Aztreonam (injection)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Aztreonam (injection) is a monobactam antibiotic that is FDA approved for the treatment of urinary tract infections (complicated and uncomplicated), lower respiratory tract infections, septicemia, skin and skin-structure infections, intra-abdominal infections and gynecologic infections. Aztreonam is indicated for the treatment of the following infections caused by susceptible Gram-negative microorganisms: urinary tract infections, lower respiratory tract infections, septicemia, skin infections, intra abdominal infection, and gynecologic infections. Common adverse reactions include chest discomfort, abdominal pain, vomiting, alkaline phosphatase raised, ALT/SGPT level raised, AST/SGOT level raised, serum creatinine raised, cough, nasal congestion, pain in throat, wheezing, and fever.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Aztreonam is indicated for the following infections:
Urinary Tract Infections (complicated and uncomplicated)
- Including pyelonephritis and cystitis (initial and recurrent) caused by Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Pseudomonas aeruginosa, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter species, and Serratia marcescens.
Lower Respiratory Tract Infections
- Including pneumonia and bronchitis caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Haemophilus influenzae, Proteus mirabilis, Enterobacter species, and Serratia marcescens.
Septicemia
- Caused by Escherichia coli, Klebsiella pneumoniae, Pseudomonas aeruginosa, Proteus mirabilis, Serratia marcescens, and Enterobacter species.
Skin and Skin-Structure Infections
- Including those associated with postoperative wounds, ulcers, and burns, caused by Escherichia coli, Proteus mirabilis, Serratia marcescens, Enterobacter species, Pseudomonas aeruginosa, Klebsiella pneumoniae, and Citrobacter species.
Intra-abdominal Infections
- Including peritonitis caused by Escherichia coli, Klebsiella species including K. pneumoniae, Enterobacter species including E. cloacae, Pseudomonas aeruginosa, Citrobacter species* including C. freundii, and Serratia species* including S. marcescens.
Gynecologic Infections
- Including endometritis and pelvic cellulitis caused by Escherichia coli, Klebsiella pneumoniae, Enterobacter species including E. cloacae, and Proteus mirabilis.
Aztreonam is indicated for adjunctive therapy to surgery in the management of infections caused by susceptible organisms, including abscesses, infections complicating hollow viscus perforations, cutaneous infections, and infections of serous surfaces. Aztreonam is effective against most of the commonly encountered Gram-negative aerobic pathogens seen in general surgery
Aztreonam, an intravenous solution in GALAXY plastic containers (PL 2040), is intended for intravenous use only. Dosage should be determined by susceptibility of the causative organisms, severity and site of infection, and the condition of the patient.

- Because of the serious nature of infections due to Pseudomonas aeruginosa, dosage of 2 g every six or eight hours is recommended, at least upon initiation of therapy, in systemic infections caused by this organism.
- The intravenous route is recommended for patients requiring single doses greater than 1 g or those with bacterial septicemia, localized parenchymal abscess (eg, intra-abdominal abscess), peritonitis, or other severe systemic or life-threatening infections.
- The duration of therapy depends on the severity of infection. Generally, Aztreonam should be continued for at least 48 hours after the patient becomes asymptomatic or evidence of bacterial eradication has been obtained. Persistent infections may require treatment for several weeks. Doses smaller than those indicated should not be used.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam in adult patients.
Non–Guideline-Supported Use
Bacterial Musculoeskeletal Infection
Febrile Neutropenia
- Used in combination with Vancomycin[3]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Aztreonam should be administered intravenously to pediatric patients with normal renal function. There are insufficient data regarding intramuscular administration to pediatric patients or dosing in pediatric patients with renal impairment.

Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Aztreonam in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Aztreonam in pediatric patients.
Contraindications
- This preparation is contraindicated in patients with known hypersensitivity to aztreonam or any other component in the formulation.
Warnings
Hypersensitivity Reactions
- Both animal and human data suggest that Aztreonam (aztreonam injection) is rarely cross-reactive with other beta-lactam antibiotics and weakly immunogenic. Treatment with aztreonam can result in hypersensitivity reactions in patients with or without prior exposure. Careful inquiry should be made to determine whether the patient has any history of hypersensitivity reactions to any allergens.
