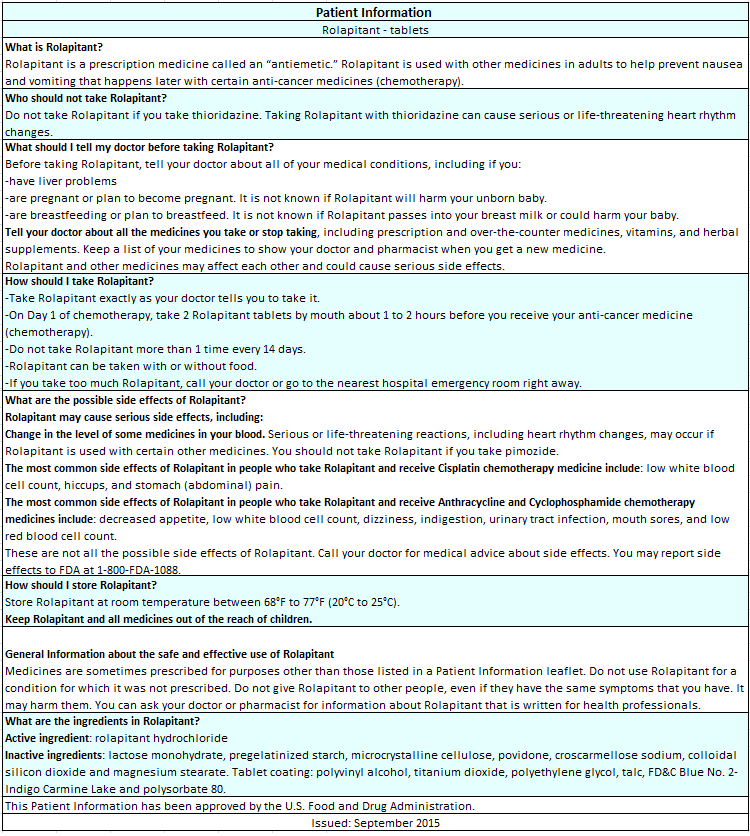
Rolapitant
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Martin Nino [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Rolapitant is a substance P/neurokinin 1 (NK1) receptor antagonist that is FDA approved for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy. Common adverse reactions include neutropenia, hiccups, decreased appetite and dizziness.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Rolapitant is indicated in combination with other antiemetic agents in adults for the prevention of delayed nausea and vomiting associated with initial and repeat courses of emetogenic cancer chemotherapy, including, but not limited to, highly emetogenic chemotherapy.
Dosage
- Prevention of Nausea and Vomiting Associated with Emetogenic Cancer Chemotherapy
The recommended dosage of rolapitant in adults in combination with a 5-HT3 receptor antagonist and dexamethasone is shown in TABLE 1. There is no drug interaction between rolapitant and dexamethasone, so no dosage adjustment for dexamethasone is required. Administer a dexamethasone dose of 20 mg on Day 1.
Administer rolapitant prior to the initiation of each chemotherapy cycle, but at no less than 2 week intervals.
Administer rolapitant without regards to meals.
- Table 1: Recommended Dosing Regimen

VARUBI: Rolapitant's Brand name
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rolapitant in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rolapitant in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and efficacy of rolapitant have not been established in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Rolapitant in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Rolapitant in pediatric patients.
Contraindications
Rolapitant is contraindicated in patients receiving thioridazine, a CYP2D6 substrate. A significant increase in plasma concentrations of thioridazine may result in QT prolongation and Torsades de Pointes.
Warnings
- Interaction with CYP2D6 Substrates with a Narrow Therapeutic Index
The inhibitory effect of rolapitant on CYP2D6 lasts at least 7 days and may last longer after a single dose administration of rolapitant. Avoid use of rolapitant in patients who are receiving pimozide, a CYP2D6 substrate. An increase in plasma concentrations of pimozide may result in QT prolongation. Monitor for adverse reactions if concomitant use of rolapitant and other CYP2D6 substrates with a narrow therapeutic index cannot be avoided.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The safety of rolapitant was evaluated in approximately 2800 patients in 4 controlled clinical trials in patients receiving emetogenic cancer chemotherapy. Rolapitant was given in combination with a 5-HT3 receptor antagonist and dexamethasone. On Day 1 of Cycle 1 of chemotherapy, 1567 patients were treated with rolapitant and 1198 of these patients continued into the optional multiple cycle extension for up to 6 cycles of chemotherapy. The median number of cycles administered 180 mg of rolapitant was four. Rolapitant 180 mg was administered to 1294 patients.
