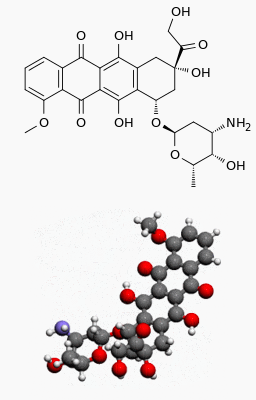
Doxorubicin hydrochloride
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
CARDIOMYOPATHY, SECONDARY MALIGNANCIES, EXTRAVASATION AND TISSUE NECROSIS, AND SEVERE MYELOSUPPRESSION
See full prescribing information for complete Boxed Warning.
|
Overview
Doxorubicin hydrochloride is an antineoplastic and antibiotic that is FDA approved for the treatment of women with axillary lymph node involvement following resection of primary breast cancer, leukemias, lymphomas and metastasis neoplasms.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include alopecia, nausea and vomiting.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Adjuvant in breast cancer
- Dosage: 60 mg/m2 administered as an intravenous bolus on day 1 of each 21-day treatment cycle, in combination with cyclophosphamide, for a total of four cycles
Metastasic Neoplasm, Lymphomas or Leukemias
- The recommended dose of doxorubicin HCl when used as a single agent is 60 to 75 mg/m2 intravenously every 21 days.
- The recommended dose of doxorubicin HCl, when administered in combination with other chemotherapy drugs, is 40 to 75 mg/m2 intravenously every 21 to 28 days.
- Consider use of the lower doxorubicin dose in the recommended dose range or longer intervals between cycles for heavily pretreated patients, elderly patients, or obese patients.
- Cumulative doses above 550 mg/m2 are associated with an increased risk of cardiomyopathy.
- Doxorubicin Hydrochloride is indicated for the following metastasic neoplasms:
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxorubicin Hydrochloride in adult patients.
Non–Guideline-Supported Use
Adrenal Carcinoma
- Multidrug dosage:
Bladder Carcinoma
- Multidrug Dosage:
- Doxorubicin: 30 mg/m2 - day 2 [2]
- Methotrexate: 30 mg/m2 - Day 1, 15 and 22
- Vinblastine: 3 mg/m2 - Day 2, 15 and 22
- Cisplatin: 70 mg/m2 - Day 2
- This treatment must be administered for 3 cycles.
Endometrial Carcinoma
Used in women with stage III and IV or recurrent endometrial carcinoma.
- Multidrug dosage:
- Doxorubicin[3]: 45 mg/m2 - Day 1
- Cisplatin: 50 mg/m2 - Day 1 (applied just after Doxorubicin)
- Paclitaxel: 160 mg/m2 - Day 2
- Filgrastim: 5 mcg/kg of weight
Primary Liver Carcinoma
Malignant Tumor of Thymus
- Multidrug Dosage:
- Scheme 1: Neoadjuvant chemotherapy
- Doxorubicin[4]: 20 mg/m3/day - Days 1, 2 and 3
- Cyclophosphamide: 500 mg/m2/day - Day 1
- Cisplatin: 30 mg/m2/day - Days 1, 2 and 3
- Prednisone: 100 mg/day - Days 1 through 5
- Scheme 2: Treatment for recurrent or metastasic thymoma
- Doxorubicin[5]: 50 mg/m2 - Each 21 days (8 cycles)
- Cyclophosphamide: 500 mg/m2 - Each 21 days (8 cycles)
- Cisplatin: 50 mg/m2 - Each 21 days (8 cycles)
- Scheme 3: treatment for Invasive thymoma
- Doxorubicin[6]: 40 mg/m2 - Day 1 (repeat each 3 weeks)
- Vincristine: 0.6 mg/m2 - Day 3 (repeat each 3 weeks)
- Cisplatin: 50 mg/m2 - Day 1 (repeat each 3 weeks)
- Cyclophosphamide: 700 mg/m2 - Day 4 (repeat each 3 weeks)
- Scheme 1: Neoadjuvant chemotherapy
Multiple Myeloma
- Multidrug Dosage:
- Doxorubicin[7]: 10 mg/m2/day
- Dexamethasone: 40 mg/day for 4 days
- Oral Thalidomide: 400 mg (Night-time).
- Etoposide: 40 mg/m2/day
- Cisplatin: 10 mg/m2/day
Transitional Cell Carcinoma of the Urinary Tract
- Multidrug dosage:
- Doxorubicin: 30 mg/m2 - Day 2
- Methotrexate: 30 mg/m2 - Day 1, 15 and 22
- Cisplatin: 70 mg/m2 - Day 2
- Vinblastine: 3 mg/m2 - Day 2, 15 and 22
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Doxorubicin hydrochloride FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doxorubicin Hydrochloride in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doxorubicin Hydrochloride in pediatric patients.
