Sandbox suveen
Cholera
Antibiotics
- Antibiotic treatments for one to three days shorten the course of the disease and reduce the severity of the symptoms.
- People can recover even without them, if sufficient hydration and electrolyte balance is maintained.
- Doxycycline is typically used first line, although some strains of V. cholerae have shown resistance.
- Doxycycline single dose 300 mg or tetracycline 12,5 mg/kg 4 time/day for 3 days
- Other antibiotics proven to be effective include cotrimoxazole, erythromycin, tetracycline, chloramphenicol, and furazolidone.[1]
- Fluoroquinolones, such as norfloxacin, also may be used, but resistance has been reported.[2]
- Young children: erythromycin 12,5 mg/kg 4 time/day for 3 days
- for children below 6 months of age: 10 mg daily for 10 days add zinc
- for children 6 months to 5 years of age: 20mg daily for 10 days add zinc
- In many areas of the world, antibiotic resistance is increasing. Testing for resistance during an outbreak can help determine appropriate future choices. In Bangladesh, for example, most cases are resistant to tetracycline, trimethoprim-sulfamethoxazole, and erythromycin. Rapid diagnostic assay methods are available for the identification of multiple drug-resistant cases.[3] New generation antimicrobials have been discovered which are effective against in in vitro studies.[4]
References
- ↑ "Cholera treatment". Molson Medical Informatics. 2007. Retrieved 2008-01-03.
- ↑ Krishna BV, Patil AB, Chandrasekhar MR (2006). "Fluoroquinolone-resistant Vibrio cholerae isolated during a cholera outbreak in India". Trans. R. Soc. Trop. Med. Hyg. 100 (3): 224–6. doi:10.1016/j.trstmh.2005.07.007. PMID 16246383. Unknown parameter
|month=ignored (help) - ↑ Mackay IM (editor) (2007). Real-Time PCR in microbiology: From diagnosis to characterization. Caister Academic Press. ISBN 978-1-904455-18-9.
- ↑ Ramamurthy T (2008). "Antibiotic resistance in Vibrio cholerae". Vibrio cholerae: Genomics and molecular biology. Caister Academic Press. ISBN 978-1-904455-33-2.
Vibrio parahaemolyticus
Antibiotic regimen
- 1. Sepsis or Soft Tissue Infection Antibiotic Management [1]
- Preferred regimen: Doxycycline 100 mg IV q12h AND Ceftazidime 2 g IV q8h OR Fluoroquinolone
- Alternative regimen (1): Cefotaxime 2 g IV q6h IV AND Ciprofloxacin 400 mg IV q12h (synergistic in vitro) OR Moxifloxacin 400 mg IV OD OR Levofloxacin 750 mg IV OD
- Alternative regimen (2): Cefotaxime AND Minocycline (synergistic in vitro)
- 2. Gastroenteritis
- Most cases self-limiting
- Maintain hydration: oral or parenteral routes
- Role of Doxycycline or Fluoroquinolones unclear, does not appear to shorten duration of non-cholera gastroenteritis
References
- ↑ Bartlett, John (2012). Johns Hopkins ABX guide : diagnosis and treatment of infectious diseases. Burlington, MA: Jones and Bartlett Learning. ISBN 978-1449625580.
Bordetella pertussis
Diagnosis
A nasopharyngeal or an oropharynx swab is sent to the bacteriology laboratory for Gram stain (Gram-negative, coccobacilli, diplococci arrangement), growth on Bordet-Gengou agar or BCYE plate with added cephalosporin to select for the organism, which shows mercury-drop-like colonies.
Several diagnostic tests are available, especially ELISA kits. These are designed to detect FHA and/or PT antibodies of the following classes: IgG, IgA, IgM. Some kits use a combination of antigens which will lead to a higher sensitivity, but might also make the interpretation of the results harder since one cannot know which antibody has been detected. Also, a new rapid molecular test is available, real-time PCR, based on the so-called FilmArray technology . This test takes about one hour and detects about 15–17 viruses and bacteria, including B. pertussis.
