Omalizumab
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Deepika Beereddy, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: ANAPHYLAXIS
See full prescribing information for complete Boxed Warning.
Anaphylaxis presenting as bronchospasm, hypotension, syncope, urticaria, and/or angioedema of the throat or tongue, has been reported to occur after administration of Xolair. Anaphylaxis has occurred as early as after the first dose of Xolair, but also has occurred beyond 1 year after beginning regularly administered treatment. Because of the risk of anaphylaxis, observe patients closely for an appropriate period of time after Xolair administration. Health care providers administering Xolair should be prepared to manage anaphylaxis that can be life-threatening. Inform patients of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care should symptoms occur.
|
Overview
Omalizumab is an anti-allergic and anti-asthmatic agent that is FDA approved for the treatment of allergic asthma and chronic idiopathic urticaria. There is a Black Box Warning for this drug as shown here. Common adverse reactions include arthralgia, pain (general), leg pain, fatigue, dizziness, fracture, arm pain, pruritus, dermatitis, earache, nausea, nasopharyngitis, sinusitis, upper respiratory tract infection, viral upper respiratory tract infection, headache, and cough.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Allergic Asthma
- Dosing Information
- Administer Xolair 150 to 375 mg by subcutaneous (SC) injection every 2 or 4 weeks. Determine doses (mg) and dosing frequency by serum total IgE level (IU/mL), measured before the start of treatment, and body weight (kg). See the dose determination charts below (Table 1 and Table 2) for appropriate dose assignment.
- Periodically reassess the need for continued therapy based upon the patient's disease severity and level of asthma control.

Dosing adjustments for Allergic Asthma
- Adjust doses for significant changes in body weight (see Table 1 and Table 2).
- Total IgE levels are elevated during treatment and remain elevated for up to one year after the discontinuation of treatment. Therefore, re-testing of IgE levels during Xolair treatment cannot be used as a guide for dose determination.
- Interruptions lasting less than one year: Dose based on serum IgE levels obtained at the initial dose determination.
- Interruptions lasting one year or more: Re-test total serum IgE levels for dose determination.
Chronic Idiopathic Urticaria
- Dosing Information
- Administer Xolair 150 or 300 mg by subcutaneous injection every 4 weeks.
- Dosing of Xolair in CIU patients is not dependent on serum IgE (free or total) level or body weight.
- The appropriate duration of therapy for CIU has not been evaluated. Periodically reassess the need for continued therapy.
Dosage forms and strengths
- 150 mg of omalizumab as lyophilized, sterile powder in a single-use 5 mL vial.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Omalizumab in adult patients.
Non–Guideline-Supported Use
Prophylaxis of Allergic Rhinitis
- Dosing Information
- Doses of 150 or 300 mg every 3 to 4 weeks have been effective in adults and children with seasonal allergic rhinitis.
Allergy to Peanuts
- Dosing Information
- Omalizumab 450 mg significantly increased the threshold of peanut sensitivity following an oral food challenge, compared with placebo in patients (12 to 60 years) with a history of peanut allergy in a randomized study.
Latex Allergy
- Dosing Information
- In a small randomized trial, omalizumab therapy was effective in reducing clinical symptoms of latex allergy compared with placebo in healthcare workers exposed to latex on a daily basis. Omalizumab was dosed according to body weight and serum IgE levels and ranged from 150 to 750 mg monthly.
Subcutaneous Immunotherapy
- Dosing Information
- The dose of omalizumab is 0.016 mg/kg/IgE (international units/mL)/month subQ every 2 to 4 weeks, depending on weight and baseline IgE levels.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Xolair is not indicated for use in pediatric patients less than 12 years of age.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Omalizumab in pediatric patients.
Non–Guideline-Supported Use
Prophylaxis of Allergic Rhinitis
- Dosing Information
- Doses of 150 or 300 mg every 3 to 4 weeks have been effective in children with seasonal allergic rhinitis.
Allergy to Peanuts
- Dosing Information
- Omalizumab 450 mg significantly increased the threshold of peanut sensitivity following an oral food challenge, compared with placebo in patients (12 to 60 years) with a history of peanut allergy in a randomized study.
Latex Allergy
- There is limited information regarding Off-Label Non-Guideline-Supported use of Omalizumab for Latex Allergy in pediatric patients.
Subcutaneous Immunotherapy
- Dosing Information
- Preseasonal treatment with omalizumab in children and adolescents with seasonal allergic rhinitis decreases symptomatic days and rescue medication use. The dose of omalizumab was 0.016 mg/kg/IgE (international units/mL/month).
Contraindications
The use of Xolair is contraindicated in the following: Severe hypersensitivity reaction to Xolair or any ingredient of Xolair.
Warnings
|
WARNING: ANAPHYLAXIS
See full prescribing information for complete Boxed Warning.
Anaphylaxis presenting as bronchospasm, hypotension, syncope, urticaria, and/or angioedema of the throat or tongue, has been reported to occur after administration of Xolair. Anaphylaxis has occurred as early as after the first dose of Xolair, but also has occurred beyond 1 year after beginning regularly administered treatment. Because of the risk of anaphylaxis, observe patients closely for an appropriate period of time after Xolair administration. Health care providers administering Xolair should be prepared to manage anaphylaxis that can be life-threatening. Inform patients of the signs and symptoms of anaphylaxis and instruct them to seek immediate medical care should symptoms occur.
|
Anaphylaxis
Anaphylaxis has been reported to occur after administration of Xolair in premarketing clinical trials and in postmarketing spontaneous reports. Signs and symptoms in these reported cases have included bronchospasm, hypotension, syncope, urticaria, and/or angioedema of the throat or tongue. Some of these events have been life-threatening. In premarketing clinical trials in allergic asthma, anaphylaxis was reported in 3 of 3507 (0.1%) patients in clinical trials. Anaphylaxis occurred with the first dose of Xolair in two patients and with the fourth dose in one patient. The time to onset of anaphylaxis was 90 minutes after administration in two patients and 2 hours after administration in one patient. In postmarketing spontaneous reports, the frequency of anaphylaxis attributed to Xolair use was estimated to be at least 0.2% of patients based on an estimated exposure of about 57,300 patients from June 2003 through December 2006. Anaphylaxis has occurred as early as after the first dose of Xolair, but also has occurred beyond one year after beginning regularly scheduled treatment.