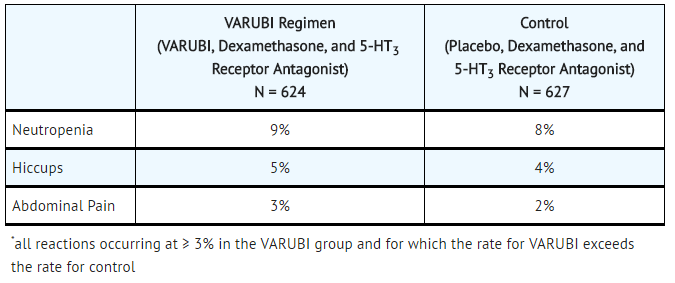
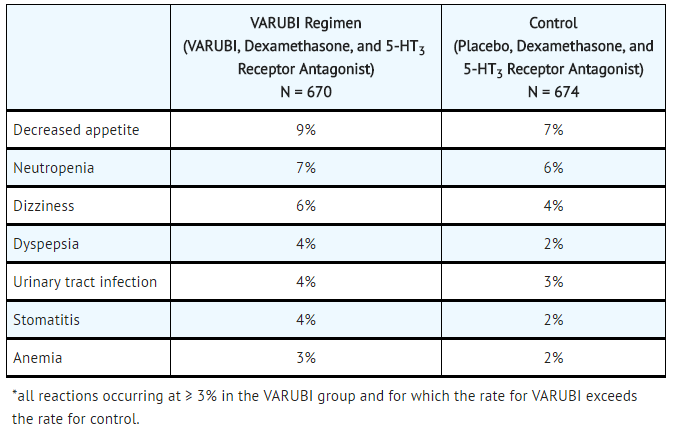
In Cycle 1 adverse reactions were reported in approximately 7% of patients treated with rolapitant compared with approximately 6% of patients treated with control therapy. The most common adverse reactions reported with an incidence of ≥3% and greater than control are listed in TABLE 2 and TABLE 3.
- Table 2: Most Common Adverse Reactions in Patients Receiving Cisplatin-Based Highly Emetogenic Chemotherapy (Cycle 1)*
VARUBI : Rolapitant's Brand name
- Table 3: Most Common Adverse Reactions in Patients Receiving Moderately Emetogenic Chemotherapy and Combinations of Anthracycline and Cyclophosphamide (Cycle 1)*
VARUBI : Rolapitant's Brand name
Adverse reactions in the multiple-cycle extensions of highly and moderately emetogenic chemotherapy studies for up to 6 cycles of chemotherapy were generally similar to that observed in Cycle 1.
Postmarketing Experience
There is limited information regarding Rolapitant Postmarketing Experience in the drug label.
Drug Interactions
Effect of rolapitant on Other Drugs
Rolapitant is not an inhibitor or inducer of CYP3A4. Therefore, no dosage adjustment for dexamethasone (CYP3A4 substrate) is needed when co-administered with rolapitant.
Rolapitant is a moderate CYP2D6 inhibitor, an inhibitor of Breast-Cancer-Resistance Protein (BCRP) and an inhibitor of P-glycoprotein (P-gp).
- CYP2D6 Substrates with a Narrow Therapeutic Index: Increased plasma concentration of CYP2D6 substrates may result in potential adverse reactions. A 3-fold increase in the exposure of dextromethorphan, a CYP2D6 substrate, was observed 7 days after a single dose of rolapitant. The duration of CYP2D6 inhibition was not studied beyond 7 days and may last longer. Concomitant use with Thioridazine is contraindicated. Avoid use of rolapitant with pimozide. Monitor for QT prolongation if concomitant use with pimozide cannot be avoided. Monitor for adverse reactions if concomitant use with CYP2D6 substrates with a narrow therapeutic index cannot be avoided.
- BCRP Substrates with a Narrow Therapeutic Index (e.g., Methotrexate, topotecan, or irinotecan): Increased plasma concentrations of BCRP substrates may result in potential adverse reactions. Monitor for adverse reactions related to the concomitant drug if use of rolapitant cannot be avoided. Use the lowest effective dose of rosuvastatin.
- P-gp Substrates with a Narrow Therapeutic Index: Increased plasma concentrations of digoxin, or other P-gp substrates, may result in potential adverse reactions. Monitor for increased digoxin concentrations. Monitor for adverse reactions if concomitant use of rolapitant with other P-gp substrates with a narrow therapeutic index cannot be avoided.
Effect of Other Drugs on rolapitant
Strong CYP3A4 Inducers (e.g., rifampin): significantly reduced plasma concentrations of rolapitant can decrease the efficacy of rolapitant; avoid use of rolapitant in patients who require chronic administration of such drugs.
