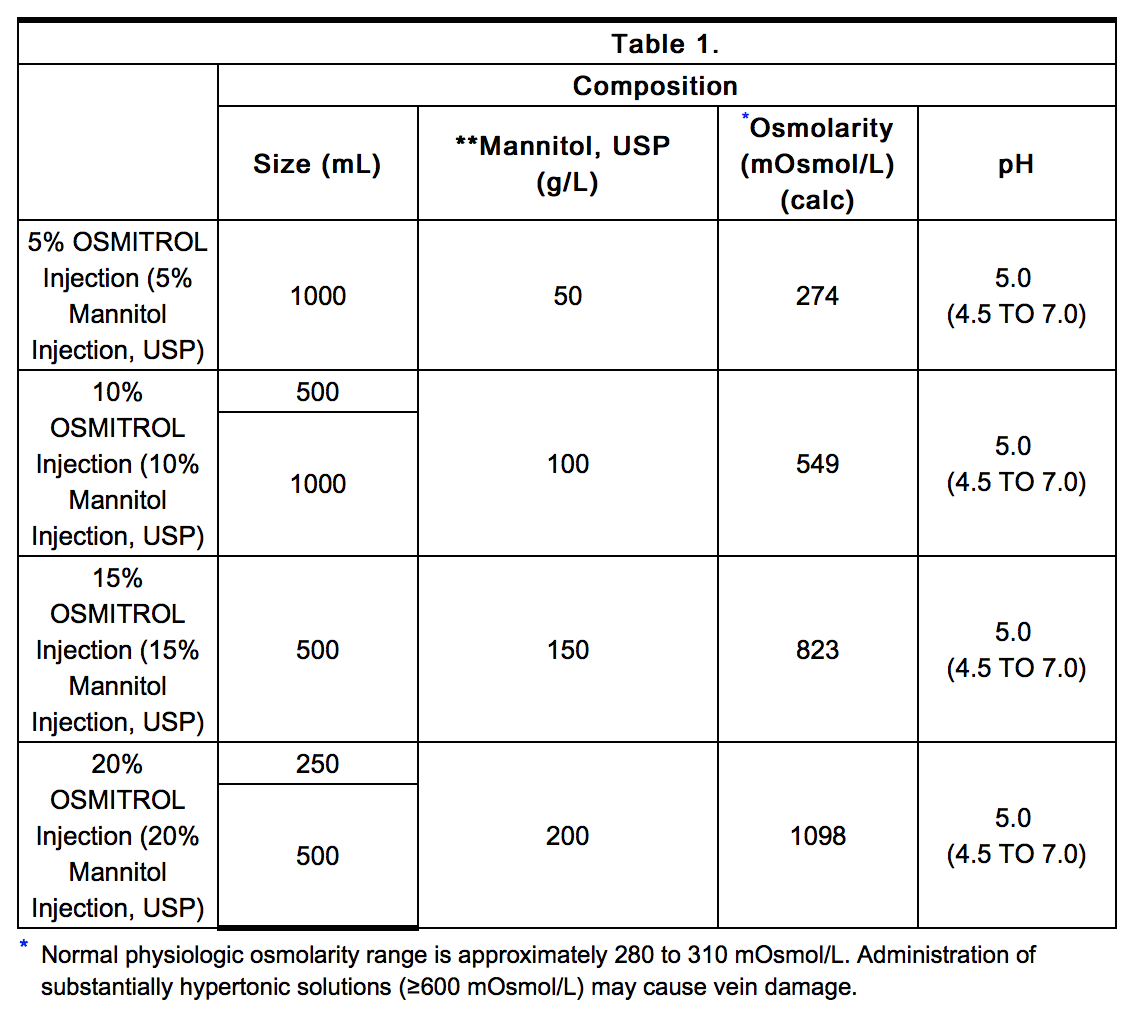
|structure=* Osmitrol Injection (Mannitol Injection, USP) is a sterile, nonpyrogenic solution of Mannitol, USP in a single dose container for intravenous administration. It contains no antimicrobial agents. Mannitol** is a six carbon sugar alcohol prepared commercially by the reduction of dextrose. Although virtually inert metabolically in humans, it occurs naturally in fruits and vegetables. Mannitol is an obligatory osmotic diuretic. The pH is adjusted with sodium hydroxide or hydrochloric acid. Composition, osmolarity, and pH are shown in Table 1. | |||
: [[File:{{PAGENAME}}01.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* The Viaflex plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies. | |||
= | <!--Pharmacodynamics--> | ||
Mannitol | |PD=* Mannitol, when administered intravenously, exerts its osmotic effect as a solute of relatively small molecular size being largely confined to the extracellular space. Only relatively small amounts of the dose administered is metabolized. Mannitol is readily diffused through the glomerulus of the kidney over a wide range of normal and impaired kidney function. In this fashion, approximately 80% of a 100 gram dose of mannitol will appear in the urine in three hours with lesser amounts thereafter. Even at peak concentrations, mannitol will exhibit less than 10% of tubular reabsorption and is not secreted by tubular cells. Mannitol will hinder tubular reabsorption of water and enhance excretion of sodium and chloride by elevating the [[osmolarity]] of the glomerular filtrate. | ||
* This increase in extracellular osmolarity effected by the intravenous administration of mannitol will induce the movement of intracellular water to the extracellular and vascular spaces. This action underlies the role of mannitol in reducing [[intracranial pressure]], intracranial [[edema]], and [[IICP|elevated intraocular pressure]]. | |||
<!--Pharmacokinetics--> | |||
|PK=There is limited information regarding <i>Pharmacokinetics</i> of {{PAGENAME}} in the drug label. | |||
<!--Nonclinical Toxicology--> | |||
|nonClinToxic=There is limited information regarding <i>Nonclinical Toxicology</i> of {{PAGENAME}} in the drug label. | |||
<!--Clinical Studies--> | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
<!--How Supplied--> | |||
|howSupplied=* Osmitrol Injection (Mannitol Injection, USP) in Viaflex plastic containers is available as follows: | |||
: [[File:{{PAGENAME}}03.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | |||
* Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product. | |||
<!--Patient Counseling Information--> | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
<!--Precautions with Alcohol--> | |||
|alcohol=* Alcohol-{{PAGENAME}} interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |||
<!--Brand Names--> | |||
|brandNames=* Osmitrol®<ref>{{Cite web | title = Osmitrol (mannitol) injection | url = http://dailymed.nlm.nih.gov/dailymed/lookup.cfm?setid=0d914965-7001-45cb-ba51-d7c5964b05bc }}</ref> | |||