Administer Xolair only in a healthcare setting by healthcare providers prepared to manage anaphylaxis that can be life-threatening. Observe patients closely for an appropriate period of time after administration of Xolair, taking into account the time to onset of anaphylaxis seen in premarketing clinical trials and postmarketing spontaneous reports. Inform patients of the signs and symptoms of anaphylaxis, and instruct them to seek immediate medical care should signs or symptoms occur.
Discontinue Xolair in patients who experience a severe hypersensitivity reaction.
Malignancy
Malignant neoplasms were observed in 20 of 4127 (0.5%) Xolair-treated patients compared with 5 of 2236 (0.2%) control patients in clinical studies of adults and adolescents (≥ 12 years of age) with asthma and other allergic disorders. The observed malignancies in Xolair-treated patients were a variety of types, with breast, non-melanoma skin, prostate, melanoma, and parotid occurring more than once, and five other types occurring once each. The majority of patients were observed for less than 1 year. The impact of longer exposure to Xolair or use in patients at higher risk for malignancy (e.g., elderly, current smokers) is not known.
Acute Asthma Symptoms
Xolair has not been shown to alleviate asthma exacerbations acutely. Do not use Xolair to treat acute bronchospasm or status asthmaticus.
Corticosteroid reduction
Do not discontinue systemic or inhaled corticosteroids abruptly upon initiation of Xolair therapy for allergic asthma. Decrease corticosteroids gradually under the direct supervision of a physician. In CIU patients, the use of Xolair in combination with corticosteroids has not been evaluated.
Eosiophilic Conditions
In rare cases, patients with asthma on therapy with Xolair may present with serious systemic eosinophilia sometimes presenting with clinical features of vasculitis consistent with Churg-Strauss syndrome, a condition which is often treated with systemic corticosteroid therapy. These events usually, but not always, have been associated with the reduction of oral corticosteroid therapy. Physicians should be alert to eosinophilia, vasculitic rash, worsening pulmonary symptoms, cardiac complications, and/or neuropathy presenting in their patients. A causal association between Xolair and these underlying conditions has not been established.
Fever, Arthralgia and Rash
In post-approval use, some patients have experienced a constellation of signs and symptoms including arthritis/arthralgia, rash, fever and lymphadenopathy with an onset 1 to 5 days after the first or subsequent injections of Xolair. These signs and symptoms have recurred after additional doses in some patients. Although circulating immune complexes or a skin biopsy consistent with a Type III reaction were not seen with these cases, these signs and symptoms are similar to those seen in patients with serum sickness. Physicians should stop Xolair if a patient develops this constellation of signs and symptoms.
Parasitic (Helminth) infection
Monitor patients at high risk of geohelminth infection while on Xolair therapy. Insufficient data are available to determine the length of monitoring required for geohelminth infections after stopping Xolair treatment. In a one-year clinical trial conducted in Brazil in patients at high risk for geohelminthic infections (roundworm, hookworm, whipworm, threadworm), 53% (36/68) of Xolair-treated patients experienced an infection, as diagnosed by standard stool examination, compared to 42% (29/69) of placebo controls. The point estimate of the odds ratio for infection was 1.96, with a 95% confidence interval (0.88, 4.36) indicating that in this study a patient who had an infection was anywhere from 0.88 to 4.36 times as likely to have received Xolair than a patient who did not have an infection. Response to appropriate anti-geohelminth treatment of infection as measured by stool egg counts was not different between treatment groups.
Laboratory Tests
Serum total IgE levels increase following administration of Xolair due to formation of Xolair:IgE complexes [see Clinical Pharmacology (12.2)]. Elevated serum total IgE levels may persist for up to 1 year following discontinuation of Xolair. Do not use serum total IgE levels obtained less than 1 year following discontinuation to reassess the dosing regimen for allergic asthma patients, because these levels may not reflect steady state free IgE levels.
Adverse Reactions
Clinical Trials Experience
Clinical Trials Experience in Allergic Asthma:
Adult and Adolescent Patients 12 years of Age and Older The data described below reflect Xolair exposure for 2076 adult and adolescent patients ages 12 and older, including 1687 patients exposed for six months and 555 exposed for one year or more, in either placebo-controlled or other controlled asthma studies. The mean age of patients receiving Xolair was 42 years, with 134 patients 65 years of age or older; 60% were women, and 85% Caucasian. Patients received Xolair 150 to 375 mg every 2 or 4 weeks or, for patients assigned to control groups, standard therapy with or without a placebo.
The adverse events most frequently resulting in clinical intervention (e.g., discontinuation of Xolair, or the need for concomitant medication to treat an adverse event) were injection site reaction (45%), viral infections (23%), upper respiratory tract infection (20%), sinusitis (16%), headache (15%), and pharyngitis (11%). These events were observed at similar rates in Xolair-treated patients and control patients.
Table 4 shows adverse reactions from four placebo-controlled asthma studies that occurred ≥ 1% and more frequently in patients receiving Xolair than in those receiving placebo. Adverse events were classified using preferred terms from the International Medical Nomenclature (IMN) dictionary. Injection site reactions were recorded separately from the reporting of other adverse events and are described following Table 4.

There were no differences in the incidence of adverse reactions based on age (among patients under 65), gender or race.
- Injection Site Reactions
- Injection site reactions of any severity occurred at a rate of 45% in Xolair-treated patients compared with 43% in placebo-treated patients. The types of injection site reactions included: bruising, redness, warmth, burning, stinging, itching, hive formation, pain, indurations, mass, and inflammation.
