Alglucosidase alfa
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sree Teja Yelamanchili, MBBS [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: RISK OF ANAPHYLAXIS, HYPERSENSITIVITY AND IMMUNE-MEDIATED REACTIONS, AND RISK OF CARDIORESPIRATORY FAILURE
See full prescribing information for complete Boxed Warning.
* Life-threatening anaphylactic reactions and severe hypersensitivity reactions, presenting as respiratory distress, hypoxia, apnea, dyspnea, bradycardia, tachycardia, bronchospasm, throat tightness, hypotension, angioedema (including tongue or lip swelling, periorbital edema, and face edema), and urticaria, have occurred in some patients during and after alglucosidase alfa infusions.
|
Overview
Alglucosidase alfa is an enzyme replacement therapy that is FDA approved for the treatment of Lysosomal alpha-1,4-glucosidase deficiency - infantile onset and lysosomal alpha-1,4-glucosidase deficiency - juvenile onset.. There is a Black Box Warning for this drug as shown here. Common adverse reactions include hypertension, chest discomfort, tachycardia, flushing, hyperhidrosis, rash, urticaria, constipation, diarrhea, vomiting, anemia, IgG antibody development, muscle twitch, tremor, cough, tachypnea, infusion reactions, and fever..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding FDA-labeled indications and dosing information of Alglucosidase alfa in adult patients.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alglucosidase alfa in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Alglucosidase alfa in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
Hydrolytic lysosomal glycogen-specific enzyme indicated for patients with Pompe disease (acid α-glucosidase (GAA) deficiency)
Dosage
Recommended Dose
- The recommended dosage of alglucosidase alfa is 20 mg/kg body weight administered every 2 weeks as an intravenous infusion.
- For injection: 50 mg of alglucosidase alfa as lyophilized powder in a single-use vial for reconstitution
Dosage form and Strengths
For injection: 50 mg of alglucosidase alfa is supplied as a sterile, nonpyrogenic, white to off-white, lyophilized cake or powder in a single-use vial for reconstitution. After reconstitution, the resultant solution concentration is 5 mg/mL.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Alglucosidase alfa in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Alglucosidase alfa in pediatric patients.
Contraindications
None.
Warnings
|
WARNING: RISK OF ANAPHYLAXIS, HYPERSENSITIVITY AND IMMUNE-MEDIATED REACTIONS, AND RISK OF CARDIORESPIRATORY FAILURE
See full prescribing information for complete Boxed Warning.
* Life-threatening anaphylactic reactions and severe hypersensitivity reactions, presenting as respiratory distress, hypoxia, apnea, dyspnea, bradycardia, tachycardia, bronchospasm, throat tightness, hypotension, angioedema (including tongue or lip swelling, periorbital edema, and face edema), and urticaria, have occurred in some patients during and after alglucosidase alfa infusions.
|
Anaphylaxis and Hypersensitivity Reactions
- Anaphylaxis and hypersensitivity reactions have been observed in patients during and up to 3 hours after alglucosidase alfa infusion.
- Some of the reactions were life-threatening and included anaphylactic shock, cardiac arrest, respiratory arrest, respiratory distress, hypoxia, apnea, dyspnea, bradycardia, tachycardia, bronchospasm, throat tightness, hypotension, angioedema (including tongue or lip swelling, periorbital edema, and face edema), and urticaria.
- Other accompanying reactions included chest discomfort/pain, wheezing, tachypnea, cyanosis, decreased oxygen saturation, convulsions, pruritus, rash, hyperhidrosis, nausea, dizziness, hypertension/increased blood pressure, flushing/feeling hot, erythema, pyrexia, pallor, peripheral coldness, restlessness, nervousness, headache, back pain, and paresthesia.
- Some of these reactions were IgE-mediated.
- If anaphylaxis or severe hypersensitivity reactions occur, immediately discontinue administration of alglucosidase alfa, and initiate appropriate medical treatment.
- Severe reactions are generally managed with infusion interruption, administration of antihistamines, corticosteroids, intravenous fluids, and/or oxygen, when clinically indicated. In some cases of anaphylaxis, epinephrine has been administered.
- Appropriate medical support, including cardiopulmonary resuscitation equipment, should be readily available when alglucosidase alfa is administered.
- The risks and benefits of re-administering alglucosidase alfa following an anaphylactic or hypersensitivity reaction should be considered.
- Some patients have been rechallenged and have continued to receive alglucosidase alfa under close clinical supervision. Extreme care should be exercised, with appropriate resuscitation measures available, if the decision is made to re-administer the product.
Immune-Mediated Reactions
- Immune-mediated cutaneous reactions have been reported with alglucosidase alfa including necrotizing skin lesions.
- Systemic immune-mediated reactions, including possible type III immune-mediated reactions have been observed with alglucosidase alfa. These reactions occurred several weeks to 3 years after initiation of alglucosidase alfa infusions.
- Skin biopsy in one patient demonstrated deposition of anti-rhGAA antibodies in the lesion.