The organism is oxidase positive, but urease, nitrate reductase, and citrate negative. It is also nonmotile.
References
Marburg hemorrhagic Fever
|
WikiDoc Resources for Sandbox suveen |
|
Articles |
|---|
|
Most recent articles on Sandbox suveen Most cited articles on Sandbox suveen |
|
Media |
|
Powerpoint slides on Sandbox suveen |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sandbox suveen at Clinical Trials.gov Trial results on Sandbox suveen Clinical Trials on Sandbox suveen at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sandbox suveen NICE Guidance on Sandbox suveen
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sandbox suveen Discussion groups on Sandbox suveen Patient Handouts on Sandbox suveen Directions to Hospitals Treating Sandbox suveen Risk calculators and risk factors for Sandbox suveen
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sandbox suveen |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2], Serge Korjian M.D.
Overview
The Marburg virus causes severe viral hemorrhagic fever in humans with case fatality rates ranging from 24% to 88%. [1] Rousettus aegypti, fruit bats of the Pteropodidae family, are considered to be natural hosts of Marburg virus. The Marburg virus is transmitted to people from fruit bats and spreads through human-to-human transmission. No specific antiviral treatment or vaccine is available.
Historical Perspective
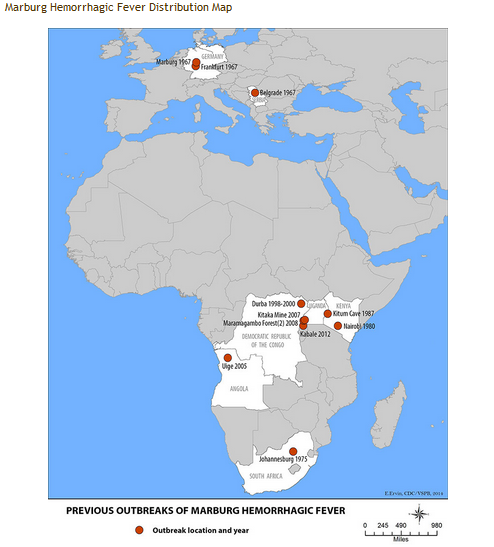
- Marburg hemorrhagic fever was initially detected in 1967 after simultaneous outbreaks in Marburg, from which the disease takes its name, Frankfurt, and Belgrade.
- Subsequently, outbreaks and sporadic cases were reported in Angola, Democratic Republic of the Congo, Kenya, South Africa, and Uganda.
| Years | Country | Apparent or suspected origin | Reported number of human cases | Reported number (%) of deaths among cases | Situation |
|---|---|---|---|---|---|
| 1967 | Germany and Yugoslavia | Uganda | 31 | 7 (23%) | Simultaneous outbreaks occurred in laboratory workers handling African green monkeys imported from Uganda. In addition to the 31 reported cases, an additional primary case was retrospectively diagnosed by serology. [2] |
| 1975 | Johannesburg, South Africa | Zimbabwe | 3 | 1 (33%) | A man with a recent travel history to Zimbabwe was admitted to hospital in South Africa. Infection spread from the man to his traveling companion and a nurse at the hospital. The man died, but both women were given vigorous supportive treatment and eventually recovered.[3] |
| 1980 | Kenya | Kenya | 2 | 1 (50%) | A man with a recent travel history to Kitum Cave in Kenya's Mount Elgon National Park. Despite specialized care in Nairobi, the male patient died. A doctor who attempted resuscitation developed symptoms 9 days later but recovered[4] |
| 1987 | Kenya | Kenya | 1 | 1 (100%) | A 15-year-old Danish boy was hospitalized with a 3-day history of headache, malaise, fever, and vomiting. Nine days prior to symptom onset, he had visited Kitum Cave in Mount Elgon National Park. Despite aggressive supportive therapy, the patient died on the 11th day of illness. No further cases were detected[5] |
| 1990 | Russia | Russia | 1 | 1 (100%) | Laboratory contamination.[6] |
| 1998-2000 | Democratic Republic of Congo (DRC) | Durba, DRC | 154 | 128 (83%) | Most cases occurred in young male workers at a gold mine in Durba, in the north-eastern part of the country, which proved to be the epicenter of the outbreak. Cases were subsequently detected in the neighboring village of Watsa.[6] |