- Severe injection site reactions occurred more frequently in Xolair-treated patients compared with patients in the placebo group (12% versus 9%).
- The majority of injection site reactions occurred within 1 hour-post injection, lasted less than 8 days, and generally decreased in frequency at subsequent dosing visits.
Clinical Trials Experience in Chronic Idiopathic Urticaria:
Adult and Adolescent Patients 12 years of Age and Older The safety of Xolair for the treatment of CIU was assessed in three placebo-controlled, multiple-dose clinical studies of 12 weeks' (CIU Study 2) and 24 weeks' duration (CIU Studies 1 and 3). In CIU Studies 1 and 2, patients received Xolair 75, 150, or 300 mg or placebo every 4 weeks in addition to their baseline level of H1 antihistamine therapy throughout the treatment period. In CIU Study 3 patients were randomized to Xolair 300 mg or placebo every 4 weeks in addition to their baseline level of H1 antihistamine therapy. The data described below reflect Xolair exposure for 733 patients enrolled and receiving at least one dose of Xolair in the three clinical trials, including 684 patients exposed for 12 weeks and 427 exposed for 24 weeks. The mean age of patients receiving Xolair 300 mg was 43 years, 75% were women, and 89% were white. The demographic profiles for patients receiving Xolair 150 mg and 75 mg were similar.
Table 5 shows adverse events that occurred in ≥ 2% of patients receiving Xolair (150 or 300 mg) and more frequently than those receiving placebo. Adverse events are pooled from Study 2 and the first 12 weeks of Studies 1 and 3.
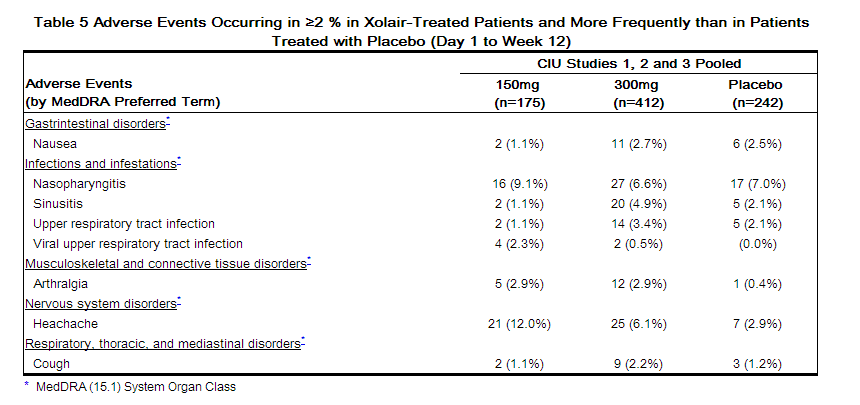
Additional events reported during the 24 week treatment period in Studies 1 and 3 [≥2% of patients receiving Xolair (150 or 300 mg) and more frequently than those receiving placebo] included: toothache, fungal infection, urinary tract infection, myalgia, pain in extremity, musculoskeletal pain, peripheral edema, pyrexia, migraine, sinus headache, anxiety, oropharyngeal pain, asthma, urticaria, and alopecia.
- Injection Site Reactions
- Injection site reactions of any severity occurred during the studies in more Xolair-treated patients [11 patients (2.7%) at 300 mg, 1 patient (0.6%) at 150 mg] compared with 2 placebo-treated patients (0.8%). The types of injection site reactions included: swelling, erythema, pain, bruising, itching, bleeding and urticaria. None of the events resulted in study discontinuation or treatment interruption.
Immunogenicity:
Antibodies to Xolair were detected in approximately 1/1723 (< 0.1%) of patients treated with Xolair in the clinical studies for approval of asthma. There were no detectable antibodies in the patients treated in the phase 3 CIU clinical trials, but due to levels of Xolair at the time of anti-therapeutic antibody sampling and missing samples for some patients, antibodies to Xolair could only have been determined in 88% of the 733 patients treated in these clinical studies. The data reflect the percentage of patients whose test results were considered positive for antibodies to Xolair in ELISA assays and are highly dependent on the sensitivity and specificity of the assays.
Additionally, the observed incidence of antibody positivity in the assay may be influenced by several factors including sample handling, timing of sample collection, concomitant medications, and underlying disease. Therefore, comparison of the incidence of antibodies to Xolair with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Xolair in adult and adolescent patients 12 years of age and older. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Anaphylaxis:
Based on spontaneous reports and an estimated exposure of about 57,300 patients from June 2003 through December 2006, the frequency of anaphylaxis attributed to Xolair use was estimated to be at least 0.2% of patients. Diagnostic criteria of anaphylaxis were skin or mucosal tissue involvement, and, either airway compromise, and/or reduced blood pressure with or without associated symptoms, and a temporal relationship to Xolair administration with no other identifiable cause. Signs and symptoms in these reported cases included bronchospasm, hypotension, syncope, urticaria, angioedema of the throat or tongue, dyspnea, cough, chest tightness, and/or cutaneous angioedema. Pulmonary involvement was reported in 89% of the cases. Hypotension or syncope was reported in 14% of cases. Fifteen percent of the reported cases resulted in hospitalization. A previous history of anaphylaxis unrelated to Xolair was reported in 24% of the cases.
Of the reported cases of anaphylaxis attributed to Xolair, 39% occurred with the first dose, 19% occurred with the second dose, 10% occurred with the third dose, and the rest after subsequent doses. One case occurred after 39 doses (after 19 months of continuous therapy, anaphylaxis occurred when treatment was restarted following a 3 month gap). The time to onset of anaphylaxis in these cases was up to 30 minutes in 35%, greater than 30 and up to 60 minutes in 16%, greater than 60 and up to 90 minutes in 2%, greater than 90 and up to 120 minutes in 6%, greater than 2 hours and up to 6 hours in 5%, greater than 6 hours and up to 12 hours in 14%, greater than 12 hours and up to 24 hours in 8%, and greater than 24 hours and up to 4 days in 5%. In 9% of cases the times to onset were unknown.