- While cross-reactivity of aztreonam with other beta-lactam antibiotics is rare, this drug should be administered with caution to any patient with a history of hypersensitivity to beta-lactams (eg, penicillins, cephalosporins, and/or carbapenems). Treatment with aztreonam can result in hypersensitivity reactions in patients with or without prior exposure to aztreonam. If an allergic reaction to aztreonam occurs, discontinue the drug and institute supportive treatment as appropriate (eg, maintenance of ventilation, pressor amines, antihistamines, corticosteroids). Serious hypersensitivity reactions may require epinephrine and other emergency measures.
Clostridium Difficile Associated Diarrhea
- Clostridium difficile–associated diarrhea (CDAD) has been reported with use of nearly all antibacterial agents, including Aztreonam, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial agents alters the normal flora of the colon leading to overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin-producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over 2 months after the administration of antibacterial agents.
- If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Rare cases of toxic epidermal necrolysis have been reported in association with aztreonam in patients undergoing bone marrow transplant with multiple risk factors including sepsis, radiation therapy, and other concomitantly administered drugs associated with toxic epidermal necrolysis.
Adverse Reactions
Clinical Trials Experience
- Local reactions (eg, phlebitis/thrombophlebitis; discomfort/swelling) following intravenous administration occurred at rates of approximately 1.9%.
- Systemic reactions (considered to be related to therapy or of uncertain etiology) occurring at an incidence of 1% to 1.3% include diarrhea, nausea and/or vomiting, and rash.
Reactions occurring at an incidence of less than 1% are listed within each body system in order of decreasing severity:
Hypersensitivity
Hematologic
Gastrointestinal
- Abdominal cramps
- CDAD
- Onset of pseudomembranous colitis symptoms may occur during or after antibiotic treatment.
Dermatologic
- Toxic epidermal necrolysis
- Purpura
- Erythema multiforme
- Exfoliative dermatitis
- urticaria
- Petechiae
- Pruritus
- Diaphoresis
Cardiovascular
- Hypotension
- Transient ECG changes (ventricular bigeminy and PVC)
- Flushing
Respiratory
Hepatobiliary
Nervous System
Musculoskeletal
Special Senses
Other
Body as a Whole
Pediatric Adverse Reactions
- Of the 612 pediatric patients who were treated with Aztreonam in clinical trials, less than 1% required discontinuation of therapy due to adverse events. The following systemic adverse events, regardless of drug relationship, occurred in at least 1% of treated patients in domestic clinical trials: rash (4.3%), diarrhea (1.4%), and fever (1.0%). These adverse events were comparable to those observed in adult clinical trials.
- In 343 pediatric patients receiving intravenous therapy, the following local reactions were noted: pain (12%), erythema (2.9%), induration (0.9%), and phlebitis (2.1%). In the US patient population, pain occurred in 1.5% of patients, while each of the remaining 3 local reactions had an incidence of 0.5%.
- The following laboratory adverse events, regardless of drug relationship, occurred in at least 1% of treated patients: increased eosinophils (6.3%), increased platelets (3.6%), neutropenia (3.2%), increased AST (3.8%), increased ALT (6.5%), and increased serum creatinine (5.8%).
- In US pediatric clinical trials, neutropenia (absolute neutrophil count less than 1000/mm3) occurred in 11.3% of patients (8/71) younger than 2 years receiving 30 mg/kg every 6 hours. AST and ALT elevations to greater than 3 times the upper limit of normal were noted in 15% to 20% of patients aged 2 years or above receiving 50 mg/kg every 6 hours. The increased frequency of these reported laboratory adverse events may be due to either increased severity of illness treated or higher doses of Aztreonam administered.
Postmarketing Experience
There is limited information regarding Aztreonam (injection) Postmarketing Experience in the drug label.
Drug Interactions
- Concomitant administration of probenecid or furosemide and aztreonam causes clinically insignificant increases in the serum levels of aztreonam. Single-dose intravenous pharmacokinetic studies have not shown any significant interaction between aztreonam and concomitantly administered gentamicin, nafcillin sodium, cephradine, clindamycin, or metronidazole. No reports of disulfiram-like reactions with alcohol ingestion have been noted; this is not unexpected since aztreonam does not contain a methyl-tetrazole side chain.
