Diphenhydramine (Injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Adeel Jamil, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Diphenhydramine (Injection) is an analgesic and antihistamine that is FDA approved for the treatment of allergic reactions to blood or plasma, uncomplicated allergic conditions of the immediate type, in anaphylaxis as an adjunct to epinephrine, motion sickness and parkinsonism. Common adverse reactions include xerostomia, dizziness, dyskinesia, sedation, somnolence, dryness of nasal mucosa, pharyngeal dryness, thick sputum.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Diphenhydramine hydrochloride injection, USP is effective in adults and pediatric patients, other than premature infants and neonates, for the following conditions when diphenhydramine hydrochloride in the oral form is impractical.
Antihistaminic
- For amelioration of allergic reactions to blood or plasma, in anaphylaxis as an adjunct to epinephrine and other standard measures after the acute symptoms have been controlled, and for other uncomplicated allergic conditions of the immediate type when oral therapy is impossible or contraindicated.
Motion sickness
- For active treatment of motion sickness.
Antiparkinsonism
- For use in parkinsonism, when oral therapy is impossible or contraindicated, as follows: parkinsonism in the elderly who are unable to tolerate more potent agents; mild cases of parkinsonism in other age groups, and in other cases of parkinsonism in combinations with centrally acting anticholinergic agents.
Dosing Information
- THIS PRODUCT IS FOR INTRAVENOUS OR INTRAMUSCULAR ADMINISTRATION ONLY.
- Diphenhydramine hydrochloride injection, USP is indicated when the oral form is impractical.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
- DOSAGE SHOULD BE INDIVIDUALIZED ACCORDING TO THE NEEDS AND THE RESPONSE OF THE PATIENT.
8 10 mg to 50 mg intravenously at a rate generally not exceeding 25 mg/min, or deep intramuscularly, 100 mg if required; maximum daily dosage is 400 mg.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Diphenhydramine (Injection) in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diphenhydramine (Injection) in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosing Information
8 Pediatric Patients, other than premature infants and neonates 5 mg/kg/24 hr or 150 mg/m2/24 hr. Maximum daily dosage is 300 mg. Divide into four doses, administered intravenously at a rate generally not exceeding 25 mg/min, or deep intramuscularly.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Diphenhydramine (Injection) in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Diphenhydramine (Injection) in pediatric patients.
Contraindications
Use in Neonates or Premature Infants
- This drug should not be used in neonates or premature infants.
Use in Nursing Mothers
- Because of the higher risk of antihistamines for infants generally, and for neonates and prematures in particular, antihistamine therapy is contraindicated in nursing mothers.
Use as a Local Anesthetic
- Because of the risk of local necrosis, this drug should not be used as a local anesthetic.
Antihistamines are also contraindicated in the following conditions:
- Hypersensitivity to diphenhydramine hydrochloride and other antihistamines of similar chemical structure.
Warnings
- Antihistamines should be used with considerable caution in patients with narrow-angle glaucoma, stenosing peptic ulcer, pyloroduodenal obstruction, symptomatic prostatic hypertrophy, or bladder-neck obstruction.
- Local necrosis has been associated with the use of subcutaneous or intradermal use of intravenous diphenhydramine hydrochloride.
Use in Pediatric Patients
- In pediatric patients, especially, antihistamines in overdosage may cause hallucinations, convulsions, or death.
- As in adults, antihistamines may diminish mental alertness in pediatric patients. In the young pediatric patient, particularly, they may produce excitation.
Use in the Elderly (approximately 60 years or older)
- Antihistamines are more likely to cause dizziness, sedation, and hypotension in elderly patients.
PRECAUTIONS
General
- Diphenhydramine hydrochloride has an atropine-like action and, therefore, should be used with caution in patients with a history of bronchial asthma, increased intraocular pressure, hyperthyroidism, cardiovascular disease or hypertension. Use with caution in patients with lower respiratory disease including asthma.