| 2004-2005 | Angola | Uige Province, Angola | 252 | 227 (90%) | Outbreak believed to have begun in Uige Province in October 2004. Most cases detected in other provinces have been linked directly to the outbreak in Uige[7] |
| 2007 | Uganda | Lead and gold mine in Kamwenge District, Uganda | 4 | 1 (25%) | Small outbreak, with 4 cases in young males working in a mine. To date, there have been no additional cases identified[6] |
| 2008 | Netherlands ex Uganda | Cave in Maramagambo forest in Uganda, at the southern edge of Queen Elizabeth National Park | 1 | 1 (100%) | A 40-year-old Dutch woman with a recent history of travel to Uganda was admitted to hospital in the Netherlands. Three days prior to hospitalization, the first symptoms (fever, chills) occurred, followed by rapid clinical deterioration. The woman died on the 10th day of the illness. |
| 2012 | Uganda | Kabale | 15 | 4 (27%) | Testing at CDC/UVRI identified a Marburg virus disease outbreak in the districts of Kabale, Ibanda, Mbarara, and Kampala over a 3 week time period[8] |
| 2014 | Uganda | Uganda | 1 | 1 (100%) | Ninety-nine individuals were quarantined after a 30-year-old male health-worker died of Marburg hemorrhagic fever on the 28th of September. |
Pathophysiology
Pathogen
 |
- Marburg virus is the causative agent of Marburg haemorrhagic fever (MHF). Marburg and Ebola viruses are the two members of the Filoviridae family (filovirus). Though caused by different viruses, the two diseases are clinically similar.
- The viral structure is typical of filoviruses, with long threadlike particles which have a consistent diameter but vary greatly in length from an average of 800 nanometers up to 14,000 nm. Peak infectious activity is at approximately 790 nm.
- Virions contain seven known structural proteins. Four proteins form the nucelocapsid of the Marburg virus: NP, VP35, VP30, and L.[11] While nearly identical to Ebola virus in structure, Marburg virus is antigenically distinct from Ebola virus.
- Marburg virus was the first filovirus to be identified.
Transmission
- Initial human infection results from prolonged exposure to mines or caves inhabited by Rousettus bat colonies. The reservoir host of Marburg virus is the African fruit bat, Rousettus aegyptiacus. Primates (including humans) can become infected with Marburg virus, and may develop serious disease with high mortality.
- Transmission is mainly human-to-human, resulting from close contact with the blood, secretions, organs or other bodily fluids of infected persons.[12]
- Transmission via infected semen can occur up to seven weeks after clinical recovery.
- Transmission to health-care workers has been reported while treating Marburg patients, mainly due to incorrect or inadequate use of personal protective equipment.
Differentiating Marburg Hemorrhagic Fever from other Diseases
Marburg hemorrhagic fever must be differentiated from other diseases that may cause fever, abdominal pain, diarrhea, vomiting and bleeding such as:
- Malaria
- Typhoid fever
- Shigellosis
- Cholera
- Leptospirosis
- Plague,
- Rickettsiosis
- Relapsing fever
- Meningitis
- Hepatitis
- Other viral hemorrhagic fevers
Treatment should be based on the most likely etiology of fever according to local epidemiology. If the fever continues after 3 days of recommended treatment, and if the patient has signs such as bleeding or shock, a viral hemorrhagic fever should be considered. It is important to review the patient’s history for any contact with someone who was ill with fever and bleeding or who died from an unexplained illness with fever and bleeding.