Twenty-three patients who experienced anaphylaxis were rechallenged with Xolair and 18 patients had a recurrence of similar symptoms of anaphylaxis. In addition, anaphylaxis occurred upon rechallenge with Xolair in 4 patients who previously experienced urticaria only.
Eosinophilic Conditions:
Eosinophilic conditions have been reported.
Fever, Arthralgia, and Rash:
A constellation of signs and symptoms including arthritis/arthralgia, rash (urticaria or other forms), fever and lymphadenopathy similar to serum sickness have been reported in post-approval use of Xolair. Hematologic: Severe thrombocytopenia has been reported. Skin: Hair loss has been reported.
Drug Interactions
No formal drug interaction studies have been performed with Xolair. In patients with allergic asthma the concomitant use of Xolair and allergen immunotherapy has not been evaluated. In patients with CIU the use of Xolair in combination with immunosuppressive therapies has not been studied.
Use in Specific Populations
Pregnancy
Pregnancy Category B:
Pregnancy Exposure Registry:
There is a pregnancy exposure registry that monitors pregnancy outcomes in women exposed to Xolair during pregnancy. Encourage patients to call 1-866-4XOLAIR (1-866-496-5247) or visit www.xolairpregnancyregistry.com for information about the pregnancy exposure registry and the enrollment procedure.
Risk Summary:
Adequate and well-controlled studies with Xolair have not been conducted in pregnant women. All pregnancies, regardless of drug exposure, have a background rate of 2 to 4% for major malformations, and 15 to 20% for pregnancy loss. In animal reproduction studies, no evidence of fetal harm was observed in Cynomolgus monkeys with subcutaneous doses of omalizumab up to 10 times the maximum recommended human dose (MRHD).
Because animal reproduction studies are not always predictive of human response, Xolair should be used during pregnancy only if clearly needed.
Clinical Considerations:
In general, monoclonal antibodies are transported across the placenta in a linear fashion as pregnancy progresses, with the largest amount transferred during the third trimester.
Data:
Animal Data:
Reproductive studies have been performed in Cynomolgus monkeys at subcutaneous doses of omalizumab up to 75 mg/kg (approximately 10 times the MRHD on a mg/kg basis). No evidence of maternal toxicity, embryotoxicity, or teratogenicity was observed when omalizumab was administered throughout organogenesis. Omalizumab did not elicit adverse effects on fetal or neonatal growth when administered throughout late gestation, delivery and nursing. Neonatal serum levels of omalizumab after in utero exposure and 28 days of nursing were between 11% and 94% of the maternal serum level. Levels of omalizumab in milk were 0.15% of maternal serum concentration.
Pregnancy Category (AUS):
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
- There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Omalizumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Omalizumab during labor and delivery.
Nursing Mothers
It is not known whether Xolair is present in human breast milk; however, IgG is present in human milk in small amounts. In Cynomolgus monkeys, milk levels of omalizumab were measured at 0.15% of the maternal serum concentration [see Use in Specific Populations (8.1)]. The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for Xolair and any potential adverse effects on the breastfed child from Xolair or from the underlying maternal condition. Exercise caution when administering Xolair to a nursing woman.
Pediatric Use
Allergic Asthma:
Safety and effectiveness of Xolair for allergic asthma were evaluated in 2 studies in 926 (Xolair 624; placebo 302) asthma patients 6 to <12 years of age. One study was a pivotal study of similar design and conduct to that of adult and adolescent Asthma Studies 1 and 2. The other study was primarily a safety study and included evaluation of efficacy as a secondary outcome. In the pivotal study, Xolair-treated patients had a statistically significant reduction in the rate of exacerbations (exacerbation was defined as worsening of asthma that required treatment with systemic corticosteroids or a doubling of the baseline ICS dose), but other efficacy variables such as nocturnal symptom scores, beta-agonist use, and measures of airflow (FEV1) were not significantly different in Xolair-treated patients compared to placebo. Considering the risk of anaphylaxis and malignancy seen in Xolair-treated patients ≥ 12 years old and the modest efficacy of Xolair in the pivotal pediatric study, the risk-benefit assessment does not support the use of Xolair in patients 6 to <12 years of age.
Although patients treated with Xolair in these two studies did not develop anaphylaxis or malignancy, the studies are not adequate to address these concerns because patients with a history of anaphylaxis or malignancy were excluded, and the duration of exposure and sample size were not large enough to exclude these risks in patients 6 to <12 years of age. Furthermore, there is no reason to expect that younger pediatric patients would not be at risk of anaphylaxis and malignancy seen in adult and adolescent patients with Xolair.
Studies in patients 0-5 years of age were not required because of the safety concerns of anaphylaxis and malignancy associated with the use of Xolair in adults and adolescents.
Chronic Idiopathic Urticaria
The safety and effectiveness of Xolair for adolescent patients with CIU were evaluated in 39 patients 12 to 17 years of age (Xolair 29, placebo 10) included in three randomized, placebo-controlled CIU studies. A numerical decrease in weekly itch score was observed, and adverse reactions were similar to those reported in patients 18 years and older.
Clinical studies with Xolair have not been conducted in CIU patients below the age of 12 years. Considering the risk of anaphylaxis and malignancy seen in Xolair-treated patients ≥ 12 years old, the risk-benefit assessment does not support the use of Xolair in patients <12 years of age. Therefore, the use of Xolair in this patient population is not recommended.
Geriatic Use
In clinical studies 134 allergic asthma patients and 37 CIU phase 3 study patients 65 years of age or older were treated with Xolair. Although there were no apparent age-related differences observed in these studies, the number of patients aged 65 and over is not sufficient to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Omalizumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Omalizumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Omalizumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Omalizumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Omalizumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Omalizumab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Preparation
- Prepare Xolair for subcutaneous injection using Sterile Water for Injection (SWFI), USP, ONLY. Each vial of Xolair is for single use only and contains no preservatives.