Use in Specific Populations
Pregnancy
- Risk Summary
There are no available data on rolapitant use in pregnant women to inform any drug-associated risks. In animal reproduction studies, there were no teratogenic or embryo-fetal effects observed with oral administration of rolapitant hydrochloride in rats and rabbits during the period of organogenesis at doses up to 1.2 times and 2.9 times, respectively, the maximum recommended human dose (MRHD). In the U.S. general population, the estimated background risk of major birth defects and miscarriage in clinically recognized pregnancies is 2% to 4% and 15% to 20%, respectively.
- Data
- Animal Data
The potential embryo-fetal toxicity of rolapitant hydrochloride was assessed in pregnant rats administered oral doses equivalent to up to 22.5 mg/kg per day rolapitant free base throughout organogenesis. Rats administered doses equivalent to 13.5 or 22.5 mg/kg per day rolapitant free base exhibited evidence of maternal toxicity including decreased body weight gain and/or body weight loss and a concomitant decrease in food consumption during the first week of dosing. No teratogenic or embryo-fetal effects were observed at doses equivalent to up to 22.5 mg/kg per day rolapitant free base (approximately 1.2 times the recommended human dose on a body surface area basis). In rabbits administered rolapitant hydrochloride throughout the period of organogenesis, oral doses equivalent to up to 27 mg/kg per day rolapitant free base (approximately 2.9 times the recommended human dose on a body surface area basis) were without effects on the developing fetus.
The pre- and postnatal developmental effects of rolapitant hydrochloride were assessed in rats administered oral doses equivalent to 2.25, 9 or 22.5 mg/kg per day rolapitant free base during the periods of organogenesis and lactation. Maternal toxicity was evident based on mortality/moribund condition, decreased body weight and food consumption, total litter loss, prolonged parturition, decreased length of gestation, and increased number of unaccounted for implantation sites at a dose equivalent to 22.5 mg/kg per day free base (approximately 1.2 times the recommended human dose on a body surface area basis). Effects on offspring at this dose included decreased postnatal survival, and decreased body weights and body weight gain, and may be related to the maternal toxicity observed. At a maternal dose equivalent to 9 mg/kg per day rolapitant free base (approximately 0.5 times the recommended human dose on a body surface area basis), there was a decrease in memory in female pups in a maze test and a decrease in pup body weight.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Rolapitant in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Rolapitant during labor and delivery.
Nursing Mothers
- Risk Summary
There are no data on the presence of rolapitant in human milk, the effects of rolapitant in the breastfed infant, or the effects of rolapitant on milk production. Rolapitant hydrochloride administered orally to lactating female rats was present in milk. The developmental and health benefits of breastfeeding should be considered along with the mother’s clinical need for rolapitant and any potential adverse effects on the breastfed infant from rolapitant or from the underlying maternal condition or the use of concomitant chemotherapy.
- Data
Radioactivity from labeled [14C] rolapitant hydrochloride was transferred into milk of lactating rats following a single oral dose equivalent to 22.5 mg/kg rolapitant free base, and the maximum radioactivity in milk was observed at 12 hours post-dose. The mean milk/plasma radioactivity concentration ratios in dams at 1 to 48 hours post-dose ranged from 1.24 to 3.25. Based on average daily consumption of milk (2 mL/day) and the maximum milk radioactivity determined, pup exposure is expected to be 0.32% of the orally administered dose.
Pediatric Use
Safety and efficacy of rolapitant have not been established in pediatric patients.
Geriatic Use
Of the 1294 subjects treated with rolapitant, 25% were 65 years and over, while 5% were 75 and over. No overall differences in safety or efficacy were reported between the elderly subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Rolapitant with respect to specific gender populations.
Race
There is no FDA guidance on the use of Rolapitant with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Rolapitant in patients with renal impairment.
Hepatic Impairment
No dosage adjustment is needed in patients with mild (Child-Pugh Class A) or moderate (Child-Pugh Class B) hepatic impairment. There are no clinical or pharmacokinetic data in patients with severe hepatic impairment (Child-Pugh Class C). Avoid use of rolapitant in patients with severe hepatic impairment. If use cannot be avoided, monitor patients for adverse reactions related to rolapitant.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Rolapitant in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Rolapitant in patients who are immunocompromised.
Administration and Monitoring
Administration
- Prevention of Nausea and Vomiting Associated with Emetogenic Cancer Chemotherapy
The recommended dosage of rolapitant in adults in combination with a 5-HT3 receptor antagonist and dexamethasone is shown in TABLE 1. There is no drug interaction between rolapitant and dexamethasone, so no dosage adjustment for dexamethasone is required. Administer a dexamethasone dose of 20 mg on Day 1.
Administer rolapitant prior to the initiation of each chemotherapy cycle, but at no less than 2 week intervals.
Administer rolapitant without regards to meals.
Monitoring
There is limited information regarding Rolapitant Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Rolapitant and IV administrations.
Overdosage
- There are no data on overdose with rolapitant.