<!--Look-Alike Drug Names--> | |||
|lookAlike=* N/A<ref name="www.ismp.org">{{Cite web | last = | first = | title = http://www.ismp.org | url = http://www.ismp.org | publisher = | date = }}</ref> | |||
<!--Drug Shortage Status--> | |||
|drugShortage= | |||
}} | |||
{{PillImage | |||
|fileName=No image.jpg | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
{{LabelImage | |||
|fileName={{PAGENAME}}04.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}05.png|This image is provided by the National Library of Medicine. | |||
}} | |||
{{LabelImage | |||
|fileName={{PAGENAME}}06.png|This image is provided by the National Library of Medicine. | |||
}} | |||
<!--Category--> | |||
[[Category: | [[Category:Cardiovascular Drugs]] | ||
[[Category: | [[Category:Drug]] | ||
Latest revision as of 16:38, 20 August 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gerald Chi
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Mannitol is a osmotic diuretic that is FDA approved for the treatment of oliguric phase of acute renal failure, cerebral edema, and elevated intraocular pressure. Mannitol is also indicated for promoting the urinary excretion of toxic substances. Common adverse reactions include nausea, vomiting, rhinitis, skin necrosis, thrombophlebitis, chills, dizziness, urticaria, hypotension, hypertension, tachycardia, fever, and angina-like chest pains.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Mannitol should be administered only by intravenous infusion. The total dosage, concentration, and rate of administration should be governed by the nature and severity of the condition being treated, fluid requirement, and urinary output.
- The usual adult dosage ranges from 20 to 100 g in a 24 hour period, but in most instances an adequate response will be achieved at a dosage of approximately 50 to 100 g in a 24 hour period. The rate of administration is usually adjusted to maintain a urine flow of at least 30 to 50 mL/hour. This outline of administration and dosage is only a general guide to therapy.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration whenever solution and container permit. Use of a final filter is recommended during administration of all parenteral solutions, where possible.
- Test Dose: A test dose of mannitol should be given prior to instituting Mannitol therapy for patients with marked oliguria, or those believed to have inadequate renal function. Such a test dose may be approximately 0.2 g/kg body weight (about 75 mL of a 20% solution or 100 mL of a 15% solution) infused in a period of three to five minutes to produce a urine flow of at least 30 to 50 mL/hour. If urine flow does not increase, a second test dose may be given; if there is an inadequate response, the patient should be reevaluated.
- Measurement of glomerular filtration rate by creatinine clearance may be useful for determination of dosage.