Shown below is a table summarizing the typical findings of the differential diagnoses of MHF.
| Disease | Findings |
|---|---|
| Shigellosis & other bacterial enteric infections | Presents with diarrhea, possibly bloody, accompanied by fever, nausea, and sometimes toxemia, vomiting, cramps, and tenesmus. Stools contain blood and mucous in a typical case. A search for possible sites of bacterial infection, together with cultures and blood smears, should be made. Presence of leucocytosis distinguishes bacterial infections. |
| Typhoid fever | Presents with fever, headache, rash, gastrointestinal symptoms, with lymphadenopathy, relative bradycardia, cough and leucopenia and sometimes sore throat. Blood and stool culture can demonstrate causative bacteria. |
| Malaria | Presents with acute fever, headache and sometime diarrhea (children). Blood smears must be examined for malaria parasites. Presence of parasites does not exclude concurrent viral infection. Antimalarial must be prescribed in an attempt at therapy. |
| Lassa fever | Disease onset is usually gradual, with fever, sore throat, cough, pharyngitis, and facial edema in the later stages. Inflammation and exudation of the pharynx and conjunctiva are common. |
| Yellow fever and other Flaviviridae | Present with hemorrhagic complications. Epidemiological investigation may reveal a pattern of disease transmission by an insect vector. Virus isolation and serological investigation serves to distinguish these virus. Confirmed history of previous yellow fever vaccination will rule out yellow fever. |
| Others | Viral hepatitis, leptospirosis, rheumatic fever, typhus, and mononucleosis produce signs and symptoms that may be confused with Ebola in the early stages of infection. |
| Table adapted from WHO Guidelines For Epidemic Preparedness And Response: Ebola Haemorrhagic Fever [13] | |
Risk of Exposure
- People with close contact with African fruit bats, human patients, or non-human primates infected with Marburg virus are at risk.
- Family members and hospital staff who care for patients infected with Marburg virus and have not used proper barrier techniques.
- Particular occupations, such as veterinarians and laboratory or quarantine facility workers who handle non-human primates from Africa, may also be at increased risk of exposure to Marburg virus.
- Exposure risk can be higher for travelers visiting endemic regions in Africa, including Uganda and other parts of central Africa, and have contact with fruit bats, or enter caves or mines inhabited by fruit bats.
Epidemiology and Demographics
Both Marburg and Ebola hemorrhagic fevers are rare and have the capacity to cause dramatic outbreaks with high fatality rates.
Two large outbreaks that occurred simultaneously in Marburg and Frankfurt in Germany, and in Belgrade, Serbia, in 1967, led to the initial recognition of the disease. The outbreak was associated with laboratory work using African green monkeys (Cercopithecus aethiops) imported from Uganda. Subsequently, outbreaks and sporadic cases have been reported in Angola, Democratic Republic of the Congo, Kenya, South Africa (in a person with recent travel history to Zimbabwe) and Uganda. In 2008, two independent cases were reported in travelers who visited a cave inhabited by Rousettus bat colonies in Uganda. In 2014, one fatal case of Marburg virus was reported in Uganda with subsequent quarantine of 99 individuals.

Natural History, Complications and Prognosis
Case fatality rates in Marburg hemorrhagic fever outbreaks have ranged from 23% to 90%.
Diagnosis
Signs and Symptoms
Because many of the signs and symptoms of Marburg hemorrhagic fever are similar to those of other infectious diseases, such as malaria or typhoid, diagnosis of the disease can be difficult, especially if only a single case is involved. The incubation period (interval from infection to onset of symptoms) varies from 5 to 10 days. The disease is spread through bodily fluids, including blood, excrement, saliva, and vomit and a history of such contact should be solicited.
- Early symptoms are often non-specific. They include the following:
- After five days, a macropapular rash is often present on the trunk.