- Reconstitution
- The lyophilized product takes 15–20 minutes to dissolve. The fully reconstituted product will appear clear or slightly opalescent and it is acceptable if there are a few small bubbles or foam around the edge of the vial. The reconstituted product is somewhat viscous; in order to obtain the full 1.2 mL dose, ALL OF THE PRODUCT MUST BE WITHDRAWN from the vial before expelling any air or excess solution from the syringe.
- Use the solution within 8 hours following reconstitution when stored in the vial at 2–8°C (36–46°F), or within 4 hours of reconstitution when stored at room temperature. Reconstituted Xolair vials should be protected from sunlight.
Preparation:
STEP 1: Draw 1.4 mL of SWFI, USP into a 3 mL syringe equipped with a 1 inch, 18-gauge needle. STEP 2: Place the vial upright on a flat surface and using standard aseptic technique, insert the needle and inject the SWFI, USP directly onto the product. STEP 3: Keeping the vial upright, gently swirl the upright vial for approximately 1 minute to evenly wet the powder. Do not shake. STEP 4: After completing STEP 3, gently swirl the vial for 5-10 seconds approximately every 5 minutes in order to dissolve any remaining solids. There should be no visible gel like particles in the solution. Do not use if foreign particles are present.
Note: If it takes longer than 20 minutes to dissolve completely, repeat STEP 4 until there are no visible gel-like particles in the solution. Do not use if the contents of the vial do not dissolve completely by 40 minutes. STEP 5: Invert the vial for 15 seconds in order to allow the solution to drain toward the stopper. Using a new 3 mL syringe equipped with a 1-inch, 18-gauge needle, insert the needle into the inverted vial.
Position the needle tip at the very bottom of the solution in the vial stopper when drawing the solution into the syringe. Before removing the needle from the vial, pull the plunger all the way back to the end of the syringe barrel in order to remove all of the solution from the inverted vial. STEP 6: Replace the 18-gauge needle with a 25-gauge needle for subcutaneous injection. STEP 7: Expel air, large bubbles, and any excess solution in order to obtain the required 1.2 mL dose. A thin layer of small bubbles may remain at the top of the solution in the syringe.
- Administration
- Administer Xolair by subcutaneous injection. The injection may take 5-10 seconds to administer because the solution is slightly viscous. Each vial delivers 1.2 mL (150 mg) of Xolair. Do not administer more than 150 mg per injection site. Divide doses of more than 150 mg among two or more injection sites (Table 3).

Monitoring
- Monitor patients at high risk of geohelminth infection while on Xolair therapy. Insufficient data are available to determine the length of monitoring required for geohelminth infections after stopping Xolair treatment.
IV Compatibility
There is limited information regarding IV Compatibility of Omalizumab in the drug label.
Overdosage
The maximum tolerated dose of Xolair has not been determined. Single intravenous doses of up to 4,000 mg have been administered to patients without evidence of dose limiting toxicities. The highest cumulative dose administered to patients was 44,000 mg over a 20 week period, which was not associated with toxicities.
Pharmacology
There is limited information regarding Omalizumab Pharmacology in the drug label.
Mechanism of Action
- Allergic Asthma
- Omalizumab inhibits the binding of IgE to the high-affinity IgE receptor (FcεRI) on the surface of mast cells and basophils. Reduction in surface-bound IgE on FcεRI-bearing cells limits the degree of release of mediators of the allergic response. Treatment with Xolair also reduces the number of FcεRI receptors on basophils in atopic patients.
- Chronic Idiopathic Urticaria
Structure
- Xolair is a recombinant DNA-derived humanized IgG1κ monoclonal antibody that selectively binds to human immunoglobulin E (IgE). The antibody has a molecular weight of approximately 149 kiloDaltons. Xolair is produced by a Chinese hamster ovary cell suspension culture in a nutrient medium containing the antibiotic gentamicin. Gentamicin is not detectable in the final product.
- Xolair is a sterile, white, preservative free, lyophilized powder contained in a single use vial that is reconstituted with Sterile Water for Injection (SWFI), USP, and administered as a subcutaneous (SC) injection. Each 202.5 mg vial of omalizumab also contains L-histidine (1.8 mg), L-histidine hydrochloride monohydrate (2.8 mg), polysorbate 20 (0.5 mg) and sucrose (145.5 mg) and is designed to deliver 150 mg of omalizumab in 1.2 mL after reconstitution with 1.4 mL SWFI, USP.
Pharmacodynamics
- Allergic Asthma
- In clinical studies, serum free IgE levels were reduced in a dose dependent manner within 1 hour following the first dose and maintained between doses. Mean serum free IgE decrease was greater than 96% using recommended doses. Serum total IgE levels (i.e., bound and unbound) increased after the first dose due to the formation of omalizumab:IgE complexes, which have a slower elimination rate compared with free IgE.
- At 16 weeks after the first dose, average serum total IgE levels were five-fold higher compared with pre-treatment when using standard assays. After discontinuation of Xolair dosing, the Xolair-induced increase in total IgE and decrease in free IgE were reversible, with no observed rebound in IgE levels after drug washout. Total IgE levels did not return to pre-treatment levels for up to one year after discontinuation of Xolair.
- Chronic Idiopathic Urticaria
- In clinical studies in CIU patients, Xolair treatment led to a dose-dependent reduction of serum free IgE and an increase of serum total IgE levels, similar to the observations in allergic asthma patients. Maximum suppression of free IgE was observed 3 days following the first subcutaneous dose. After repeat dosing once every 4 weeks, predose serum free IgE levels remained stable between 12 and 24 weeks of treatment. Total IgE levels in serum increased after the first dose due to the formation of omalizumab-IgE complexes which have a slower elimination rate compared with free IgE. After repeat dosing once every 4 weeks at 75 mg up to 300 mg, average predose serum total IgE levels at Week 12 were two-to three-fold higher compared with pre-treatment levels, and remained stable between 12 and 24 weeks of treatment. After discontinuation of Xolair dosing, free IgE levels increased and total IgE levels decreased towards pre-treatment levels over a 16-week follow-up period.