Adverse Reactions
Clinical Trials Experience
- The most frequent adverse reactions are underscored.
General:
- Urticaria, drug rash, anaphylactic shock, photosensitivity, excessive perspiration, chills, dryness of mouth, nose, and throat
Cardiovascular System:
Hematologic System:
Nervous System:
- Sedation, sleepiness, dizziness, disturbed coordination, fatigue, confusion, restlessness, excitation, nervousness, tremor, irritability, insomnia, euphoria, paresthesia, blurred vision, diplopia, vertigo, tinnitus, acute labyrinthitis, neuritis, convulsions
GI System:
GU System:
- Urinary frequency, difficult urination, urinary retention, early menses
Respiratory System:
- Thickening of bronchial secretions, tightness of chest or throat and wheezing, nasal stuffiness
Postmarketing Experience
There is limited information regarding Diphenhydramine (Injection) Postmarketing Experience in the drug label.
Drug Interactions
- Diphenhydramine hydrochloride has additive effects with alcohol and other CNS depressants (hypnotics, sedatives, tranquilizers, etc.)
- MAO inhibitors prolong and intensify the anticholinergic (drying) effects of antihistamines.
Use in Specific Populations
Pregnancy
- Reproduction studies have been performed in rats and rabbits at doses up to 5 times the human dose and have revealed no evidence of impaired fertility or harm to the fetus due to diphenhydramine hydrochloride. There are, however, no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Diphenhydramine (Injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Diphenhydramine (Injection) during labor and delivery.
Nursing Mothers
There is no FDA guidance on the use of Diphenhydramine (Injection) in women who are nursing.
Pediatric Use
- Diphenhydramine hydrochloride should not be used in neonates and premature infants.
- Diphenhydramine hydrochloride may diminish mental alertness, or, in the young pediatric patient, cause excitation. Overdosage may cause hallucinations, convulsions, or death.
Geriatic Use
There is no FDA guidance on the use of Diphenhydramine (Injection) in geriatric settings.
Gender
There is no FDA guidance on the use of Diphenhydramine (Injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Diphenhydramine (Injection) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Diphenhydramine (Injection) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Diphenhydramine (Injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Diphenhydramine (Injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Diphenhydramine (Injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intramuscular
- Intravenous
Monitoring
There is limited information regarding Diphenhydramine (Injection) Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Diphenhydramine (Injection) and IV administrations.
Overdosage
- Antihistamine overdosage reactions may vary from central nervous system depression to stimulation. Stimulation is particularly likely in pediatric patients. Atropine-like signs and symptoms; dry mouth; fixed, dilated pupils; flushing; and gastrointestinal symptoms may also occur.
- Stimulants should not be used.
- Vasopressors may be used to treat hypotension.
Pharmacology
Mechanism of Action
- Diphenhydramine hydrochloride is an antihistamine with anticholinergic (drying) and sedative side effects. Antihistamines appear to compete with histamine for cell receptor sites on effector cells.
Structure
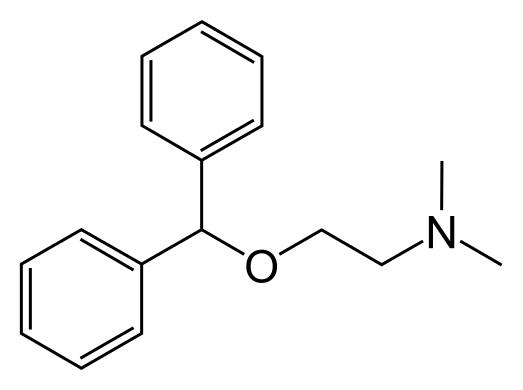
- Diphenhydramine hydrochloride is an antihistamine drug having the chemical name 2-(Diphenylmethoxy)-N, N-dimethylethylamine hydrochloride. It occurs as a white, crystalline powder, is freely soluble in water and alcohol and has a molecular weight of 291.82. The molecular formula is C17H21NO • HCl. The structural formula is as follows:

Pharmacodynamics
There is limited information regarding Diphenhydramine (Injection) Pharmacodynamics in the drug label.