Prevention of Acute Renal Failure (Oliguria)
- Dosing Information
- When used during cardiovascular and other types of surgery, 50 to 100 g of mannitol as a 5, 10, or 15% solution may be given. The concentration will depend upon the fluid requirements of the patient.
Treatment of Oliguria
- Dosing Information
- The usual dose for treatment of oliguria is 100 g administered as a 15 or 20% solution.
Reduction of Intraocular Pressure
- Dosing Information
- A dose of 1.5 to 2.0 g/kg as a 20% solution (7.5 to 10 mL/kg) or as a 15% solution (10 to 13 mL/kg) may be given over a period as short as 30 minutes in order to obtain a prompt and maximal effect. When used preoperatively the dose should be given one to one and one-half hours before surgery to achieve maximal reduction of intraocular pressure before operation.
Reduction of Intracranial Pressure
- Dosing Information
- Usually a maximum reduction in intracranial pressure in adults can be achieved with a dose of 0.25 g/kg given not more frequently than every six to eight hours. An osmotic gradient between the blood and cerebrospinal fluid of approximately 10 mOsmol will yield a satisfactory reduction in intracranial pressure.
Adjunctive Therapy for Intoxications
- Dosing Information
- As an agent to promote diuresis in intoxications, 5%, 10%, 15% or 20% mannitol is indicated. The concentration will depend upon the fluid requirement and urinary output of the patient.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mannitol in adult patients.
Non–Guideline-Supported Use
Dysequilibrium Syndrome
- Dosing Information
Intracranial Tumor
- Dosing Information
- 10 mL (2.5 g) as a 25% solution over 30 to 60 seconds[3]
Subdural Hematoma
- Dosing Information
- 0.6 to 0.7 g/kg[4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Safety and effectiveness in children below the age of 12 have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Mannitol in pediatric patients.
Non–Guideline-Supported Use
Dysequilibrium Syndrome
- Dosing Information
- 40 g/m2 over 1 hour orally as a 5.5% or a 20% solution[5]
Contraindications
- Well established anuria due to severe renal disease
- Severe pulmonary congestion or frank pulmonary edema
- Active intracranial bleeding except during craniotomy
- Severe dehydration
- Progressive renal damage or dysfunction after institution of mannitol therapy, including increasing oliguria and azotemia
- Progressive heart failure or pulmonary congestion after institution of mannitol therapy
Warnings
- In patients with severe impairment of renal function, a test dose should be utilized (see Dosage and Administration). A second test dose may be tried if there is an inadequate response, but no more than two test doses should be attempted.
- The obligatory diuretic response following rapid infusion of 15% or 20% mannitol injection may further aggravate preexisting hemoconcentration. Excessive loss of water and electrolytes may lead to serious imbalances. Serum sodium and potassium should be carefully monitored during mannitol administration.
- If urine output continues to decline during mannitol infusion, the patient’s clinical status should be closely reviewed and mannitol infusion suspended if necessary. Accumulation of mannitol may result in overexpansion of the extracellular fluid which may intensify existing or latent congestive heart failure.
- Excessive loss of water and electrolytes may lead to serious imbalances. With continued administration of mannitol, loss of water in excess of electrolytes can cause hypernatremia. Electrolyte measurements, including sodium and potassium, are therefore, of vital importance in monitoring the infusion of mannitol.
- Osmotic nephrosis, a reversible vacuolization of the tubules of unknown clinical significance, may proceed to severe irreversible nephrosis, so that the renal function must be closely monitored during mannitol infusion.
Precautions
- The cardiovascular status of the patient should be carefully evaluated before rapidly administering mannitol since sudden expansion of the extracellular fluid may lead to fulminating congestive heart failure.
- Shift of sodium free intracellular fluid into the extracellular compartment following mannitol infusion may lower serum sodium concentration and aggravate preexisting hyponatremia.
- By sustaining diuresis, mannitol administration may obscure and intensify inadequate hydration or hypovolemia.
- Electrolyte free mannitol injections should not be given conjointly with blood. If it is essential that blood be given simultaneously, at least 20 mEq of sodium chloride should be added to each liter of mannitol solution to avoid pseudoagglutination.
- When exposed to low temperatures, solutions of mannitol may crystallize. Concentrations greater than 15% have a greater tendency to crystallization. Inspect for crystals prior to administration. If crystals are visible, redissolve by warming the solution up to 70°C, with agitation. Allow the solution to cool to room temperature before reinspection for crystals. Administer intravenously using sterile, filter-type administration set.