- There is no antidote for rolapitant overdose. Discontinue rolapitant in the event of overdose, and institute general supportive measures and close observation.
Pharmacology
| |
Rolapitant
| |
| Systematic (IUPAC) name | |
| (5S,8S)-8-({(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy}methyl)- 8-phenyl-1,7-diazaspiro[4.5]decan-2-one | |
| Identifiers | |
| CAS number | |
| ATC code | A04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 500.476 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Protein binding | 99.8% |
| Metabolism | CYP3A4 |
| Half life | 169–183 hours |
| Excretion | Feces (52–89%), urine (9–20%)[1] |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | By mouth (tablets) |
Mechanism of Action
Rolapitant is a selective and competitive antagonist of human substance P/NK1 receptors. Rolapitant does not have significant affinity for the NK2 or NK3 receptors or for a battery of other receptors, transporters, enzymes and ion channels. Rolapitant is also active in animal models of chemotherapy-induced emesis.
Structure
Rolapitant hydrochloride is chemically described as (5S,8S)-8- { [(1R)-1-[3,5-bis(trifluoromethyl)phenyl]ethoxy]methyl]}-8-phenyl 1,7-diazaspiro[4.5]decan-2-one monohydrochloride monohydrate. Its empirical formula is C25H26F6N2O2. HCl.H2O and its structural formula is:
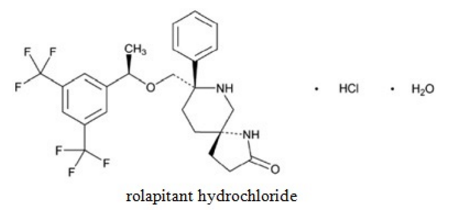
Rolapitant hydrochloride is a white to off-white powder, with a molecular weight of 554.95. Solubility of rolapitant hydrochloride in aqueous solution is pH-dependent and is more soluble at lower pH. Rolapitant hydrochloride has good solubility in common pharmaceutical solvents such as ethanol, propylene glycol, and 40% hydroxypropyl beta cyclodextrin.
Each tablet for oral administration contains 90 mg rolapitant and the following inactive ingredients: lactose monohydrate, pregelatinized starch, microcrystalline cellulose, povidone, croscarmellose sodium, colloidal silicon dioxide and magnesium stearate. The tablets are coated in non-functional blue and clear coats. The tablet coating comprises the following inactive ingredients: polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, FD&C Blue No. 2- Indigo Carmine Lake and polysorbate 80.
Pharmacodynamics
- NK1 Receptor Occupancy
A human Positron Emission Tomography (PET) study with rolapitant demonstrated that rolapitant crosses the blood brain barrier and occupies brain NK1 receptors. A dose-dependent increase in mean NK1 receptor occupancy was observed in the dose range from 4.5 mg to 180 mg of rolapitant. At the 180 mg dose of rolapitant, the mean NK1 receptor occupancy was 73% in the striatum at 120 hours after a single dose administration in healthy subjects. The relationship between NK1 receptor occupancy and the clinical efficacy of rolapitant has not been established.
In a thorough QT study, rolapitant at doses up to 4 times higher than the recommended dose had no significant effects on the QT intervals.
Pharmacokinetics
Following a single dose administration of 180 mg rolapitant under fasting conditions to healthy subjects, rolapitant was measurable in plasma between 30 minutes and the peak plasma concentration (Cmax) for rolapitant was reached in about 4 hours and mean Cmax was 968 ng/mL (%CV:28%).
Following multiple oral doses 9 to 45 mg once daily of rolapitant, accumulation of rolapitant was approximately 5-fold.
The systemic exposures (Cmax and AUC) to rolapitant increased in a dose-proportional manner when the dose of rolapitant increased from 4.5 mg to 180 mg. With an increase in dose by 4 times from the recommended clinical dose of 180 mg, the Cmax and AUC of rolapitant increased by 3.1 fold and 3.7 fold, respectively.
Concomitant administration of a high fat meal did not significantly affect the pharmacokinetics of rolapitant after administration of 180 mg rolapitant.
Rolapitant was highly protein bound to human plasma (99.8%). The apparent volume of distribution (Vd/F) was 460 L in healthy subjects, indicating an extensive tissue distribution of rolapitant. In a population pharmacokinetic analysis of rolapitant, the Vd/F was 387 L in cancer patients.
Following single oral doses (4.5 to 180 mg) of rolapitant, the mean terminal half-life (t1/2) of rolapitant ranged from 169 to 183 hours (approximately 7 days) and was independent of dose. In a population pharmacokinetic analysis the apparent total clearance (CL/F) of rolapitant was 0.96 L/hour in cancer patients.