Contraindications
- Severe myocardial insufficiency
- Recent myocardial infarction
- Severe persistent drug-induced myelosuppression
- Severe hepatic impairment
- Hypersensitivity to doxorubicin HCl
Warnings
|
CARDIOMYOPATHY, SECONDARY MALIGNANCIES, EXTRAVASATION AND TISSUE NECROSIS, AND SEVERE MYELOSUPPRESSION
See full prescribing information for complete Boxed Warning.
|
Cardiomyopathy
- Doxorubicin HCl can result in myocardial damage, including acute left ventricular failure. The risk of cardiomyopathy is generally proportional to the cumulative exposure. Include prior doses of other anthracyclines or anthracenediones in calculations of total cumulative dosage for doxorubicin HCl. Cardiomyopathy may develop during treatment or up to several years after completion of treatment and can include decrease in LVEF and signs and symptoms of congestive heart failure (CHF). The probability of developing cardiomyopathy is estimated to be 1 to 2% at a total cumulative dose of 300 mg/m2 of doxorubicin HCl, 3 to 5% at a dose of 400 mg/m2, 5 to 8% at a dose of 450 mg/m2, and 6 to 20% at a dose of 500 mg/m2, when doxorubicin HCl is administered every 3 weeks. There is an additive or potentially synergistic increase in the risk of cardiomyopathy in patients who have received radiotherapy to the mediastinum or concomitant therapy with other known cardiotoxic agents such as cyclophosphamide and trastuzumab. Pericarditis and myocarditis have also been reported during or following doxorubicin HCl treatment.
- Assess left ventricular cardiac function (e.g., MUGA or echocardiogram) prior to initiation of doxorubicin HCl, during treatment to detect acute changes, and after treatment to detect delayed cardiotoxicity. Increase the frequency of assessments as the cumulative dose exceeds 300 mg/m2. Use the same method of assessment of LVEF at all time points. Consider the use of dexrazoxane to reduce the incidence and severity of cardiomyopathy due to doxorubicin HCl administration in patients who have received a cumulative doxorubicin HCl dose of 300 mg/m2 and who will continue to receive doxorubicin HCl.
Arrhythmias
- Doxorubicin HCl can result in arrhythmias, including life-threatening arrhythmias, during or within a few hours after doxorubicin HCl administration and at any time point during treatment. Tachyarrhythmias, including sinus tachycardia, premature ventricular contractions, and ventricular tachycardia, as well as bradycardia may occur. Electrocardiographic changes including non-specific ST-T wave changes, atrioventricular and bundle-branch block can also occur. These electrocardiographic changes may be transient and self-limited and may not require dose-modifications of doxorubicin HCl.
Secondary Malignancies
- The risk of developing secondary acute myelogenous leukemia (AML) and myelodysplastic syndrome (MDS) is increased following treatment with doxorubicin HCl. Cumulative incidences ranged from 0.2% at five years to 1.5% at 10 years in two separate trials involving the adjuvant treatment of women with breast cancer. These leukemias generally occur within 1 to 3 years of treatment.
Extravasation and Tissue Necrosis
- Extravasation of doxorubicin HCl can result in severe local tissue injury manifesting as blistering, ulceration, and necrosis requiring wide excision of the affected area and skin grafting. When given via a peripheral venous line, infuse doxorubicin over 10 minutes or less to minimize the risk of thrombosis or perivenous extravasation. If signs or symptoms of extravasation occur, immediately terminate the injection or infusion. Extravasation may be present in patients who do not experience a stinging or burning sensation or when blood return is present on aspiration of the infusion needle. If extravasation is suspected, apply ice to the site intermittently for 15 minutes, 4 times a day for 3 days. If appropriate, administer dexrazoxane at the site of extravasation as soon as possible and within the first 6 hours after extravasation.
Severe Myelosuppression
- Doxorubicin HCl can cause myelosuppression. In Study 1, the incidence of severe myelosuppression was: grade 4 leukopenia (0.3%), grade 3 leukopenia (3%), and grade 4 thrombocytopenia (0.1%). A dose-dependent, reversible neutropenia is the predominant manifestation of hematologic toxicity from doxorubicin HCl. When doxorubicin HCl is administered every 21 days, the neutrophil count reaches its nadir 10 to 14 days after administration with recovery usually occurring by the 21st day. Obtain baseline assessment of blood counts and carefully monitor patients during treatment for possible clinical complications due to myelosuppression.
Use in Patients with Hepatic Impairment
- The clearance of doxorubicin is decreased in patients with elevated serum bilirubin with an increased risk of toxicity. Reduce the dose of doxorubicin HCl in patients with serum bilirubin levels of 1.2–5.0 mg/dL. Doxorubicin is contraindicated in patients with severe hepatic impairment (defined as Child Pugh Class C or serum bilirubin level greater than 5 mg/dL). Obtain liver tests including SGOT, SGPT, alkaline phosphatase, and bilirubin prior to and during doxorubicin HCl therapy.
Tumor Lysis Syndrome
- Doxorubicin HCl may induce tumor lysis syndrome in patients with rapidly growing tumors. Evaluate blood uric acid levels, potassium, calcium, phosphate, and creatinine after initial treatment. Hydration, urine alkalinization, and prophylaxis with allopurinol to prevent hyperuricemia may minimize potential complications of tumor lysis syndrome.
