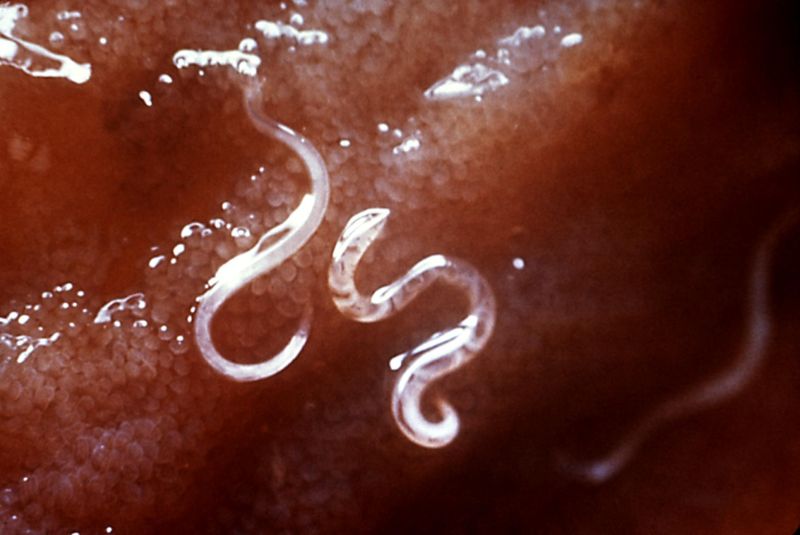
Hookworm
| Necator americanus and Ancylostoma duodenale | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
 | ||||||||||||
| Scientific classification | ||||||||||||
| ||||||||||||
| Species | ||||||||||||
|
Species N. americanus and A. duodenale |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
The hookworm is a parasitic nematode worm that lives in the small intestine of its host, which may be a mammal such as a dog, cat, or human. Two species of hookworms commonly infect humans, Ancylostoma duodenale and Necator americanus. Necator americanus predominates in the Americas, Sub-Saharan Africa, Southeast Asia, China and Indonesia, while A. duodenale predominates in the Middle East, North Africa, India and (formerly) in southern Europe. Hookworms are thought to infect 800 million people worldwide. The A. braziliense and A. tubaeforme species infect cats, while A. caninum infects dogs. Uncinaria stenocephala infects both dogs and cats.
Hookworms are much smaller than the large roundworm, Ascaris lumbricoides, and the complications of tissue migration and mechanical obstruction so frequently observed with roundworm infestation are less frequent in hookworm infestation. The most significant risk of hookworm infection is anemia, secondary to loss of iron (and protein) in the gut. The worms suck blood voraciously and damage the mucosa. However, the blood loss in the stools is occult blood loss (not visibly apparent).
Ankylostomiasis, alternatively spelled anchylostomiasis and also called helminthiasis, "miners' anaemia", "tunnel disease", "brickmaker's anaemia", "Egyptian chlorosis" and in Germany Wurmkrankheit, is the disease caused by hookworms. It is caused when hookworms, present in large numbers, produce an iron deficiency anemia by voraciously sucking blood from the host's intestinal walls. The name is derived from Greek ancylo "crooked, bent" and stoma "mouth."
Hookworm is commonly called "larva migratoria" in Spanish and "bicho do pé" in Portuguese.
Hookworm is a leading cause of maternal and child morbidity in the developing countries of the tropics and subtropics. In susceptible children hookworms cause intellectual, cognitive and growth retardation, intrauterine growth retardation, prematurity, and low birth weight among newborns born to infected mothers. Hookworm infection is rarely fatal, but anemia can be significant in the heavily infected individual.
History
The symptoms now attributed to hookworm appear in papyrus papers of ancient Egypt (c. 1600 B.C.), described as a derangement characterized by anemia. Avicenna, a Persian physician of the 11th century, discovered the worm in several of his patients and related it to their disease. In later times, the condition was noticeably prevalent in the mining industry in England, France, Germany, Belgium, North Queensland and elsewhere.
Italian physician Angelo Dubini was the modern-day discoverer of the worm in 1838 after an autopsy of a peasant woman. Dubini published details in 1843 and identified the species as A. duodenale. Working in the Egyptian medical system in 1852 German physician Theodor Bilharz, drawing upon the work of colleague Wilhelm Griesinger, found these worms during autopsies and went a step further in linking them to local endemic occurrences of chlorosis, which would probably be called iron deficiency anemia today.