Use in Specific Populations
Pregnancy
- In pregnant women, aztreonam crosses the placenta and enters the fetal circulation.
- Developmental toxicity studies in pregnant rats and rabbits with daily doses of aztreonam up to 1800 and 1200 mg/kg, respectively, revealed no evidence of embryotoxicity or fetotoxicity or teratogenicity. These doses, based on body surface area, are 2.2- and 2.9-fold greater than the MRHD for adults of 8 g per day. A peri/postnatal study in rats revealed no drug-induced changes in any maternal, fetal, or neonatal parameters. The highest dose used in this study, 1800 mg/kg/day, is 2.2 times the MRHD based on body surface area.
- There are no adequate and well-controlled studies of aztreonam on human pregnancy outcomes. Because animal reproduction studies are not always predictive of human response, aztreonam should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Aztreonam (injection) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Aztreonam (injection) during labor and delivery.
Nursing Mothers
- Aztreonam is excreted in human milk in concentrations that are less than 1% of concentrations determined in simultaneously obtained maternal serum; consideration should be given to temporary discontinuation of nursing and use of formula feedings.
Pediatric Use
- The safety and effectiveness of intravenous Aztreonam have been established in the age groups 9 months to 16 years. Use of Aztreonam in these age groups is supported by evidence from adequate and well-controlled studies of Aztreonam in adults with additional efficacy, safety, and pharmacokinetic data from noncomparative clinical studies in pediatric patients. Sufficient data are not available for pediatric patients under 9 months of age or for the following treatment indications/pathogens: septicemia and skin and skin-structure infections (where the skin infection is believed or known to be due to H. influenzae type b). In pediatric patients with cystic fibrosis, higher doses of Aztreonam may be warranted.
Geriatic Use
- Renal status is a major determinant of dosage in the elderly; these patients in particular may have diminished renal function. Serum creatinine may not be an accurate determinant of renal status. Therefore, as with all antibiotics eliminated by the kidneys, estimates of creatinine clearance should be obtained and appropriate dosage modifications made if necessary.
Gender
There is no FDA guidance on the use of Aztreonam (injection) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Aztreonam (injection) with respect to specific racial populations.
Renal Impairment
- Prolonged serum levels of aztreonam may occur in patients with transient or persistent renal insufficiency. Therefore, the dosage of Aztreonam should be halved in patients with estimated creatinine clearances between 10 and 30 mL/min/1.73 m2 after an initial loading dose of 1 or 2 g.
- When only the serum creatinine concentration is available, the following formula (based on sex, weight, and age of the patient) may be used to approximate the creatinine clearance (Clcr). The serum creatinine should represent a steady state of renal function.

- In patients with severe renal failure (creatinine clearance less than 10 mL/min/1.73 m2), such as those supported by hemodialysis, the usual dose of 500 mg, 1 g, or 2 g should be given initially. The maintenance dose should be one-fourth of the usual initial dose given at the usual fixed interval of 6, 8, or 12 hours. For serious or life-threatening infections, in addition to the maintenance doses, one-eighth of the initial dose should be given after each hemodialysis session.
Hepatic Impairment
There is no FDA guidance on the use of Aztreonam (injection) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Aztreonam (injection) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Aztreonam (injection) in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Aztreonam (injection) Administration in the drug label.
Monitoring
There is limited information regarding Aztreonam (injection) Monitoring in the drug label.
IV Compatibility
Infusion of Aztreonam should be completed within a 20- to 60-minute period. The plastic container is a single-dose unit; discard any unused portion remaining in the container.