Radiation Sensitization and Radiation Recall
- Doxorubicin HCl can increase radiation-induced toxicity to the myocardium, mucosa, skin, and liver. Radiation recall, including but not limited to cutaneous and pulmonary toxicity, can occur in patients who receive doxorubicin HCl after prior radiation therapy.
Embryofetal Toxicity
- Doxorubicin HCl can cause fetal harm when administered to a pregnant woman. Doxorubicin HCl was teratogenic and embryotoxic in rats and rabbits at doses lower than the recommended human dose. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to a fetus. Advise female patients of reproductive potential to use highly effective contraception during treatment with doxorubicin HCl and for 6 months after treatment. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking doxorubicin HCl.
Adverse Reactions
Clinical Trials Experience
The Following Adverse Reactions Are Discussed In More Detail In Other Sections Of The Labeling
- Cardiomyopathy and Arrhythmias
- Secondary Malignancies
- Extravasation and Tissue Necrosis
- Severe Myelosuppression
- Tumor Lysis Syndrome
- Radiation Sensitization and Radiation Recall
Clinical Trial Experience in Breast Cancer
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
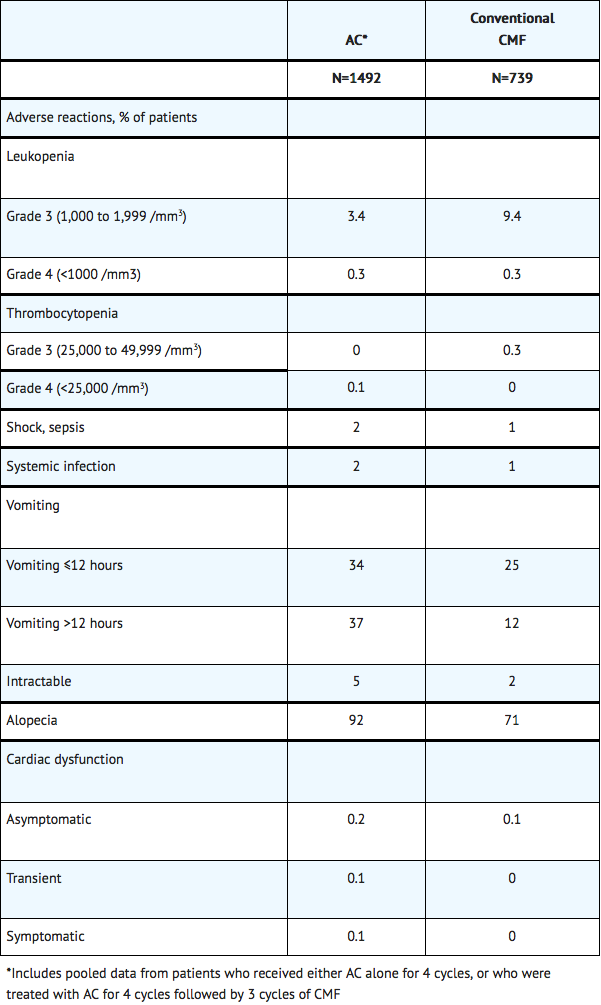
The safety data below were collected from 1492 women who received doxorubicin hydrochloride at a dose of 60 mg/m2 and cyclophosphamide at a dose of 600 mg/m2 (AC) every 3 weeks for 4 cycles for the adjuvant treatment of axillary lymph node positive breast cancer. The median number of cycles received was 4. Selected adverse reactions reported in this study are provided in Table 1. No treatment-related deaths were reported in patients on either arm of the study.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of doxorubicin HCl. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
Cardiac
- Cardiogenic shock
Cutaneous
- Skin and nail hyperpigmentation
- Onycholysis
- Rash
- Itching
- Photosensitivity
- Urticaria
- Acral erythema
- Palmar and plantar erythrodysesthesia
Gastrointestinal
- Nausea
- Mucositis
- Stomatitis
- Necrotizing colitis
- Typhlitis
- Gastric erosions
- Gastrointestinal bleeding
- Hematochezia
- Esophagitis
- Anorexia
- Abdominal pain
- Dehydration
- Diarrhea
- Hyperpigmentation of the oral mucosa
Hypersensitivity
Laboratory Abnormalities
- Increased alanine aminotransferase
- Increased aspartate aminotransferase
Neurological
- Peripheral sensory and motor neuropathy
- Seizures
- Coma
Ocular
Vascular
Other
Drug Interactions
Effect of CYP3A4 Inhibitors, Inducers and P-gp
- Doxorubicin is a major substrate of cytochrome P450 CYP3A4 and CYP2D6, and P-glycoprotein (P-gp). Clinically significant interactions have been reported with inhibitors of CYP3A4, CYP2D6, and/or P-gp (e.g., verapamil), resulting in increased concentration and clinical effect of doxorubicin. Inducers of CYP3A4 (e.g., phenobarbital, phenytoin, St. John's Wort) and P-gp inducers may decrease the concentration of doxorubicin. Avoid concurrent use of doxorubicin HCl with inhibitors and inducers of CYP3A4, CYP2D6, or P-gp.