Laboratory Tests
- Although blood levels of mannitol can be measured, there is little if any clinical virtue in doing so. The appropriate monitoring of blood levels of sodium and potassium; degree of hemoconcentration or hemodilution, if any; indices of renal, cardiac and pulmonary function are paramount in avoiding excessive fluid and electrolyte shifts. The routine features of physical examination and clinical chemistries suffice in achieving an adequate degree of appropriate patient monitoring.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Mannitol in the drug label.
Postmarketing Experience
- Extensive use of mannitol over the last several decades has produced recorded adverse events, in a variety of clinical settings, that are isolated or idiosyncratic in nature. None of these adverse reactions have occurred with any great frequency nor with any security in attributing them to mannitol.
- The inability to clearly exclude the drug related nature of such events in these isolated reports prompts the necessity to list the reactions that have been observed in patients during or following mannitol infusion. In this fashion, patients have exhibited nausea, vomiting, rhinitis, local pain, skin necrosis and thrombophlebitis at the site of injection, chills, dizziness, urticaria, hypotension, hypertension, tachycardia, fever and angina-like chest pains.
- Of far greater clinical significance is a variety of events that are related to inappropriate recognition and monitoring of fluid shifts. These are not intrinsic adverse reactions to the drug but the consequence of manipulating osmolarity by any agency in a therapeutically inappropriate manner. Failure to recognize severe impairment of renal function with the high likelihood of nondiuretic response can lead to aggravated dehydration of tissues and increased vascular fluid load. Induced diuresis in the presence of preexisting hemoconcentration and preexisting deficiency of water and electrolytes can lead to serious imbalances. Expansion of the extracellular space can aggravate cardiac decompensation or induce it in the presence of latent heart failure. Pulmonary congestion or edema can be seriously aggravated with the expansion of the extracellular and therefore intravascular fluid load. Hemodilution and dilution of the extracellular fluid space by osmotic shift of water can induce or aggravate preexisting hyponatremia.
- If unrecognized, such fluid and/or electrolyte shift can produce the reported adverse reactions of pulmonary congestion, acidosis, electrolyte loss, dryness of mouth, thirst, edema, headache, blurred vision, convulsions and congestive cardiac failure.
- These are not truly adverse reactions to the drug and can be appropriately prevented by evaluation of degree of renal failure with a test dose response to mannitol when indicated; evaluation of hypervolemia and hypovolemia; sodium and potassium levels; hemodilution or hemoconcentration; and evaluation of renal, cardiac and pulmonary function at the onset of therapy.
Drug Interactions
There is limited information regarding Drug Interactions of Mannitol in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category C
- Animal reproduction studies have not been conducted with mannitol. It is also not known whether mannitol can cause fetal harm when administered to a pregnant woman or can affect reproduction capacity. Mannitol should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Mannitol in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Mannitol during labor and delivery.
Nursing Mothers
- It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when mannitol is administered to a nursing woman.
Pediatric Use
- Safety and effectiveness in children below the age of 12 have not been established.
Geriatic Use
- Clinical studies of Osmitrol Injection (Mannitol Injection, USP) did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy.
- This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Mannitol with respect to specific gender populations.
Race
There is no FDA guidance on the use of Mannitol with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Mannitol in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Mannitol in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Mannitol in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Mannitol in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
- Intravenous
Monitoring
- Electrolyte measurements, including sodium and potassium are of vital importance in monitoring the infusion of mannitol.
- Osmotic nephrosis, a reversible vacuolization of the tubules of unknown clinical significance, may proceed to severe irreversible nephrosis, so that the renal function must be closely monitored during mannitol infusion.
IV Compatibility
There is limited information regarding IV Compatibility of Mannitol in the drug label.
Overdosage
Acute Overdose
There is limited information regarding Acute Overdose of Mannitol in the drug label.
Chronic Overdose
There is limited information regarding Chronic Overdose of Mannitol in the drug label.
Pharmacology
| |

| |
D-Mannitol
| |
| Systematic (IUPAC) name | |
| (2R,3R,4R,5R)-Hexan-1,2,3,4,5,6-hexol | |
| Identifiers | |
| CAS number | |
| ATC code | A06 B05BC01 (WHO) B05CX04 (WHO) R05CB16 (WHO) |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 182.172 |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ~7% |
| Metabolism | Hepatic, negligible. |
| Half life | 100 minutes |
| Excretion | Renal: 90% |
| Therapeutic considerations | |
| Pregnancy cat. |
C: (USA) |
| Legal status | |
| Routes | Intravenous Oral |
Mechanism of Action
- Osmitrol Injection (Mannitol Injection, USP) is one of the nonelectrolyte, obligatory, osmotic diuretics. It is freely filterable at the renal glomerulus, only poorly reabsorbed by the renal tubule, not secreted by the tubule, and is pharmacologically inert.