Rolapitant is metabolized primarily by CYP3A4 to form a major active metabolite, M19 (C4- pyrrolidine-hydroxylated rolapitant). In a mass balance study, the metabolite M19 was the major circulating metabolite. The formation of M19 was significantly delayed with the median Tmax of 120 hours (range: 24-168 hours) and the mean half-life of M19 was 158 hours.
The exposure ratio of M19 to rolapitant was approximately 50% in plasma.
Rolapitant is eliminated primarily through the hepatic/biliary route. Following administration of a single oral 180-mg dose of [14C]-rolapitant, on average 14.2% (range 9% to 20%) and 73% (range 52% to 89%) of the dose was recovered in the urine and feces, respectively over 6 weeks. In pooled samples collected over 2 weeks, 8.3% of the dose was recovered in the urine primarily as metabolites and 37.8% of the dose was recovered in the feces primarily as unchanged rolapitant. Unchanged rolapitant or M19 were not found in pooled urine sample.
- Specific Populations
- Age, Sex and Race/Ethnicity
Population pharmacokinetic analyses indicated that age, sex and race had no significant impact on the pharmacokinetics of rolapitant.
Following administration of a single dose of 180 mg rolapitant to patients with mild hepatic impairment (Child-Pugh Class A), the pharmacokinetics of rolapitant were comparable with those of healthy subjects. In patients with moderate hepatic impairment (Child-Pugh Class B), the mean Cmax was 25% lower while mean AUC of rolapitant was similar compared to those of healthy subjects. The median Tmax for M19 was delayed to 204 hours in patients with mild or moderate hepatic impairment compared to 168 hours in healthy subjects. The pharmacokinetics of rolapitant was not studied in patients with severe hepatic impairment (Child-Pugh Class C).
In population pharmacokinetic analyses, creatinine clearance (CLcr) at baseline did not show a significant effect on rolapitant pharmacokinetics in cancer patients with mild (CLcr: 60 to 90 mL/min) or moderate (CLcr: 30 to 60 mL/min) renal impairment compared to cancer patients with normal kidney function. Information is insufficient for the effect of severe renal impairment. The pharmacokinetics of rolapitant was not studied in patients with end-stage renal disease requiring hemodialysis.
- Drug Interaction Studies
- Effect of Other Drugs on rolapitant
Rolapitant is a substrate for CYP3A4.
- CYP3A4 inducers
Concomitant administration of a CYP3A4 inducer significantly decreased the systemic exposure to rolapitant. When 600 mg rifampin was administered once daily for 7 days before and 7 days after administration of a single dose of 180 mg rolapitant , the mean Cmax of rolapitant was reduced by 30% and the mean AUC was reduced by 85% compared to administration of rolapitant alone. The mean half-life of rolapitant decreased from 176 hours without rifampin to 41 hours with concurrent rifampin.
- CYP3A4 inhibitors
No clinically significant effect was seen on the pharmacokinetics of rolapitant when ketoconazole, a strong CYP3A4 inhibitor was administered with rolapitant. Concurrent administration of 400 mg ketoconazole once daily for 21 days following a single 90 mg dose of rolapitant , did not significantly affect the Cmax of rolapitant while the AUC increased by 21%.
- Effect of rolapitant on Other Drugs
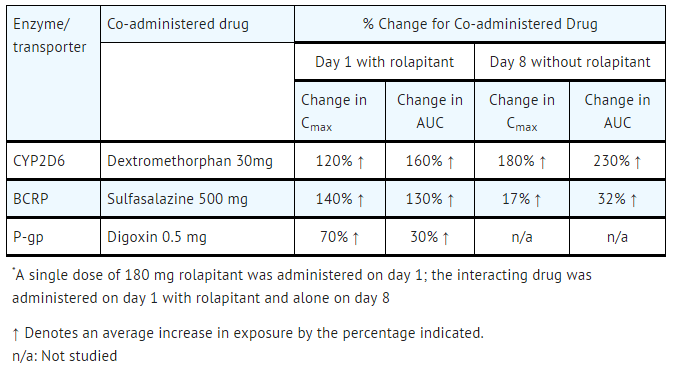
The effect of rolapitant on CYP450 enzymes and transporters is summarized below. See TABLE 4 for a summary of the effects of the clinical dose of rolapitant on the pharmacokinetics of co- administered drugs.
- CYP3A4 substrates
Rolapitant is neither an inhibitor nor an inducer of CYP3A4.
Midazolam: A single dose of 180 mg rolapitant had no significant effects on the pharmacokinetics of midazolam when oral midazolam 3 mg was co-administered on Day 1 and administered alone on Days 6, and 9.
Ondansetron: Rolapitant had no significant effects on the pharmacokinetics of intravenous ondansetron when concomitantly administered with a single 180 mg dose of rolapitant on the same day.