Trastuzumab
- Concurrent use of trastuzumab and doxorubicin HCl results in an increased risk of cardiac dysfunction. Avoid concurrent administration of doxorubicin and trastuzumab. The appropriate interval for administering doxorubicin following trastuzumab therapy has not been determined
Paclitaxel
- Paclitaxel, when given prior to doxorubicin HCl, increases the plasma-concentrations of doxorubicin and its metabolites. Administer doxorubicin HCl prior to paclitaxel if used concomitantly.
Dexrazoxane
- Do not administer dexrazoxane as a cardioprotectant at the initiation of doxorubicin HCl-containing chemotherapy regimens. In a randomized trial in women with metastatic breast cancer, initiation of dexrazoxane with doxorubicin HCl-based chemotherapy resulted in a significantly lower tumor response rate (48% vs. 63%; p=0.007) and shorter time to progression than in women who received doxorubicin HCl-based chemotherapy alone.
6-Mercaptopurine
- Doxorubicin HCl may potentiate 6-mercaptopurine-induced hepatotoxicity. In 11 patients with refractory leukemia treated with 6-mercaptopurine (500 mg/m2 intravenously daily for 5 days per cycle every 2–3 weeks) and doxorubicin HCl (50 mg/m2 intravenous once per cycle every 2–3 weeks) alone or with vincristine and prednisone, all developed hepatic dysfunction manifested by elevations of total serum bilirubin, alkaline phosphatase and aspartate aminotransferase.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): D
Doxorubicin HCl can cause fetal harm when administered to a pregnant woman. Doxorubicin HCl was teratogenic and embryotoxic in rats and rabbits at doses approximately 0.07 times (based on body surface area) the recommended human dose of 60 mg/m2. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, apprise the patient of the potential hazard to a fetus.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Doxorubicin hydrochloride in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Doxorubicin hydrochloride during labor and delivery.
Nursing Mothers
Doxorubicin has been detected in the milk of at least one lactating patient. Because of the potential for serious adverse reactions in nursing infants from doxorubicin HCl, a decision should be made whether to discontinue nursing or discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Based on postmarketing reports, pediatric patients treated with doxorubicin HCl are at risk for developing late cardiovascular dysfunction. Risk factors include young age at treatment (especially < 5 years), high cumulative doses and receipt of combined modality therapy. Long-term periodic cardiovascular monitoring is recommended for all pediatric patients who have received doxorubicin HCl. Doxorubicin HCl, as a component of intensive chemotherapy regimens administered to pediatric patients, may contribute to prepubertal growth failure and may also contribute to gonadal impairment, which is usually temporary.
There are no recommended dose adjustments based on age. Doxorubicin clearance was increased in patients aged 2 years to 20 years as compared to adults, while doxorubicin clearance was similar in children less than 2 years as compared to adults.
Geriatic Use
Clinical experience in patients who were 65 years of age and older who received doxorubicin HCl-based chemotherapy regimens for metastatic breast cancer showed no overall differences in safety and effectiveness compared with younger patients.
Gender
Females
Doxorubicin HCl can cause fetal harm when administered during pregnancy. Advise female patients of reproductive potential to use highly effective contraception during treatment with doxorubicin HCl and for 6 months after treatment. Advise patients to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, while taking doxorubicin HCl.
Males
Doxorubicin HCl may damage spermatozoa and testicular tissue, resulting in possible genetic fetal abnormalities. Males with female sexual partners of reproductive potential should use effective contraception during and for 6 months after treatment.
Race
There is no FDA guidance on the use of Doxorubicin hydrochloride with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Doxorubicin hydrochloride in patients with renal impairment.
Hepatic Impairment
The clearance of doxorubicin was reduced in patients with elevated serum bilirubin levels. Reduce the dose of doxorubicin HCl in patients with serum bilirubin levels greater than 1.2 mg/dL. Doxorubicin HCl is contraindicated in patients with severe hepatic impairment (defined as Child Pugh Class C or serum bilirubin levels greater than 5 mg/dL).
Females of Reproductive Potential and Males
Females
In females of reproductive potential, doxorubicin HCl may cause infertility and result in amenorrhea. Premature menopause can occur. Recovery of menses and ovulation is related to age at treatment.
Males
Doxorubicin HCl may result in oligospermia, azoospermia, and permanent loss of fertility. Sperm counts have been reported to return to normal levels in some men. This may occur several years after the end of therapy.
Immunocompromised Patients
There is no FDA guidance one the use of Doxorubicin hydrochloride in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Doxorubicin hydrochloride Administration in the drug label.
Monitoring
There is limited information regarding Doxorubicin hydrochloride Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Doxorubicin hydrochloride and IV administrations.
Overdosage
Few cases of overdose have been described. A 58-year-old man with acute lymphoblastic leukemia received 10-fold overdose of doxorubicin HCl (300 mg/m2) in one day. He was treated with charcoal filtration, hemopoietic growth factor (G-CSF), proton pump inhibitor and antimicrobial prophylaxis. The patient suffered sinus tachycardia, grade 4 neutropenia and thrombocytopenia for 11 days, severe mucositis and sepsis. The patient recovered completely 26 days after the overdose. A 17-year-old girl with osteogenic sarcoma received 150 mg of doxorubicin HCl daily for 2 days (intended dose was 50 mg per day for 3 days). The patient developed severe mucositis on days 4–7 after the overdose and chills and pyrexia on day 7. The patient was treated with antibiotics and platelets and recovered 18 days after overdose.