A breakthrough came 25 years later following a diarrhea and anemia epidemic that took place among Italian workmen employed on the Gotthard Rail Tunnel. In an 1880 paper, physicians Camillo Bozzolo, Edoardo Perroncito, and Luigi Pagliani correctly hypothesized that hookworm was linked to the fact that workers had to defecate inside the 15 km tunnel, and that many wore worn-out shoes. In 1897, it was established that the skin was the principal avenue of infection and the biological life cycle of the hookworm was clarified. In 1899, American zoologist Charles Wardell Stiles brought this evidence to bear on health issues in the southeast United States, identifying "progressive pernicious anemia" seen in the southern United States was caused by A. duodenale and he also identified the other important hookworm species: U. Necator. Testing in the 1900s revealed very heavy infestations in schoolage children.
On October 26, 1909 the Rockefeller Sanitary Commission for the Eradication of Hookworm Disease was organized as a result of a gift of US$1 million from John D. Rockefeller, Sr. Though humanitarian reasons are cited, some speculateTemplate:Weasel-inline that the motive was to open markets in the Appalachian region by creating more disposable income.[citation needed] Nevertheless the five-year program was a remarkable success and a great contribution to United States public health, instilling public education, medication, field work and modern government health departments in eleven southern states. The hookworm exhibit was a prominent part of the 1910 Mississippi state fair. The program nearly eradicated hookworm. The program would flourish afterwards with new funding as the Rockefeller Foundation International Health Division.
In the 1920s, hookworm eradication reached the Caribbean and Latin America, where great mortality was reported among blacks in the West Indies towards the end of the 18th century, as well as through descriptions sent from Brazil and various other tropical and sub-tropical regions.
Early treatment was with thymol to kill the worms, followed by epsom salt to clear the body of the worms. Later on, tetrachloroethylene was the leading method. It was not until later in the mid-20th century when new organic drug compounds were developed.
Pathology
Most individuals with hookworm infection are asymptomatic (without symptoms). Generally, very high loads of the parasite coupled with poor nutrition (inadequate intake of protein and iron) eventually lead to anemia.
The disease was linked to nematode worms (Ankylostoma duodenalis) from one-third to half an inch long in the intestine chiefly through the labours of Theodor Bilharz and Griesinger in Egypt (1854).
The symptoms can be linked to inflammation in the gut stimulated by feeding hookworms, such as nausea, abdominal pain and intermittent diarrhea, and to progressive anemia in prolonged disease: capricious appetite, pica (or dirt-eating), obstinate constipation followed by diarrhea, palpitations, thready pulse, coldness of the skin, pallor of the mucous membranes, fatigue and weakness, shortness of breath and in cases running a fatal course, dysentery, haemorrhages and oedema.
Blood tests in early infection often show a rise in numbers of eosinophils, a type of white blood cell that is preferentially stimulated by worm infections in tissues (large numbers of eosinophils are also present in the local inflammatory response). Falling blood hemoglobin levels will be seen in cases of prolonged infection with anemia.
In contrast to most intestinal helminthiases where the heaviest parasitic loads tend to occur in children, hookworm prevalence and intensity can be higher among adult males. The explanation for this is that hookworm infection tends to be occupational, so that plantation workers, coalminers and other groups maintain a high prevalence of infection among themselves by contaminating their work environment. However, in most endemic areas, adult women are the most severely affected by anemia, mainly because they have much higher physiological needs for iron (menstruation, repeated pregnancy), but also because customarily they have access to much poorer food than the men. In some communities, it is also these women who are most heavily exposed occupationally to hookworm, e.g. in rubber plantations, where women do the latex-tapping, working barefoot, and without latrines.
An interesting consequence of this in the case of Ancylostoma duodenale infection is translactational transmission of infection: the skin-invasive larvae of this species do not all immediately pass through the lungs and on into the gut, but spread around the body via the circulation, to become dormant inside muscle fibers. In a pregnant woman, after childbirth some or all of these larvae are stimulated to re-enter the circulation (presumably by sudden hormonal changes), then to pass into the mammary glands, so that the newborn baby can receive a large dose of infective larvae through its mother's milk. This accounts for otherwise inexplicable cases of very heavy, even fatal, hookworm infections in children a month or so of age, in places such as China, India and northern Australia. (An identical phenomenon is much more commonly seen with Ancylostoma caninum infections in dogs, where the newborn pups can even die of hemorrhaging from their intestines caused by massive numbers of feeding hookworms. This also reflects the close evolutionary link between the human and canine parasites, which probably have a common ancestor dating back to when humans and dogs first started living closely together.)