- Later-stage Marburg infection is acute and can include the following:
- Jaundice
- Pancreatitis
- Weight loss
- Delirium and neuropsychiatric symptoms
- Hemorrhage
- hypovolemic shock
- Multi-organ dysfunction with liver failure[14][15]
Many patients develop severe hemorrhagic manifestations between 5 and 7 days, and fatal cases usually have some form of bleeding, often from multiple areas. Fresh blood in vomitus and feces is often accompanied by bleeding from the nose, gums, and vagina. Spontaneous bleeding at venipuncture sites can also occur. The time course varies but the disease lasts betweeen one and three weeks. The fatality rate ranges from 23 to 90%. Death occurs most often between 8 and 9 days after symptom onset, usually preceded by severe blood loss and shock. If a patient survives, recovery is usually prompt and complete, though it may be prolonged in some cases. Persistent symptoms may include inflammation or secondary infection of various organs, including: orchitis, hepatitis, transverse myelitis, uveitis, or parotitis. Involvement of the central nervous system can result in confusion, irritability, and aggression. Orchitis has been reported occasionally in the late phase of disease (15 days).
Laboratory Studies
Marburg virus infections can be diagnosed definitively only in laboratories, by a number of different tests as follows.
| Lab test for Marburg virus detection |
|---|
| Enzyme-linked immunosorbent assay (ELISA) |
| Reverse-transcriptase polymerase chain reaction (RT-PCR) assay |
| Serum neutralization test |
| Antigen detection tests |
| Virus isolation by cell culture |
Tests on clinical samples present an extreme biohazard risk and are conducted only under maximum biological containment conditions. In deceased patients, immunohistochemistry, virus isolation, or PCR of blood or tissue specimens may be used to diagnose Marburg HF retrospectively.
Treatment
Acute Medical Therapy
Severe cases require intensive supportive care, as patients are frequently in need of intravenous fluids or oral rehydration with solutions containing electrolytes. No specific treatment or vaccine is yet available for Marburg hemorrhagic fever.
Primary Prevention and Vaccines
- No specific treatment or vaccine is yet available for MHF. Several vaccine candidates are being tested but it could be several years before any are available. New drug therapies have shown promising results in laboratory studies and are currently being evaluated.
- Avoiding fruit bats, and sick non-human primates in central Africa, is one way to protect against infection.
Precautionary measures for pig farms in endemic zones
Precautionary measures are needed in pig farms in Africa to avoid pigs becoming infected through contact with fruit bats. Such infection could potentially amplify the virus and cause or contribute to Marburg hemorrhagic fever outbreaks.
Reducing the risk of infection in people
In the absence of effective treatment and human vaccine, raising awareness of the risk factors for Marburg infection and the protective measures individuals can take to reduce human exposure to the virus, are the only ways to reduce human infections and deaths.
During MHF outbreaks in Africa, public health educational messages for risk reduction should focus on:
Reducing the risk of bat-to-human transmission arising from prolonged exposure to mines or caves inhabited by fruit bats colonies. During work or research activities or tourist visits in mines or caves inhabited by fruit bat colonies, people should wear gloves and other appropriate protective clothing (including masks). Reducing the risk of human-to-human transmission in the community arising from direct or close contact with infected patients, particularly with their body fluids. Close physical contact with Marburg patients should be avoided. Gloves and appropriate personal protective equipment should be worn when taking care of ill patients at home. Regular hand washing should be performed after visiting sick relatives in hospital, as well as after taking care of ill patients at home. Communities affected by Marburg should make efforts to ensure that the population is well informed, both about the nature of the disease itself and about necessary outbreak containment measures, including burial of the dead. People who have died from Marburg should be promptly and safely buried.
Controlling infection in health-care settings
Human-to-human transmission of Marburg virus is primarily associated with direct contact with blood and body fluids, and Marburg virus transmission associated with provision of health care has been reported when appropriate infection control measures have not been observed.