Pharmacology
Mechanism of Action
The cytotoxic effect of doxorubicin HCl on malignant cells and its toxic effects on various organs are thought to be related to nucleotide base intercalation and cell membrane lipid binding activities of doxorubicin. Intercalation inhibits nucleotide replication and action of DNA and RNA polymerases. The interaction of doxorubicin with topoisomerase II to form DNA-cleavable complexes appears to be an important mechanism of doxorubicin HCl cytocidal activity.
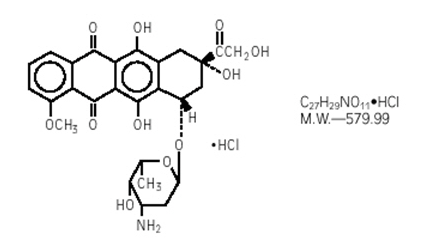
Structure
Doxorubicin Hydrochloride is a cytotoxic, anthracycline, topoisomerase II inhibitor isolated from cultures of Streptomyces peucetius var. caesius. Chemically, doxorubicin hydrochloride is: 5,12-Naphthacenedione, 10-[(3-amino-2,3,6-trideoxy-α-L-lyxo-hexopyranosyl)oxy]-7,8,9,10-tetrahydro-6,8,11-trihydroxy-8-(hydroxylacetyl)-1-methoxy-, hydrochloride (8S-cis)-. The chemical structure of doxorubicin HCl is:
Pharmacodynamics
There is limited information regarding Doxorubicin hydrochloride Pharmacodynamics in the drug label.
Pharmacokinetics
Pharmacokinetics
- Pharmacokinetic studies conducted in patients with various types of tumors have shown that doxorubicin follows multiphasic disposition after intravenous injection. The distribution half-life is approximately 5 minutes, while the terminal half-life is 20 to 48 hours. In four patients, doxorubicin demonstrated dose-independent pharmacokinetics across a dose range of 30 to 70 mg/m2.
Distribution
- Steady-state distribution volume ranges from 809 to 1214 L/m2. Binding of doxorubicin and its major metabolite, doxorubicinol, to plasma proteins is about 75% and is independent of plasma concentration of doxorubicin up to 1.1 µg/mL. Doxorubicin was measured in the milk of one lactating patient after therapy with 70 mg/m2 of doxorubicin HCl given as a 15-minute intravenous infusion. The peak milk concentration at 24 hours after treatment was 4.4-fold greater than the corresponding plasma concentration. Doxorubicin was detectable in the milk up to 72 hours. Doxorubicin does not cross the blood brain barrier.
Metabolism
- Enzymatic reduction at the 7 position and cleavage of the daunosamine sugar yields aglycones which are accompanied by free radical formation, the local production of which may contribute to the cardiotoxic activity of doxorubicin HCl. Disposition of doxorubicinol in patients is formation rate limited, with the terminal half-life of doxorubicinol being similar to doxorubicin. The relative exposure of doxorubicinol, i.e., the ratio between the AUC of doxorubicinol and the AUC of doxorubicin is approximately 0.5.
Excretion
- Plasma clearance is in the range 324 to 809 mL/min/m2 and is predominately by metabolism and biliary excretion. Approximately 40% of the dose appears in the bile in 5 days, while only 5 to 12% of the drug and its metabolites appear in the urine during the same time period. In urine, <3% of the dose was recovered as doxorubicinol over 7 days.
- Systemic clearance of doxorubicin is significantly reduced in obese women with ideal body weight greater than 130%. There was a significant reduction in clearance without any change in volume of distribution in obese patients when compared with normal patients with less than 115% ideal body weight.
Nonclinical Toxicology
- Doxorubicin HCl treatment results in an increased risk of secondary malignancies based on postmarketing reports. Doxorubicin HCl was mutagenic in the in vitro Ames assay, and clastogenic in multiple in vitro assays (CHO cell, V79 hamster cell, human lymphoblast, and SCE assays) and the in vivo mouse micronucleus assay.
- Doxorubicin HCl decreased fertility in female rats at the doses of 0.05 and 0.2 mg/kg/day (approximately 0.005 and 0.02 times the recommended human dose, based on body surface area).
- A single intravenous dose of 0.1 mg/kg doxorubicin HCl (approximately 0.01 times the recommended human dose based on body surface area) was toxic to male reproductive organs in animal studies, producing testicular atrophy, diffuse degeneration of the seminiferous tubules, and oligospermia/hypospermia in rats. Doxorubicin HCl induces DNA damage in rabbit spermatozoa and dominant lethal mutations in mice.