The following infusion solutions are compatible with Aztreonam (aztreonam injection) in GALAXY plastic container (PL 2040):
- Sodium Chloride Injection, USP, 0.9%
- Ringer’s Injection, USP
- Lactated Ringer’s Injection, USP
- Dextrose Injection, USP, 5% or 10%
- Dextrose and Sodium Chloride Injection, USP, 5%:0.9%, 5%:0.45%, or 5%:0.2%
- Sodium Lactate Injection, USP (M/6 Sodium Lactate)
- Ionosol ® B and 5% Dextrose
- Isolyte ® E
- Isolyte ® E with 5% Dextrose
- Isolyte ® M with 5% Dextrose
- Normosol ®-R
- Normosol ®-R and 5% Dextrose
- Normosol ®-M and 5% Dextrose
- Mannitol Injection, USP, 5% or 10%
- Lactated Ringer’s and 5% Dextrose Injection
- Plasma-Lyte M and 5% Dextrose
Overdosage
- If necessary, aztreonam may be cleared from the serum by hemodialysis and/or peritoneal dialysis.
Pharmacology
Mechanism of Action
- Aztreonam is a bactericidal agent that acts by inhibition of bacterial cell wall synthesis. Aztreonam has activity in the presence of some beta-lactamases, both penicillinases and cephalosporinases, of Gram-negative and Gram-positive bacteria.
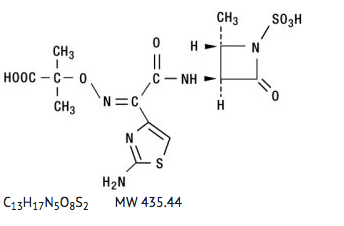
Structure
- Aztreonam is designated chemically as (Z)-2-[(2-amino-4-thiazolyl)(2S,3S)-2-methyl-4-oxo-1-sulfo-3-azetidinyl]carbamoyl]methylene]amino]oxy]-2-methylpropionic acid. Structural formula:
Pharmacodynamics
There is limited information regarding Aztreonam (injection) Pharmacodynamics in the drug label.
Pharmacokinetics
Single 30-minute intravenous infusions of 500 mg, 1 g, and 2 g doses of Aztreonam in healthy subjects produced aztreonam peak serum levels of 54 mcg/mL, 90 mcg/mL, and 204 mcg/mL, respectively, immediately after administration; at 8 hours, serum levels were 1 mcg/mL, 3 mcg/mL, and 6 mcg/mL, respectively (Figure 1). Single 3-minute intravenous injections of the same doses resulted in serum levels of 58 mcg/mL, 125 mcg/mL, and 242 mcg/mL at 5 minutes following completion of injection.
Serum concentrations of aztreonam following completion of single intravenous infusions of 500 mg, 1 g, and 2 g doses are depicted in Figure 1.

- The serum levels of aztreonam following single 500 mg, 1 g, or 2 g intravenous doses of Aztreonam exceed the MIC90 for Neisseria sp., Haemophilus influenzae, and most genera of the Enterobacteriaceae for 8 hours (for Enterobacter sp., the 8-hour serum levels exceed the MIC for 80% of strains). For Pseudomonas aeruginosa, a single 2 g intravenous dose produces serum levels that exceed the MIC90 for approximately 4 to 6 hours. All of the above doses of Aztreonam result in average urine levels of aztreonam that exceed the MIC90 for the same pathogens for up to 12 hours.
- When aztreonam pharmacokinetics were assessed for adult and pediatric patients, they were found to be comparable (down to 9 months old). The serum half-life of aztreonam averaged 1.7 hours (1.5-2.0) in subjects with normal renal function, independent of the dose. In healthy subjects, based on a 70 kg person, the serum clearance was 91 mL/min and renal clearance was 56 mL/min; the apparent mean volume of distribution at steady-state averaged 12.6 liters, approximately equivalent to extracellular fluid volume.
- In elderly patients, the mean serum half-life of aztreonam increased and the renal clearance decreased, consistent with the age-related decrease in creatinine clearance.1-4 The dosage of Aztreonam should be adjusted accordingly. In patients with impaired renal function, the serum half-life of aztreonam is prolonged. The serum half-life of aztreonam is only slightly prolonged in patients with hepatic impairment since the liver is a minor pathway of excretion.