Pharmacokinetics
After SC administration, omalizumab was absorbed with an average absolute bioavailability of 62%. Following a single SC dose in adult and adolescent patients with asthma, omalizumab was absorbed slowly, reaching peak serum concentrations after an average of 7-8 days. In patients with CIU, the peak serum concentration was reached at a similar time after a single SC dose. The pharmacokinetics of omalizumab was linear at doses greater than 0.5 mg/kg. In patients with asthma, following multiple doses of Xolair, areas under the serum concentration-time curve from Day 0 to Day 14 at steady state were up to 6-fold of those after the first dose. In patients with CIU, omalizumab exhibited linear pharmacokinetics across the dose range of 75 mg to 600 mg given as single subcutaneous dose. Following repeat dosing from 75 to 300 mg every 4 weeks, trough serum concentrations of omalizumab increased proportionally with the dose levels.
In vitro, omalizumab formed complexes of limited size with IgE. Precipitating complexes and complexes larger than 1 million daltons in molecular weight were not observed in vitro or in vivo. Tissue distribution studies in Cynomolgus monkeys showed no specific uptake of 125I-omalizumab by any organ or tissue. The apparent volume of distribution of omalizumab in patients with asthma following SC administration was 78 ± 32 mL/kg. In patients with CIU, based on population pharmacokinetics, distribution of omalizumab was similar to that in patients with asthma.
Clearance of omalizumab involved IgG clearance processes as well as clearance via specific binding and complex formation with its target ligand, IgE. Liver elimination of IgG included degradation in the liver reticuloendothelial system (RES) and endothelial cells. Intact IgG was also excreted in bile. In studies with mice and monkeys, omalizumab:IgE complexes were eliminated by interactions with Fcγ receptors within the RES at rates that were generally faster than IgG clearance. In asthma patients omalizumab serum elimination half-life averaged 26 days, with apparent clearance averaging 2.4 ± 1.1 mL/kg/day. Doubling body weight approximately doubled apparent clearance. In CIU patients, at steady state, based on population pharmacokinetics, omalizumab serum elimination half-life averaged 24 days and apparent clearance averaged 240 mL/day (corresponding to 3.0 mL/kg/day for an 80 kg patient).
Special Populations
Allergic Asthma
The population pharmacokinetics of omalizumab was analyzed to evaluate the effects of demographic characteristics in patients with allergic asthma. Analyses of these data suggested that no dose adjustments are necessary for age (12-76 years), race, ethnicity, or gender.
Chronic Idiopathic Urticaria
The population pharmacokinetics of omalizumab was analyzed to evaluate the effects of demographic characteristics and other factors on omalizumab exposure in patients with CIU. Covariate effects were evaluated by analyzing the relationship between omalizumab concentrations and clinical responses. These analyses demonstrate that no dose adjustments are necessary for age (12 to 75 years), race/ethnicity, gender, body weight, body mass index or baseline IgE level.
Nonclinical Toxicology
- Carcinogenesis, Mutagenesis, Impairment of Fertility
- No long-term studies have been performed in animals to evaluate the carcinogenic potential of Xolair.
- There were no effects on fertility and reproductive performance in male and female Cynomolgus monkeys that received Xolair at subcutaneous doses up to 75 mg/kg/week (approximately 10 times the maximum recommended human dose on a mg/kg basis).
Clinical Studies
Allergic Asthma
Adult and Adolescent Patients 12 Years of Age and Older The safety and efficacy of Xolair were evaluated in three randomized, double-blind, placebo-controlled, multicenter trials.
The trials enrolled patients 12 to 76 years old, with moderate to severe persistent (NHLBI criteria) asthma for at least one year, and a positive skin test reaction to a perennial aeroallergen. In all trials, Xolair dosing was based on body weight and baseline serum total IgE concentration. All patients were required to have a baseline IgE between 30 and 700 IU/mL and body weight not more than 150 kg. Patients were treated according to a dosing table to administer at least 0.016 mg/kg/IU (IgE/mL) of Xolair or a matching volume of placebo over each 4-week period. The maximum Xolair dose per 4 weeks was 750 mg.
In all three trials an exacerbation was defined as a worsening of asthma that required treatment with systemic corticosteroids or a doubling of the baseline ICS dose. Most exacerbations were managed in the out-patient setting and the majority were treated with systemic steroids. Hospitalization rates were not significantly different between Xolair and placebo-treated patients; however, the overall hospitalization rate was small. Among those patients who experienced an exacerbation, the distribution of exacerbation severity was similar between treatment groups.
Asthma Studies 1 and 2:
At screening, patients in Asthma Studies 1 and 2 had a forced expiratory volume in one second (FEV1) between 40% and 80% predicted. All patients had a FEV1 improvement of at least 12% following beta2-agonist administration. All patients were symptomatic and were being treated with inhaled corticosteroids (ICS) and short acting beta2-agonists. Patients receiving other concomitant controller medications were excluded, and initiation of additional controller medications while on study was prohibited. Patients currently smoking were excluded.
Each study was comprised of a run-in period to achieve a stable conversion to a common ICS (beclomethasone dipropionate), followed by randomization to Xolair or placebo. Patients received Xolair for 16 weeks with an unchanged corticosteroid dose unless an acute exacerbation necessitated an increase. Patients then entered an ICS reduction phase of 12 weeks during which ICS dose reduction was attempted in a step-wise manner.
The distribution of the number of asthma exacerbations per patient in each group during a study was analyzed separately for the stable steroid and steroid-reduction periods.