Clinical Studies
- The clinical efficacy of doxorubicin HCl-containing regimens for the post-operative, adjuvant treatment of surgically resected breast cancer was evaluated in a meta-analysis conducted by the Early Breast Cancer Trialists Collaborative Group (EBCTCG). The EBCTCG meta-analyses compared cyclophosphamide, methotrexate, and 5-fluorouracil (CMF) to no chemotherapy (19 trials including 7523 patients) and doxorubicin HCl-containing regimens with CMF as an active control (6 trials including 3510 patients). Data from the meta-analysis of trials comparing CMF to no therapy were used to establish the historical treatment effect size for CMF regimens. The major efficacy outcome measures were disease-free survival (DFS) and overall survival (OS).
- Of the 3510 women (2157 received doxorubicin HCl-containing regimens and 1353 received CMF treatment) with early breast cancer involving axillary lymph nodes included in the six trials from the meta-analyses, approximately 70% were premenopausal and 30% were postmenopausal. At the time of the meta-analysis, 1745 first recurrences and 1348 deaths had occurred. The analyses demonstrated that doxorubicin HCl-containing regimens retained at least 75% of the historical CMF adjuvant effect on DFS with a hazard ratio (HR) of 0.91 (95% CI, 0.82–1.01) and on OS with a HR of 0.91 (95% CI, 0.81–1.03).
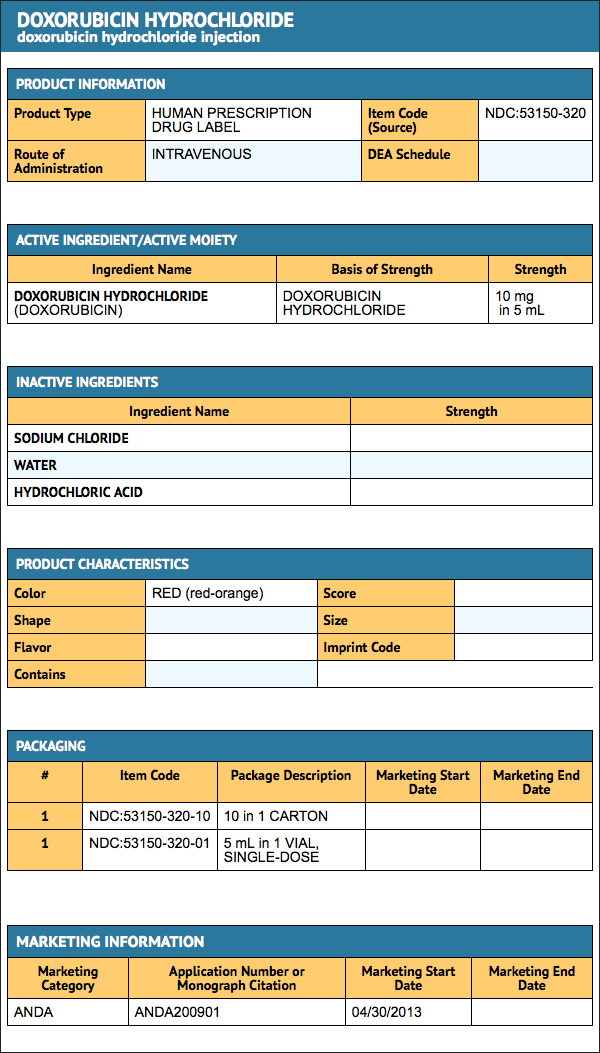
How Supplied
Doxorubicin Hydrochloride Injection, USP is a sterile parenteral, isotonic solution, available in polypropylene (CYTOSAFE)® vials in single vial packs as:
- SINGLE-DOSE VIALS:
- 10 mg/5 mL (2 mg/mL) NDC 0069-3030-20
- 20 mg/10 mL (2 mg/mL) NDC 0069-3031-20
- 50 mg/25 mL (2 mg/mL) NDC 0069-3032-20
(Retain in carton until time of use. Discard unused portion).
- MULTIPLE-DOSE VIALS:
- 150 mg/75 mL (2 mg/mL) NDC 0069-3033-20
- 200 mg/100 mL (2 mg/mL) NDC 0069-3034-20
- Retain in carton until contents are used.
Storage
Store all vials at room temperature 15° to 30°C (59° to 86°F). Protect from light.