Dexamethasone: Rolapitant had no significant effects on the pharmacokinetics of dexamethasone when oral dexamethasone was administered on Days 1 to 3 after a single 180 mg dose of rolapitant was co-administered on Day 1.
- CYP2D6 substrates
Rolapitant is a moderate inhibitor of CYP2D6.
- BCRP transporter
Rolapitant is an inhibitor of BCRP transporter.
- P-glycoprotein substrates
Rolapitant is an inhibitor of P-gp transporter.
In vitro studies suggest that rolapitant is not an inhibitor of CYP1A2 and CYP2E1. A clinically meaningful drug interaction via an inhibition of CYP2A6 appears unlikely based on in vitro study.
No clinically significant interaction was seen on the pharmacokinetics of the following drugs when administered with a single dose of 180 mg rolapitant on Day 1 and without rolapitant on Day 8: repaglinide (CYP2C8 substrate; no effect on repaglinide 0.25 mg on Day 1; on Day 8 : 29% and 24% increase in Cmax and AUC, respectively), efavirenz (CYP2B6 substrate; 18% decrease in Cmax and no effect on AUC of efavirenz 600 mg on Day 1; on Day 8: no effect on Cmax and 28% increase in AUC), tolbutamide (CYP2C9 substrate; no effect on tolbutamide 500 mg on Day 1 and on Day 8), or omeprazole (CYP2C19 substrate; 44% increase in Cmax and 23% increase in AUC of omeprazole 40 mg on Day 1; on Day 8: 37% and 15% increase in Cmax and AUC, respectively).
- Table 4: Effect of Rolapitant on the Pharmacokinetics of Co-administered Drugs*
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Carcinogenic potential of rolapitant hydrochloride was assessed in 2-year carcinogenicity studies in CD-1 mice and Sprague-Dawley rats. In mice, there were no drug-related neoplastic findings at doses equivalent to up to 135 mg/kg per day rolapitant free base (approximately 3.6 times the recommended human dose on a body surface area basis). In rats, there were no drug-related neoplastic findings at doses equivalent to up to 90 mg/kg per day rolapitant free base (approximately 4.9 times the recommended human dose on a body surface area basis).
Rolapitant hydrochloride was not genotoxic in an Ames test, a human peripheral blood lymphocyte chromosome aberration test, and a mouse micronucleus test.
In a fertility and early embryonic development study in female rats, rolapitant hydrochloride at an oral dose equivalent to 9 mg/kg per day free base (approximately 0.5 times the recommended human dose on a body surface area basis) caused a transient decrease in maternal body weight gain and increases in the incidence of pre- and post-implantation loss. At a dose equivalent to 4.5 mg/kg per day free base (approximately 0.2 times the recommended human dose on a body surface area basis), there were slight decreases in the number of corpora lutea and implantation sites. Rolapitant hydrochloride did not affect the fertility or general reproductive performance of male rats at doses equivalent to up to 90 mg/kg per day rolapitant free base (approximately 4.9 times the recommended human dose on a body surface area basis).
Clinical Studies
- Cisplatin-Based Highly Emetogenic Chemotherapy (HEC)
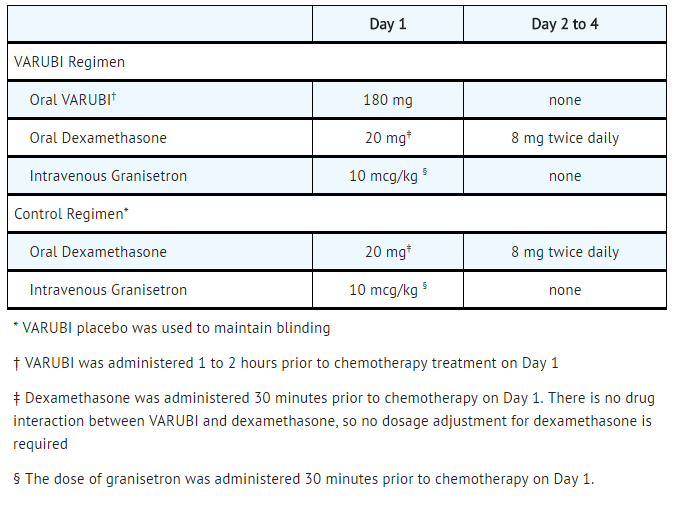
In two multicenter, randomized, double-blind, parallel group, controlled clinical studies (Study 1 and Study 2), the rolapitant regimen (rolapitant, granisetron and dexamethasone) was compared with control therapy (placebo, granisetron and dexamethasone) in patients receiving a chemotherapy regimen that included cisplatin >60 mg/m2. See TABLE 5 for the treatment regimens.