Hookworm in therapy
Moderate hookworm infections have been demonstrated to have beneficial effects on hosts suffering from diseases linked to overactive immune systems. This is possibly explained by the hygiene hypothesis[citation needed]. Research at the University of Nottingham conducted in Ethiopia has demonstrated that people with hookworm infections are half as likely to experience asthma[1] or hay fever.[2] It may also help sufferers of multiple sclerosis[3], Crohn's Disease[4] and diabetes.[5]
Hookworm therapy to treat a number of these diseases is currently in the trial stage at the University.[5] Also, some Westerners have independently infected themselves with hookworms and reported positive results.[6]
Life cycle

See the image for the biological life cycle of the hookworms where it thrives in warm earth where temperatures are over 18°C. They exist primarily in sandy or loamy soil and cannot live in clay or muck. Rainfall averages must be more than 1000 mm (40 inches) a year. Only if these conditions exist can the eggs hatch. Infective larvae of Necator americanus can survive at higher temperatures, whereas those of Ancylostoma duodenale are better adapted to cooler climates. Generally, they live for only a few weeks at most under natural conditions, and die almost immediately on exposure to direct sunlight or desiccation. Once in the host gut, Necator tends to cause a prolonged infection, as some adult worms have been recorded to live for 15 years or more. On the other hand, Ancylostoma adults are shortlived, surviving on average for only about 6 months. However, infection can be prolonged because dormant larvae can be "recruited" sequentially from tissue "stores" (see Pathology, above) over many years, to replace expired adult worms. This can give rise to seasonal fluctuations in infection prevalence and intensity (apart from normal seasonal variations in transmission).
Because it takes 5-7 weeks for adult worms to mature, mate and produce eggs, in the early stages of very heavy infection, acute symptoms might occur without any eggs being detected in the patient's feces. This can make diagnosis very difficult.
Prevention
The infective larvae develop and survive in an environment of damp dirt, particularly sandy and loamy soil. They cannot survive in clay or muck. The main lines of precaution are those dictated by sanitary science:
- Prevent skin/soil contact: do not walk barefoot
- Do not defecate outside latrines, toilets etc.
- Do not use human excrement or raw sewage as manure/fertilizer in agriculture
- Worm pet dogs — canine and feline hookworms rarely develop to adulthood in humans (Ancylostoma caninum, the common dog hookworm, occasionally develops into an adult to cause eosinophilic enteritis in people), but their invasive larvae can cause an itchy rash called cutaneous larva migrans.
Moxidectin has been released in the United States as part of Advantage Multi™ (imidacloprid + moxidectin) Topical Solution for dogs and cats. It utilizes moxidectin for control and prevention of roundworms, hookworms, heartworms, and whipworms.
In the late 1800s and early 1900s, many Mississippians were plagued by hookworms. They did not have indoor plumbing or proper sanitation facilities. As a result, hookworms, spread by fecal contamination of the environment, were very prevalent (as well as other diseases caused by lack of sanitation).
Differentiating Hookworm infections from other diseases
The table below summarizes the findings that differentiate Hookworm infections from other nematode infections:
| Differentiating Hookworm infections from other Nematode infections[7][8][9] | |||||||
|---|---|---|---|---|---|---|---|
| Infection | Nematode | Transmission | Direct Person-Person Transmission | Duration of Infection | Pulmonary Manifestation | Location of Adult worm(s) | Treatment |
| Hookworm infection | Necator americanus and Ancylostoma duodenale | Skin penetration by filariform larvae | No |
|
|
Attached to the mucosa of mid-upper portion of the small bowel | |
| Strongyloidiasis | Strongyloides stercoralis | Filariform larvae penetrate skin or bowel mucosa | Yes |
|
|
Embedded in the mucosa of the duodenum, jejunum | |
| Trichuriasis | Trichuris trichiura
(whipworm) |
Ingestion of infective ova | No | 1-3 years |
|
Anchored in the superficial mucosa of cecum and colon | |
| Ascariasis | Ascaris lumbricoides | Ingestion of infective ova | No | 1-2 years |
|
Free air in the lumen of the small bowel
(primarily jejunum) |
|
| Enterobiasis | Enterobius vermicularis
(pinworm) |
Ingestion of infective ova | Yes |
|
|
Free air in the lumen of cecum, appendix, adjacent colon | |
Diagnosis
There are no specific symptoms or signs of hookworm infection. As mentioned above, they arise from a combination of intestinal inflammation and progressive iron/protein-deficiency anemia. Larval invasion of the skin might give rise to intense, local itching, usually on the foot or lower leg, which can be followed by lesions that look like insect bites, can blister ("ground itch"), and last for a week or more. Coughing, chest pain, wheezing, and fever will sometimes be experienced by people who have been exposed to very large numbers of larvae. Epigastric pains, indigestion, nausea vomiting, constipation, and diarrhea can occur early or in later stages as well, although gastrointestional symptoms tend to improve with time. Signs of advanced severe infection are those of anemia and protein deficiency, including emaciation, cardiac failure and abdominal distension with ascites.
Diagnosis depends on finding characteristic worm eggs on microscopic examination of the stools, although this is not possible in early infection. As the eggs of both Ancylostoma and Necator (and most other hookworm species) are indistinguishable, to identify the genus, they must be cultured in the lab to allow larvae to hatch out. If the fecal sample is left for a day or more under tropical conditions, the larvae will have hatched out, so eggs might no longer be evident. In such a case, it is essential to distinguish hookworms from Strongyloides larvae, as infection with the latter has more serious implications and requires different management. The larvae of the two hookworm species can also be distinguished microscopically, although this would not be done routinely, but usually for research purposes. Adult worms are rarely seen (except via endoscopy, surgery or autopsy), but if found, would allow definitive identification of the species.