Images
Drug Images
{{#ask: Page Name::Doxorubicin hydrochloride |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
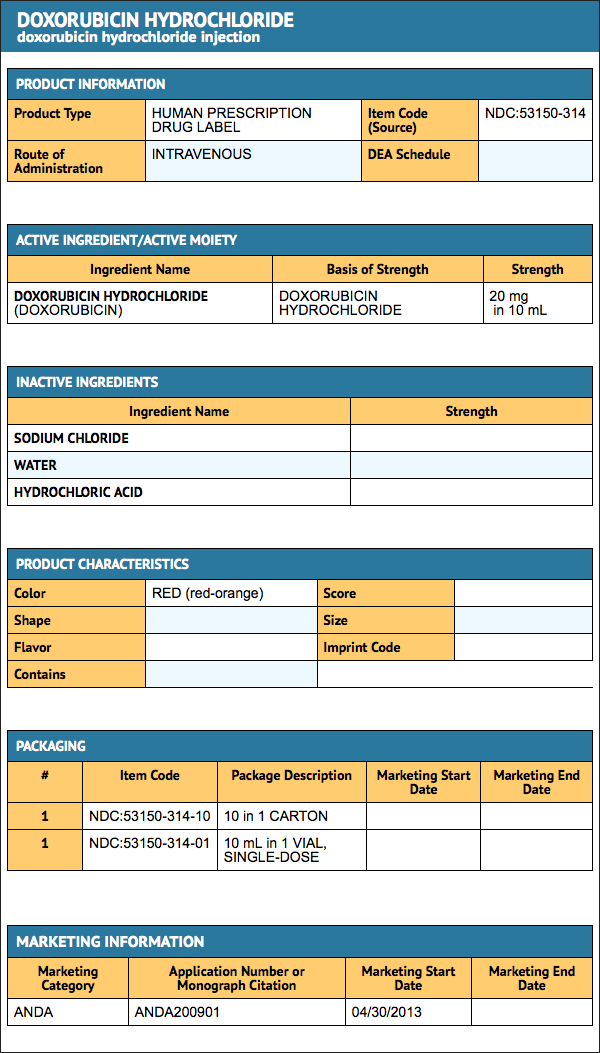
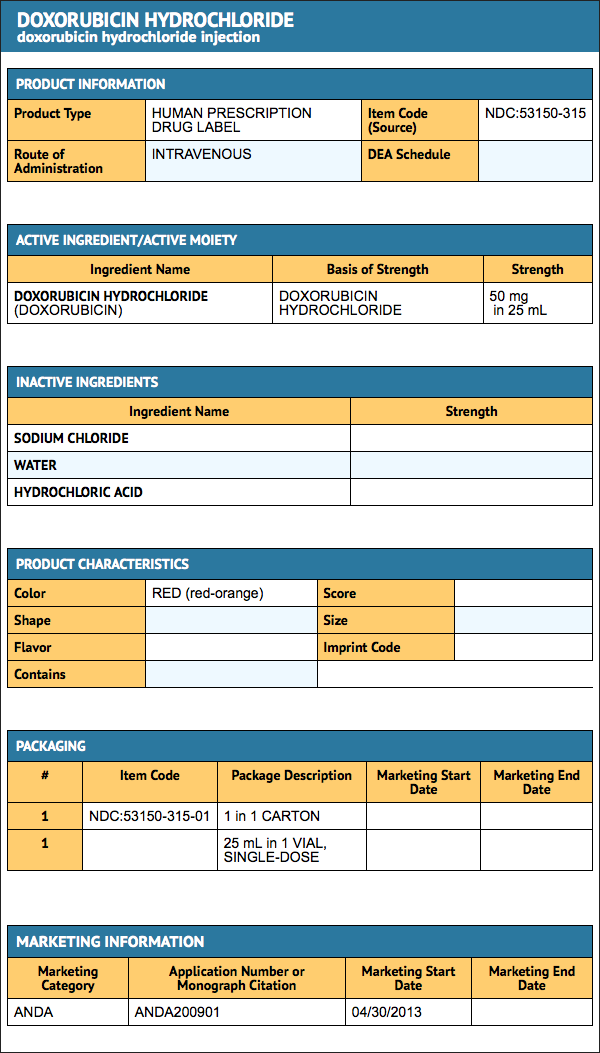
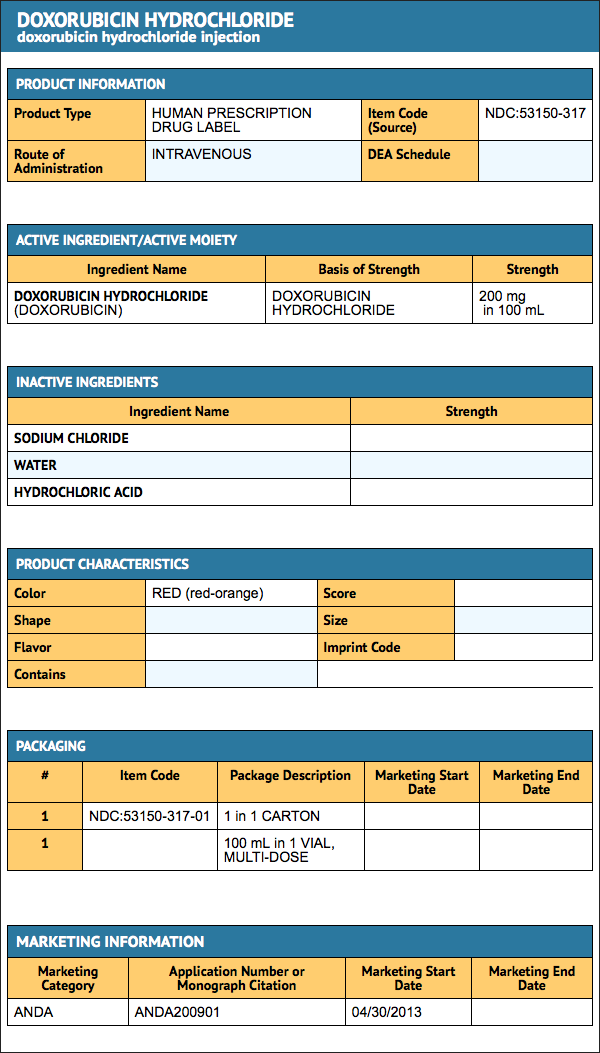
{{#ask: Label Page::Doxorubicin hydrochloride |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Inform patients of the following:
- Doxorubicin HCl can cause irreversible myocardial damage. Advise patients to contact a healthcare provider for symptoms of heart failure during or after treatment with doxorubicin HCl.