- Table 5: Treatment Regimens in Studies 1 and 2
VARUBI : Rolapitant's Brand name
- Study 1
A total of 532 patients were randomized to either the rolapitant regimen (N =266) or control therapy (N =266). A total of 526 patients were included in the evaluation of efficacy. Of those randomized 42% were women, 58 % men, 67% White, 23% Asian, 1% Black, and 9% multi racial/other/unknown. The proportion of patients from North America was 16%. Patients in this clinical study ranged from 20 to 90 years of age, with a mean age of 57 years. In Study 1, 26% of patients were 65 years or older, with 3% of patients being 75 years or older. The mean cisplatin dose was 77 mg/m2.
During this study, 82% of the patients received a concomitant chemotherapeutic agent in addition to protocol-mandated cisplatin. The most common concomitant chemotherapeutic agents administered during Cycle 1 were: gemcitabine (17%), paclitaxel (12%), fluorouracil (11%), etoposide (10%), vinorelbine (9%), docetaxel (9%), pemetrexed (7%), doxorubicin (6%) and cyclophosphamide (5%).
- Study 2
A total of 555 patients were randomized to either the rolapitant regimen (N =278) or control therapy (N =277). A total of 544 patients were included in the evaluation of efficacy. Of those randomized, 32% were women, 68 % men, 81% White, 14% Asian, 1% Black, and 5% multi racial/other/unknown. The proportion of patients from North America was 7%. Patients in this clinical study ranged from 18 to 83 years of age, with a mean age of 58 years. In this study, 27% of patients were 65 years or older, with 3% of patients being 75 years or older. The mean cisplatin dose was 76 mg/m2.
During this study, 85% of the patients received a concomitant chemotherapeutic agent in addition to protocol-mandated cisplatin. The most common concomitant chemotherapeutic agents administered during Cycle 1 were: vinrorelbine (16%), gemcitabine (15%), fluorouracil (12%), etoposide (11%), pemetrexed (9%), docetaxel (7%), paclitaxel (7%), epirubicin (5%) and capecitabine (4%).
The primary endpoint in both studies was complete response (defined as no emetic episodes and no rescue medication) in the delayed phase (25 to 120 hours) of chemotherapy-induced nausea and vomiting.
- Moderately Emetogenic Chemotherapy (MEC) and Combinations of Anthracycline and Cyclophosphamide Chemotherapy
- Study 3
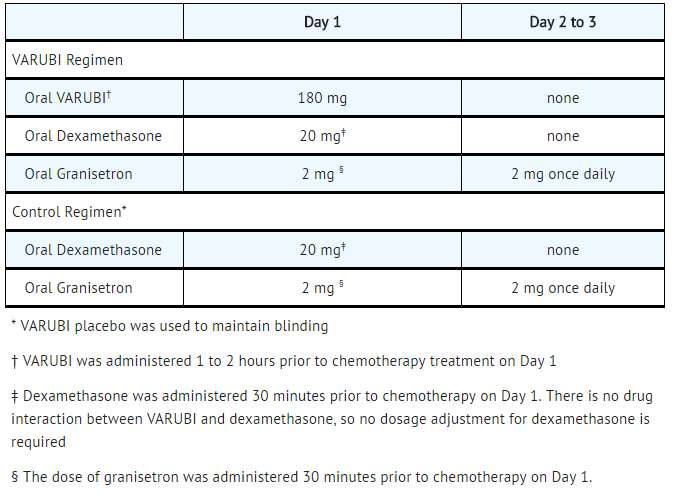
In Study 3, a multicenter, randomized, double-blind, parallel group, controlled clinical study in moderately emetogenic chemotherapy, the rolapitant regimen (rolapitant, granisetron and dexamethasone) was compared with control therapy (placebo, granisetron and dexamethasone) in patients receiving a moderately emetogenic chemotherapy regimen that included at least 50% of patients receiving a combination of anthracycline and cyclophosphamide. The percentage of patients who received carboplatin in Cycle 1 was 30%. Treatment regimens for the rolapitant and control arms are summarized in TABLE 6.
- Table 6: Treatment Regimens in Study 3
VARUBI : Rolapitant's Brand name
A total of 1369 patients were randomized to either the rolapitant regimen (N = 684) or control therapy (N = 685). A total of 1332 patients were included in the evaluation of efficacy. Of those randomized 80% were women, 20% men, 77% White, 13% Asian, 4% Black, and 6% multi- racial/other/unknown. The proportion of patients from North America was 33%. Patients in this clinical study ranged from 22 to 88 years of age, with a mean age of 57 years. In this study, 28% of patients were 65 years or older, with 7% of patients being 75 years or older.
The primary endpoint was complete response (defined as no emetic episodes and no rescue medication) in the delayed phase (25 to 120 hours) of chemotherapy-induced nausea and vomiting.