Structure
- Osmitrol Injection (Mannitol Injection, USP) is a sterile, nonpyrogenic solution of Mannitol, USP in a single dose container for intravenous administration. It contains no antimicrobial agents. Mannitol** is a six carbon sugar alcohol prepared commercially by the reduction of dextrose. Although virtually inert metabolically in humans, it occurs naturally in fruits and vegetables. Mannitol is an obligatory osmotic diuretic. The pH is adjusted with sodium hydroxide or hydrochloric acid. Composition, osmolarity, and pH are shown in Table 1.
- The Viaflex plastic container is fabricated from a specially formulated polyvinyl chloride (PL 146 Plastic). The amount of water that can permeate from inside the container into the overwrap is insufficient to affect the solution significantly. Solutions in contact with the plastic container can leach out certain of its chemical components in very small amounts within the expiration period, e.g., di-2-ethylhexyl phthalate (DEHP), up to 5 parts per million. However, the safety of the plastic has been confirmed in tests in animals according to USP biological tests for plastic containers as well as by tissue culture toxicity studies.
Pharmacodynamics
- Mannitol, when administered intravenously, exerts its osmotic effect as a solute of relatively small molecular size being largely confined to the extracellular space. Only relatively small amounts of the dose administered is metabolized. Mannitol is readily diffused through the glomerulus of the kidney over a wide range of normal and impaired kidney function. In this fashion, approximately 80% of a 100 gram dose of mannitol will appear in the urine in three hours with lesser amounts thereafter. Even at peak concentrations, mannitol will exhibit less than 10% of tubular reabsorption and is not secreted by tubular cells. Mannitol will hinder tubular reabsorption of water and enhance excretion of sodium and chloride by elevating the osmolarity of the glomerular filtrate.
- This increase in extracellular osmolarity effected by the intravenous administration of mannitol will induce the movement of intracellular water to the extracellular and vascular spaces. This action underlies the role of mannitol in reducing intracranial pressure, intracranial edema, and elevated intraocular pressure.
Pharmacokinetics
There is limited information regarding Pharmacokinetics of Mannitol in the drug label.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Mannitol in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Mannitol in the drug label.
How Supplied
- Osmitrol Injection (Mannitol Injection, USP) in Viaflex plastic containers is available as follows:
- Exposure of pharmaceutical products to heat should be minimized. Avoid excessive heat. It is recommended the product be stored at room temperature (25°C); brief exposure up to 40°C does not adversely affect the product.
Storage
There is limited information regarding Mannitol Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Mannitol |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Mannitol |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Mannitol in the drug label.
Precautions with Alcohol
- Alcohol-Mannitol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Osmitrol®[6]
Look-Alike Drug Names
- N/A[7]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Rosa, A. A. (1981). "The importance of osmolality fall and ultrafiltration rate on hemodialysis side effects. Influence of intravenous mannitol". Nephron. 27 (3): 134–141. ISSN 0028-2766. PMID 6783970. Unknown parameter
|coauthors=ignored (help) - ↑ Warren, S. E. (1981-03). "Mannitol". Archives of Internal Medicine. 141 (4): 493–497. ISSN 0003-9926. PMID 6782963. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Fortin, D. (2000-05). "Iatrogenic arterial spasm relieved by intraarterial mannitol infusion". AJNR. American journal of neuroradiology. 21 (5): 968–970. ISSN 0195-6108. PMID 10815679. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Cruz, J. (2001-10). "Improving clinical outcomes from acute subdural hematomas with the emergency preoperative administration of high doses of mannitol: a randomized trial". Neurosurgery. 49 (4): 864–871. ISSN 0148-396X. PMID 11564247. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ Poulton, A. (1987-07). "Oral mannitol in control of fluid balance". Archives of Disease in Childhood. 62 (7): 729–731. ISSN 1468-2044. PMC 1779249. PMID 3115192. Unknown parameter
|coauthors=ignored (help); Check date values in:|date=(help) - ↑ "Osmitrol (mannitol) injection".
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Mannitol
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Mannitol |Label Name=Mannitol04.png
}}
{{#subobject:
|Label Page=Mannitol |Label Name=Mannitol05.png
}}
{{#subobject:
|Label Page=Mannitol |Label Name=Mannitol06.png
}}