- Average urine concentrations of aztreonam were approximately 1100 mcg/mL, 3500 mcg/mL, and 6600 mcg/mL within the first 2 hours following single 500 mg, 1 g, and 2 g intravenous doses of Aztreonam (30-minute infusions), respectively. The range of average concentrations for aztreonam in the 8- to 12-hour urine specimens in these studies was 25 to 120 mcg/mL. In healthy subjects, aztreonam is excreted in the urine about equally by active tubular secretion and glomerular filtration. Approximately 60% to 70% of an intravenous dose was recovered in the urine by 8 hours. Urinary excretion of a single intravenous dose was essentially complete by 12 hours after injection. About 12% of a single intravenous radiolabeled dose was recovered in the feces. Unchanged aztreonam and the inactive beta-lactam ring hydrolysis product of aztreonam were present in feces and urine.
- Intravenous administration of a single 500 mg or 1 g dose of Aztreonam every 8 hours for 7 days to healthy subjects produced no apparent accumulation of aztreonam or modification of its disposition characteristics; serum protein binding averaged 56% and was independent of dose.
- Renal function was monitored in healthy subjects given aztreonam; standard tests (serum creatinine, creatinine clearance, BUN, urinalysis, and total urinary protein excretion) as well as special tests (excretion of N-acetyl-β-glucosaminidase, alanine aminopeptidase, and β2-microglobulin) were used. No abnormal results were obtained.
Aztreonam achieves measurable concentrations in the following body fluids and tissues:
- The concentration of aztreonam in saliva at 30 minutes after a single 1 g intravenous dose (9 patients) was 0.2 mcg/mL; in human milk at 2 hours after a single 1 g intravenous dose (6 patients), 0.2 mcg/mL; in amniotic fluid at 6 to 8 hours after a single 1 g intravenous dose (5 patients), 2 mcg/mL. The concentration of aztreonam in peritoneal fluid obtained 1 to 6 hours after multiple 2 g intravenous doses ranged between 12 mcg/mL and 90 mcg/mL in 7 of 8 patients studied.
- Aztreonam given intravenously rapidly reaches therapeutic concentrations in peritoneal dialysis fluid; conversely, aztreonam given intraperitoneally in dialysis fluid rapidly produces therapeutic serum levels.
- Concomitant administration of probenecid or furosemide and aztreonam causes clinically insignificant increases in the serum levels of aztreonam. Single-dose intravenous pharmacokinetic studies have not shown any significant interaction between aztreonam and concomitantly administered gentamicin, nafcillin sodium, cephradine, clindamycin, or metronidazole. No reports of disulfiram-like reactions with alcohol ingestion have been noted; this is not unexpected since aztreonam does not contain a methyl-tetrazole side chain.
Nonclinical Toxicology
There is limited information regarding Aztreonam (injection) Nonclinical Toxicology in the drug label.
Clinical Studies
There is limited information regarding Aztreonam (injection) Clinical Studies in the drug label.
How Supplied
- Aztreonam® (aztreonam injection) in GALAXY plastic container (PL 2040) is supplied as a frozen, 50 mL single-dose intravenous solution as follows:

Storage
- Store at or below –20°C (–4°F)
Images
Drug Images
{{#ask: Page Name::Aztreonam (injection) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel

{{#ask: Label Page::Aztreonam (injection) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Aztreonam (injection) Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Aztreonam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Aztreonam (injection) Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Simons WJ, Lee TJ (1985). "Aztreonam in the treatment of bone and joint infections caused by gram-negative bacilli". Rev Infect Dis. 7 Suppl 4: S783–8. PMID 2934786.
- ↑ Conrad DA, Williams RR, Couchman TL, Lentnek AL (1991). "Efficacy of aztreonam in the treatment of skeletal infections due to Pseudomonas aeruginosa". Rev Infect Dis. 13 Suppl 7: S634–9. PMID 2068473.
- ↑ "Clinical Practice Guideline for the Use of Antimicrobial Agents in Neutropenic Patients with Cancer: 2010 Update by the Infectious Diseases Society of America".
{{#subobject:
|Label Page=Aztreonam (injection) |Label Name=Aztreonam 1g.png
}}
{{#subobject:
|Label Page=Aztreonam (injection) |Label Name=Aztreonam 2g.png
}}