A summary of the study results from HEC Studies 1 and 2, and for the MEC Study 3 is shown in TABLE 7.
- Table 7: Percent of Patients Receiving Emetogenic Chemotherapy Responding by Treatment Group for the HEC Studies 1 and 2 and for the MEC Study 3

VARUBI : Rolapitant's Brand name
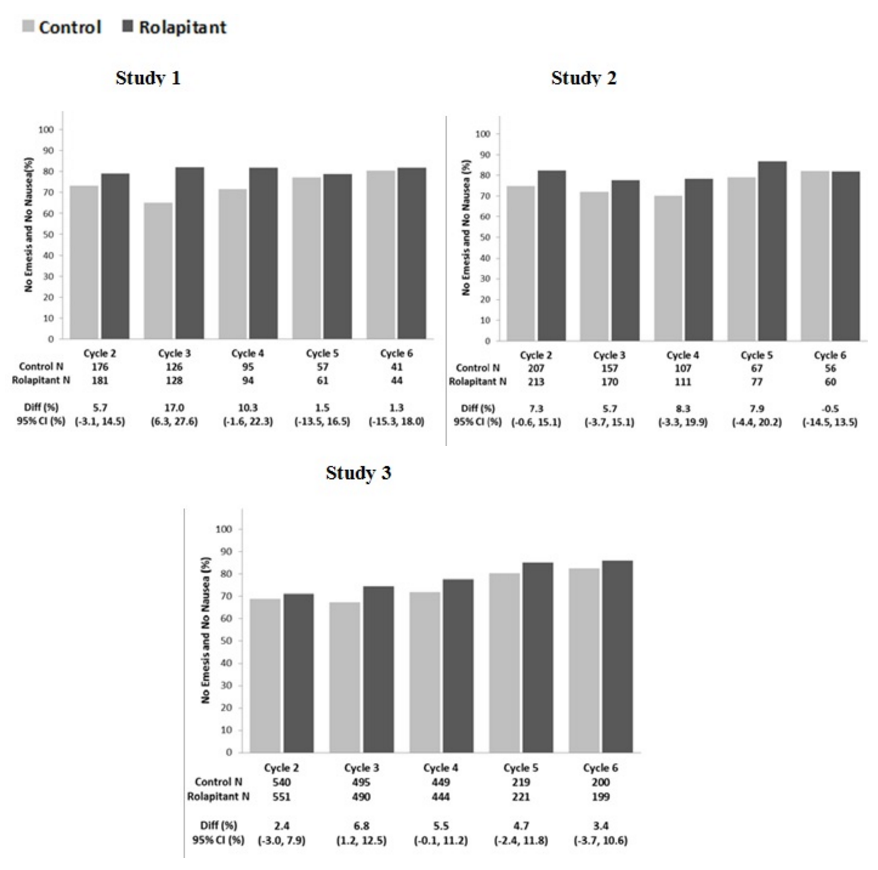
Multiple-Cycle Extension: In Studies 1, 2, and 3, patients had the option of continuing into a multiple-cycle extension for up to 5 additional cycles of chemotherapy receiving the same treatment as assigned in cycle 1. At day 6 to 8 following initiation of chemotherapy, patients were asked to recall whether they had any episode of vomiting or retching or nausea that interfered with normal daily life. The results are summarized by study and treatment group in the figure below.
- Figure 1: No Emesis and No Nausea Interfering with Daily Life over Cycles 2-6
How Supplied
Rolapitant is available as film-coated, capsule shaped, blue tablets, debossed with T0101 on one side and 100 on the other side. Each tablet contains 90 mg rolapitant. Rolapitant tablets are packaged in an Aclar blister shell with aluminum foil backing and supplied as follows:
NDC 69656-101-02 A single dose package (2 tablets as one set of twinned blisters)
Storage
Store at 20°C to 25°C (68°F to 77°F); excursions are permitted between 15°C to 30°C (59°F to 86°F)
Images
Drug Images
{{#ask: Page Name::Rolapitant |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Rolapitant |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Advise the patient to read the FDA-approved patient labeling.
- Drug Interactions
Advise patients to tell their healthcare provider when they start or stop taking any concomitant medications. Rolapitant is a moderate CYP2D6 inhibitor and can increase plasma concentrations of CYP2D6 substrates if they are co-administered. The inhibitory effect of rolapitant on CYP2D6 lasts at least 7 days and may last longer than 7 days after a single dose.
Precautions with Alcohol
Alcohol-Rolapitant interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
VARUBI™
Look-Alike Drug Names
There is limited information regarding Rolapitant Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "Varubi (rolapitant) Tablets, for Oral Use. Full Prescribing Information" (PDF). TESARO, Inc. 1000 Winter St., #3300, Waltham, MA 02451.