Health-care workers caring for patients with suspected or confirmed Marburg virus should apply infection control precautions to avoid any exposure to blood and body fluids and to direct unprotected contact with possibly contaminated environment. Therefore, provision of health care for suspected or confirmed Marburg patients requires specific control measures and reinforcement of standard precautions, particularly hand hygiene, use of personal protective equipment (PPE), safe injection practices, and safe burial practices.
Laboratory workers are also at risk. Samples taken from suspected human and animal Marburg cases for diagnosis should be handled by trained staff and processed in suitably equipped laboratories.
Secondary Prevention
- If a patient is either suspected or confirmed to have Marburg hemorrhagic fever, barrier nursing techniques should be used to prevent direct physical contact with the patient.
- Barrier nursing techniques include the following measures.
- Wearing of protective gowns, gloves, and masks
- Placing the infected individual in strict isolation
- Sterilization or proper disposal of needles, equipment, and patient excretions.
References
- ↑ http://www.who.int/mediacentre/factsheets/fs_marburg/en/
- ↑ Feldmann H, Slenczka W, Klenk HD (1996). "Emerging and reemerging of filoviruses". Arch Virol Suppl. 11: 77–100. PMID 8800808.
- ↑ "WHO". Missing or empty
|url=(help) - ↑ Smith DH, Johnson BK, Isaacson M, Swanapoel R, Johnson KM, Killey M; et al. (1982). "Marburg-virus disease in Kenya". Lancet. 1 (8276): 816–20. PMID 6122054.
- ↑ Mehedi M, Groseth A, Feldmann H, Ebihara H (2011). "Clinical aspects of Marburg hemorrhagic fever". Future Virol. 6 (9): 1091–1106. doi:10.2217/fvl.11.79. PMC 3201746. PMID 22046196.
- ↑ 6.0 6.1 6.2 "Marburg". Missing or empty
|url=(help) - ↑ Towner JS, Khristova ML, Sealy TK, Vincent MJ, Erickson BR, Bawiec DA; et al. (2006). "Marburgvirus genomics and association with a large hemorrhagic fever outbreak in Angola". J Virol. 80 (13): 6497–516. doi:10.1128/JVI.00069-06. PMC 1488971. PMID 16775337.
- ↑ Kuhn JH, Bao Y, Bavari S, Becker S, Bradfute S, Brister JR; et al. (2013). "Virus nomenclature below the species level: a standardized nomenclature for natural variants of viruses assigned to the family Filoviridae". Arch Virol. 158 (1): 301–11. doi:10.1007/s00705-012-1454-0. PMC 3535543. PMID 23001720.
- ↑ 9.0 9.1 "The Centers for Disease Control and Prevention".
- ↑ "http://phil.cdc.gov/phil/details.asp". External link in
|title=(help) - ↑ Becker S, Rinne C, Hofsäss U, Klenk HD, Mühlberger E (1998). "Interactions of Marburg virus nucleocapsid proteins". Virology. 249 (2): 406–17. doi:10.1006/viro.1998.9328. PMID 9791031.
- ↑ Borio L, Inglesby T, Peters CJ, Schmaljohn AL, Hughes JM, Jahrling PB; et al. (2002). "Hemorrhagic fever viruses as biological weapons: medical and public health management". JAMA. 287 (18): 2391–405. PMID 11988060.
- ↑ "WHO Guidelines For Epidemic Preparedness And Response: Ebola Haemorrhagic Fever".
- ↑ "CDC special pathogins branch- Marburg page". Retrieved 2007-05-03.
- ↑ "World Health Orginization - Report after final death 2004-2005 outbreak". Retrieved 2007-05-03.
Sources
WHO Fact sheet http://www.who.int/mediacentre/factsheets/fs_marburg/en/ The Centers for Disease Control and Prevention http://www.cdc.gov/vhf/marburg/index.html