In both Asthma Studies 1 and 2 the number of exacerbations per patient was reduced in patients treated with Xolair compared with placebo (Table 6).
Measures of airflow (FEV1) and asthma symptoms were also evaluated in these studies. The clinical relevance of the treatment-associated differences is unknown. Results from the stable steroid phase Asthma Study 1 are shown in Table 7. Results from the stable steroid phase of Asthma Study 2 and the steroid reduction phases of both Asthma Studies 1 and 2 were similar to those presented in Table 7.


Asthma Study 3:
In Asthma Study 3, there was no restriction on screening FEV1, and unlike Asthma Studies 1 and 2, long-acting beta2-agonists were allowed. Patients were receiving at least 1000 µg/day fluticasone propionate and a subset was also receiving oral corticosteroids. Patients receiving other concomitant controller medications were excluded, and initiation of additional controller medications while on study was prohibited. Patients currently smoking were excluded.
The study was comprised of a run-in period to achieve a stable conversion to a common ICS (fluticasone propionate), followed by randomization to Xolair or placebo. Patients were stratified by use of ICS-only or ICS with concomitant use of oral steroids. Patients received Xolair for 16 weeks with an unchanged corticosteroid dose unless an acute exacerbation necessitated an increase. Patients then entered an ICS reduction phase of 16 weeks during which ICS or oral steroid dose reduction was attempted in a step-wise manner.
The number of exacerbations in patients treated with Xolair was similar to that in placebo-treated patients (Table 8). The absence of an observed treatment effect may be related to differences in the patient population compared with Asthma Studies 1 and 2, study sample size, or other factors.

In all three of the studies, a reduction of asthma exacerbations was not observed in the Xolair-treated patients who had FEV1 > 80% at the time of randomization. Reductions in exacerbations were not seen in patients who required oral steroids as maintenance therapy.
- Pediatric Patients 6 to < 12 Years of Age
- Clinical studies with Xolair in pediatric patients 6 to 11 years of age have been conducted.
- Pediatric Patients <6 Years of Age
- Clinical studies with Xolair in pediatric patients less than 6 years of age have not been conducted.
Chronic Idiopathic Urticaria
Adult and Adolescent Patients 12 Years of Age and Older:
The safety and efficacy of Xolair for the treatment of CIU was assessed in two placebo-controlled, multiple-dose clinical studies of 24 weeks' duration (CIU Study 1; n= 319) and 12 weeks' duration (CIU Study 2; n=322). Patients received Xolair 75, 150, or 300 mg or placebo by SC injection every 4 weeks in addition to their baseline level of H1 antihistamine therapy for 24 or 12 weeks, followed by a 16-week washout observation period. A total of 640 patients (165 males, 475 females) were included for the efficacy analyses. Most patients were white (84%) and the median age was 42 years (range 12–72).
Disease severity was measured by a weekly urticaria activity score (UAS7, range 0–42), which is a composite of the weekly itch severity score (range 0–21) and the weekly hive count score (range 0–21). All patients were required to have a UAS7 of ≥ 16, and a weekly itch severity score of ≥ 8 for the 7 days prior to randomization, despite having used an H1 antihistamine for at least 2 weeks.
The mean weekly itch severity scores at baseline were fairly balanced across treatment groups and ranged between 13.7 and 14.5 despite use of an H1 antihistamine at an approved dose. The reported median durations of CIU at enrollment across treatment groups were between 2.5 and 3.9 years (with an overall subject-level range of 0.5 to 66.4 years).
In both CIU Studies 1 and 2, patients who received Xolair 150 mg or 300 mg had greater decreases from baseline in weekly itch severity scores and weekly hive count scores than placebo at Week 12. Representative results from CIU Study 1 are shown (Table 9); similar results were observed in CIU Study 2. The 75-mg dose did not demonstrate consistent evidence of efficacy and is not approved for use.

The mean weekly itch severity score at each study week by treatment groups is shown in Figure 1. Representative results from CIU Study 1 are shown; similar results were observed in CIU Study 2. The appropriate duration of therapy for CIU with Xolair has not been determined.

In CIU Study 1, a larger proportion of patients treated with Xolair 300 mg (36%) reported no itch and no hives (UAS7=0) at Week 12 compared to patients treated with Xolair 150 mg (15%), Xolair 75 mg (12%), and placebo group (9%). Similar results were observed in CIU Study 2.
How Supplied
- Xolair is supplied as a lyophilized, sterile powder in a single-use, 5 mL vial without preservatives. Each vial delivers 150 mg of Xolair upon reconstitution with 1.4 mL SWFI, USP. Each carton contains one single-use vial of Xolair® (omalizumab) NDC 50242-040-62.
Storage
Xolair should be shipped at controlled ambient temperature (≤ 30°C [ ≤ 86°F]). Store Xolair under refrigerated conditions 2–8°C (36–46°F). Do not use beyond the expiration date stamped on carton.
Use the solution for subcutaneous administration within 8 hours following reconstitution when stored in the vial at 2–8°C (36–46°F), or within 4 hours of reconstitution when stored at room temperature.
Reconstituted Xolair vials should be protected from direct sunlight.
Images
Drug Images
{{#ask: Page Name::Omalizumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Omalizumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Information for Patients
- Provide and instruct patients to read the accompanying Medication Guide before starting treatment and before each subsequent treatment. The complete text of the Medication Guide is reprinted at the end of this document.
- Inform patients of the risk of life-threatening anaphylaxis with Xolair including the following points.
- There have been reports of anaphylaxis occurring up to 4 days after administration of Xolair
- Xolair should only be administered in a healthcare setting by healthcare providers
- Patients should be closely observed following administration
- Patients should be informed of the signs and symptoms of anaphylaxis
- Patients should be instructed to seek immediate medical care should such signs or symptoms occur
- Instruct patients receiving Xolair not to decrease the dose of, or stop taking any other asthma or CIU medications unless otherwise instructed by their physician. Inform patients that they may not see immediate improvement in their asthma or CIU symptoms after beginning Xolair therapy.