- There is an increased risk of treatment-related leukemia from doxorubicin HCl.
- Doxorubicin HCl can reduce the absolute neutrophil count resulting in an increased risk of infection. Advise patients to contact a healthcare provider for new onset fever or symptoms of infection.
- Doxorubicin HCl can cause fetal harm when administered during pregnancy. Advise females of reproductive potential to use effective contraception during treatment with doxorubicin HCl and for 6 months after treatment, and to contact their healthcare provider if they become pregnant, or if pregnancy is suspected, during treatment with doxorubicin HCl.
- Doxorubicin HCl may induce chromosomal damage in sperm, which may lead to loss of fertility and offspring with birth defects. Advise patients to use effective contraception during and for 6 months after treatment.
- Doxorubicin HCl can cause premature menopause in females and loss of fertility in males.
- Discontinue nursing while receiving doxorubicin HCl.
- Doxorubicin HCl can cause nausea, vomiting, diarrhea, mouth/oral pain and sores. Advise patients to contact a healthcare provider should they develop any severe symptoms that prevent them from eating and drinking.
- Doxorubicin HCl causes alopecia.
- Doxorubicin HCl can cause their urine to appear red for 1 to 2 days after administration.
Precautions with Alcohol
Alcohol-Doxorubicin Hydrochloride interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Doxorubicin hydrochloride Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Berruti A, Terzolo M, Sperone P, Pia A, Della Casa S, Gross DJ; et al. (2005). "Etoposide, doxorubicin and cisplatin plus mitotane in the treatment of advanced adrenocortical carcinoma: a large prospective phase II trial". Endocr Relat Cancer. 12 (3): 657–66. doi:10.1677/erc.1.01025. PMID 16172198.
- ↑ "Adjuvant Treatment in Bladder Carcinoma".
- ↑ Fleming GF, Brunetto VL, Cella D, Look KY, Reid GC, Munkarah AR; et al. (2004). "Phase III trial of doxorubicin plus cisplatin with or without paclitaxel plus filgrastim in advanced endometrial carcinoma: a Gynecologic Oncology Group Study". J Clin Oncol. 22 (11): 2159–66. doi:10.1200/JCO.2004.07.184. PMID 15169803.
- ↑ Kim ES, Putnam JB, Komaki R, Walsh GL, Ro JY, Shin HJ; et al. (2004). "Phase II study of a multidisciplinary approach with induction chemotherapy, followed by surgical resection, radiation therapy, and consolidation chemotherapy for unresectable malignant thymomas: final report". Lung Cancer. 44 (3): 369–79. doi:10.1016/j.lungcan.2003.12.010. PMID 15140551.
- ↑ Loehrer PJ, Kim K, Aisner SC, Livingston R, Einhorn LH, Johnson D; et al. (1994). "Cisplatin plus doxorubicin plus cyclophosphamide in metastatic or recurrent thymoma: final results of an intergroup trial. The Eastern Cooperative Oncology Group, Southwest Oncology Group, and Southeastern Cancer Study Group". J Clin Oncol. 12 (6): 1164–8. PMID 8201378.
- ↑ Fornasiero A, Daniele O, Ghiotto C, Piazza M, Fiore-Donati L, Calabró F; et al. (1991). "Chemotherapy for invasive thymoma. A 13-year experience". Cancer. 68 (1): 30–3. PMID 2049749.
- ↑ Lee CK, Barlogie B, Munshi N, Zangari M, Fassas A, Jacobson J; et al. (2003). "DTPACE: an effective, novel combination chemotherapy with thalidomide for previously treated patients with myeloma". J Clin Oncol. 21 (14): 2732–9. doi:10.1200/JCO.2003.01.055. PMID 12860952.
- ↑ 8.0 8.1 8.2 "(doxorubicin) dosing, indications, interactions, adverse effects, and more". Medscape Reference. WebMD. Retrieved 15 April 2014.
- ↑ Brayfield, A, ed. (19 December 2013). "Doxorubicin". Martindale: The Complete Drug Reference. Pharmaceutical Press. Retrieved 15 April 2014.
{{#subobject:
|Label Page=Doxorubicin hydrochloride |Label Name=10en5.png
}}
{{#subobject:
|Label Page=Doxorubicin hydrochloride |Label Name=20en10.png
}}
{{#subobject:
|Label Page=Doxorubicin hydrochloride |Label Name=50en25.png
}}
{{#subobject:
|Label Page=Doxorubicin hydrochloride |Label Name=150en75.png
}}
{{#subobject:
|Label Page=Doxorubicin hydrochloride |Label Name=200en100.png
}}