Treatment
The hookworm can be treated with local cryotherapy when it is still in the skin.
Albendazole is effective both in the intestinal stage and during the stage the parasite is still migrating under the skin.
In case of anemia, iron supplementation can cause relief symptoms of iron deficiency anemia. However, as red blood cell levels are restored, shortage of other essentials such as folic acid or vitamin B12 may develop, so this might also be supplemented.
Quick Facts
| Genus and Species | Necator americanus | Ancylostoma duodenale |
|---|---|---|
| Common Name | New world hookworm, American murderer | Old world hookworm |
| Etiologic Agent of: | Necatoriasis, Uncinariasis | Ancylostomiasis, Wakana disease |
| Infective stage | Filariform larva | Filariform larva |
| Definitive Host | Man | Man |
| Portal of Entry | Usually via skin penetration rather than ingestion | Usually via ingestion rather than skin penetration |
| Mode of Transmission | Skin > Mouth | Mouth > Skin |
| Habitat | Small Intestine | Small Intestine |
| Pathogenic Stage | L3 Larva | L3 Larva |
| Mode of Attachment | Oral attachment to mucosa by sucking | Same |
| Mode of Nutrition | Sucking and Ingesting of blood | Same |
| Pathogenesis | Larva – ground / dew itch, creeping eruption
Adult – IDA Microcytic, Hypochromic Anemia |
Same |
| Laboratory diagnosis | Concentration methods and Direct Fecal Smear | Same |
| Treatment | Albendazole, Mebendazole, or Pyrantel Pamoate | Same |
| Diagnostic Feature - Adult | Semi-lunar cutting plate; Bipartite dorsal ray | Male – Tripartite dorsal ray |
| Diagnostic Feature - Egg | In Morula | Same |
See also
- Cutaneous larva migrans (creeping eruption)
Gallery
-
Human hookworm lifecycle. From Public Health Image Library (PHIL). [10]
External links
- Moxidectin Facts, Usage, and Safety Information
- CDC Department of Parasitic Diseases images of the hookworm life cycle
- http://www.cdc.gov/ncidod/dpd/parasites/hookworm/factsht_hookworm.htm
- Personal MD, more hookworm info
- Human Hookworm Vaccine Initiative
- http://archive.rockefeller.edu/feature/hookworm.php
- http://mshistory.k12.ms.us/features/feature31/hookworm.html
- http://www.wellcome.ac.uk/doc_WTX026496.html
- How to cure your asthma or hayfever using hookworm - a practical guide
- Amateur photographs of a hookworm infection
References
- ↑ BBC Health Worm infestation 'beats asthma'
- ↑ BBC Health Eat worms - feel better
- ↑ Annals of Neurology Association between parasite infection and immune responses in multiple sclerosis
- ↑ British Medical Journal A proof of concept study establishing Necator americanus in Crohn’s patients and reservoir donors
- ↑ 5.0 5.1 Daily Mail. The bloodsucking worm that fights allergies from inside your tummy 14-09-2007.
- ↑ How to cure your asthma or hayfever using hookworm - a practical guide. 01-05-2006.
- ↑ Durand, Marlene (2015). "Chapter 288:Intestinal Nematodes (Roundworms)". Mandell, Douglas, and Bennett's Principles and Practice of Infectious Diseases Updated Edition, Eighth Edition. Elsevier. pp. 3199–3207. ISBN 978-1-4557-4801-3.
- ↑ Kim, Kami; Weiss, Louis; Tanowitz, Herbert (2016). "Chapter 39:Parasitic Infections". Murray and Nadel's Textbook of Respiratory Medicine Sixth Edition. Elsevier. pp. 682–698. ISBN 978-1-4557-3383-5.
- ↑ Serpytis M, Seinin D (2012). "Fatal case of ectopic enterobiasis: Enterobius vermicularis in the kidneys". Scand J Urol Nephrol. 46 (1): 70–2. doi:10.3109/00365599.2011.609834. PMID 21879805.
- ↑ "Public Health Image Library (PHIL)".
- Albanese G.; Venturi C,; Galbiati G (2001): Treatment of larva migrans cutanea (creeping eruption): a comparison between albendazole and traditional therapy. Int J Dermatol: 40(1): 67-71
- Hotez P.; Pritchard D. (1995): Hookworm infection. Sci Am June: 68-74
![Human hookworm lifecycle. From Public Health Image Library (PHIL). [10]](/images/f/f8/Ancylostoma_braziliense03.jpeg)