- Pregnancy Exposure Registry
- Encourage pregnant women exposed to Xolair to enroll in the Xolair Pregnancy Exposure Registry [1-866-4XOLAIR (1-866-496-5247)] or visit www.xolairpregnancyregistry.com.
MEDICATION GUIDE
XOLAIR®(ZOHL-air)
(omalizumab)
Injection
Read this Medication Guide before you start receiving and before each dose of Xolair. This Medication Guide does not take the place of talking with your healthcare provider about your medical condition or your treatment.
- What is the most important information I should know about Xolair?
- A severe allergic reaction called anaphylaxis can happen when you receive Xolair. The reaction can occur after the first dose, or after many doses. It may also occur right after a Xolair injection or days later. Anaphylaxis is a life-threatening condition and can lead to death.
- Go to the nearest emergency room right away if you have any of these symptoms of an allergic reaction:
- wheezing, shortness of breath, cough, chest tightness, or trouble breathing
- low blood pressure, dizziness, fainting, rapid or weak heartbeat, anxiety, or feeling of "impending doom"
- flushing, itching, hives, or feeling warm
- swelling of the throat or tongue, throat tightness, hoarse voice, or trouble swallowing
- Your healthcare provider will monitor you closely for symptoms of an allergic reaction while you are receiving Xolair and for a period of time after your injection. Your healthcare provider should talk to you about getting medical treatment if you have symptoms of an allergic reaction after leaving the healthcare provider's office or treatment center.
- What is Xolair?
- Xolair is an injectable prescription medicine used to treat adults and children 12 years of age and older with:
- moderate to severe persistent allergic asthma who have had a skin or blood test that is positive for allergic asthma and whose asthma symptoms are not controlled by asthma medicines called inhaled corticosteroids.
- chronic idiopathic urticaria (CIU; chronic hives without a known cause) who continue to have hives that are not controlled by H1 antihistamine treatment.
- Xolair is not used to treat other allergic conditions, other forms of urticaria, acute bronchospasm or status asthmaticus.
- Xolair is not for use in children less than 12 years of age.
- Do not receive Xolair if you:
- are allergic to omalizumab or any of the ingredients in Xolair. See the end of this Medication Guide for a complete list of ingredients in Xolair.
- Before receiving Xolair, tell your healthcare provider about all of your medical conditions, including if you:
- have any other allergies (such as food allergy or seasonal allergies)
- have sudden breathing problems (bronchospasm)
- have or have had low white blood cell count (ask your doctor if you are not sure)
- have or have had a parasitic infection
- have or have had cancer
- are pregnant or plan to become pregnant. It is not known if Xolair may harm your unborn baby.
- if you become pregnant while taking Xolair, talk to your healthcare provider about registering with the Xolair Pregnancy Registry. You can get more information and register by calling 1-866-4XOLAIR (1-866-496-5247) or visit www.xolairpregnancyregistry.com. The purpose of this registry is to monitor pregnancy outcomes in women receiving Xolair during pregnancy.
- are breastfeeding or plan to breastfeed. It is not known if Xolair passes into your breast milk. Talk with your healthcare provider about the best way to feed your baby while you receive Xolair.
- Tell your healthcare provider about all the medicines you take, including prescription and over-the-counter medicines, vitamins, or herbal supplements.
- How should I receive Xolair?
- Xolair should be given by your healthcare provider, in a healthcare setting.
- Xolair is given in 1 or more injections under the skin (subcutaneous), 1 time every 2 or 4 weeks.
- Your healthcare provider may do certain tests and change your Xolair dose as needed.
- Do not stop taking any of your other asthma or hive medicine unless your healthcare providers tell you to.
- You may not see improvement in your symptoms right away after Xolair treatment.
- What are the possible side effects of Xolair?
- Xolair may cause serious side effects, including:
- See, "What is the most important information I should know about Xolair?"
- Cancer. People who receive treatment with Xolair may have a higher chance for getting certain types of cancer.
- Fever, muscle aches, and rash. Some people who take Xolair get these symptoms 1 to 5 days after receiving a Xolair injection. If you have any of these symptoms, tell your healthcare provider.
- Parasitic infection. Some people who are at a high risk for parasite (worm) infections, get a parasite infection after receiving Xolair. Your healthcare provider can test your stool to check if you have a parasite infection.
- High blood levels of a certain antibody (Serum total IgE)
- The most common side effects of Xolair:
- In people with allergic asthma: pain especially in your arms and legs, dizziness, feeling tired, skin rash, bone fractures, and pain or discomfort of your ears.
- In people with chronic idiopathic urticaria: nausea, headaches, swelling of the inside of your nose, throat or sinuses, cough, joint pain, and upper respiratory tract infection.
- These are not all the possible side effects of Xolair. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088.
- General information about the safe and effective use of Xolair.
- Medicines are sometimes prescribed for purposes other than those listed in a Medication Guide. You can ask your pharmacist or healthcare provider for information about Xolair that is written for health professionals. Do not use Xolair for a condition for which it was not prescribed.
- For more information, go to www.xolair.com or call 1-866-4XOLAIR (1-866-496-5247).
- What are the ingredients in Xolair?
- Active ingredient: omalizumab
- Inactive ingredients: L-histidine, L-histidine hydrochloride monohydrate, polysorbate 20 and sucrose

PRINCIPAL DISPLAY PANEL


Precautions with Alcohol
- Alcohol-Omalizumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Xolair®
Look-Alike Drug Names
- N/A[1]
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ "http://www.ismp.org". External link in
|title=(help)
{{#subobject:
|Page Name=Omalizumab
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Omalizumab |Label Name=Omalizumab11.png
}}
{{#subobject:
|Label Page=Omalizumab |Label Name=Omalizumab11.png
}}