Pharmacokinetics
- Diphenhydramine hydrochloride in the injectable form has a rapid onset of action. Diphenhydramine hydrochloride is widely distributed throughout the body, including the CNS. A portion of the drug is excreted unchanged in the urine, while the rest is metabolized via the liver. Detailed information on the pharmacokinetics of Diphenhydramine Hydrochloride Injection is not available.
Nonclinical Toxicology
There is limited information regarding Diphenhydramine (Injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Diphenhydramine (Injection) Clinical Studies in the drug label.
How Supplied
Diphenhydramine Hydrochloride Injection, USP is supplied as a sterile solution containing 50 mg diphenhydramine hydrochloride in each milliliter of solution with 0.1 mg/mL benzethonium chloride as a germicidal agent. Available in multi-dose 10 mL (NDC 67457-124-10) vials.
Manufactured for: Mylan Institutional LLC Rockford, IL 61103 U.S.A.
Manufactured by: Mylan Institutional Galway, Ireland
0499L104 MI:DIHYIJ:R2 REVISED JANUARY 2013
Storage
- Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.] Protect from freezing and light.
Images
Drug Images
{{#ask: Page Name::Diphenhydramine (Injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PRINCIPAL DISPLAY PANEL - 50 mg/mL
NDC 67457-124-10 10 mL
DiphenhydrAMINE Hydrochloride Injection, USP 500 mg/10 mL (50 mg/mL)
For Intravenous or Intramuscular Use
HIGH POTENCY Rx only Multi-Dose Vial
Usual Adult Dosage: 0.2 mL to 1 mL intravenously or intramuscularly, or more as required. See accompanying prescribing information.
Store at 20° to 25°C (68° to 77°F). [See USP Controlled Room Temperature.]
Protect from freezing and light.
MI:124:1C:R3
Sterile. Pyrogen-Free.
Each mL contains: Diphenhydramine Hydrochloride 50 mg, Benzethonium Chloride 0.1 mg, as a germicidal agent in Water for Injection. The pH may have been adjusted with Sodium Hydroxide and/or Hydrochloric Acid.
Manufactured for: Mylan Institutional LLC Rockford, IL 61103 U.S.A.
Manufactured by: Mylan Institutional Galway, Ireland
Mylan.com

{{#ask: Label Page::Diphenhydramine (Injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients taking diphenhydramine hydrochloride should be advised that this drug may cause drowsiness and has an additive effect with alcohol. Patients should be warned about engaging in activities requiring mental alertness such as driving a car or operating appliances, machinery, etc.
Precautions with Alcohol
Alcohol-Diphenhydramine (Injection) interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Benadryl
- Benylin Decongestant Cough
- Diphenhist
- Diphenyl
- Genahist
- Geridryl
- Nytol Quickcaps
- Nytol Quickgels
Look-Alike Drug Names
- Benadryl - Benazepril
- DiphenhydrAMINE - DimenhyDRINATE
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Paton DM, Webster DR (1985). "Clinical pharmacokinetics of H1-receptor antagonists (the antihistamines)". Clin. Pharmacokinet. 10 (6): 477–97. doi:10.2165/00003088-198510060-00002. PMID 2866055.
- ↑ "Showing Diphenhydramine (DB01075)". DrugBank. Retrieved 5 September 2009.
- ↑ 3.0 3.1 3.2 Simons KJ, Watson WT, Martin TJ, Chen XY, Simons FE (July 1990). "Diphenhydramine: pharmacokinetics and pharmacodynamics in elderly adults, young adults, and children". J. Clin. Pharmacol. 30 (7): 665–71. doi:10.1002/j.1552-4604.1990.tb01871.x. PMID 2391399.
- ↑ Garnett WR (February 1986). "Diphenhydramine". Am. Pharm. NS26 (2): 35–40. PMID 3962845.