- Another patient developed severe inflammatory arthropathy in association with pyrexia and elevated erythrocyte sedimentation rate.
- Nephrotic syndrome secondary to membranous glomerulonephritis was observed in some Pompe disease patients treated with alglucosidase alfa who had persistently positive anti-rhGAA IgG antibody titers.
- In these patients, renal biopsy was consistent with immune complex deposition.
- Patients improved following treatment interruption. Therefore, patients receiving alglucosidase alfa should undergo periodic urinalysis.
- Patients should be monitored for the development of systemic immune-mediated reactions involving skin and other organs while receiving alglucosidase alfa.
- If immune-mediated reactions occur, consider discontinuation of the administration of alglucosidase alfa, and initiate appropriate medical treatment.
- The risks and benefits of re-administering alglucosidase alfa following an immune-mediated reaction should be considered. Some patients have been able to be rechallenged and have continued to receive alglucosidase alfa under close clinical supervision.
Risk of Acute Cardiorespiratory Failure
- Patients with acute underlying respiratory illness or compromised cardiac and/or respiratory function may be at risk of serious exacerbation of their cardiac or respiratory compromise during infusions.
- Appropriate medical support and monitoring measures should be readily available during alglucosidase alfa infusion, and some patients may require prolonged observation times that should be individualized based on the needs of the patient.
- Acute cardiorespiratory failure has been observed in infantile-onset Pompe disease patients with underlying cardiac hypertrophy, possibly associated with fluid overload with intravenous administration of alglucosidase alfa.
Risk of Cardiac Arrhythmia and Sudden Cardiac Death During General Anesthesia for Central Venous Catheter Placement
- Administration of general anesthesia can be complicated by the presence of severe cardiac and skeletal (including respiratory) muscle weakness. Therefore, caution should be used when administering general anesthesia.
- Ventricular arrhythmias and bradycardia, resulting in cardiac arrest or death, or requiring cardiac resuscitation or defibrillation have been observed in infantile-onset Pompe disease patients with cardiac hypertrophy during general anesthesia for central venous catheter placement.
Risk of Antibody Development
- As with all therapeutic proteins, there is potential for immunogenicity.
- In clinical studies, the majority of patients developed IgG antibodies to alglucosidase alfa, typically within 3 months of treatment.
- There is evidence to suggest that some patients who develop high and sustained IgG antibody titers may experience reduced clinical efficacy to alglucosidase alfa treatment, such as loss of motor function, ventilator dependence, or death. The effect of antibody development on the long term efficacy of alglucosidase alfa is not fully understood.
- Patients should be monitored for IgG antibody formation every 3 months for 2 years and then annually thereafter.
- Testing for IgG titers may also be considered if patients develop hypersensitivity reactions, other immune-mediated reactions, or lose clinical response.
- Patients who experience reduced clinical response may also be tested for inhibitory antibody activity.
- Patients who experience anaphylactic or hypersensitivity reactions may also be tested for IgE antibodies to alglucosidase alfa and other mediators of anaphylaxis.
- There are currently no marketed tests for antibodies against alglucosidase alfa; however, a testing service is provided by Genzyme.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in clinical practice.
The following serious adverse reactions are described below and elsewhere in the labeling:
- Anaphylaxis and hypersensitivity reactions
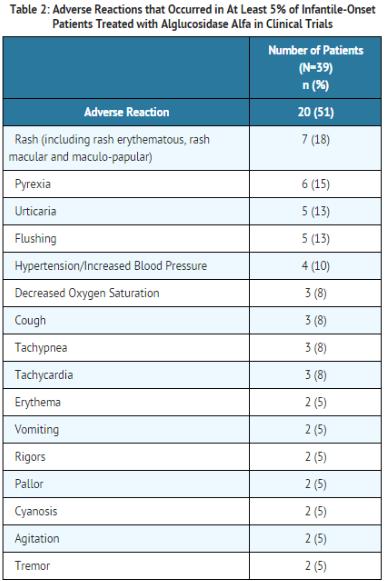
- In clinical trials, the most common adverse reactions (≥ 5%) following alglucosidase alfa treatment were hypersensitivity reactions, and included anaphylaxis, rash, pyrexia, flushing/feeling hot, urticaria, headache, hyperhidrosis, nausea, cough, decreased oxygen saturation, tachycardia, tachypnea, chest discomfort, dizziness, muscle twitching, agitation, cyanosis, erythema, hypertension/increased blood pressure, pallor, rigors, tremor, vomiting, fatigue, and myalgia.
Clinical Trials in Infantile-Onset and Juvenile-Onset Pompe Disease
- Two multicenter, open-label clinical trials were conducted in 39 infantile-onset Pompe disease patients, ages 1 month to 3.5 years old.
- Approximately half of the patients (54%) were male.
- Patients were treated with alglucosidase alfa 20 or 40 mg/kg every other week for periods ranging from 1 to 106 weeks (mean: 61 weeks).
- The most serious adverse reactions reported with alglucosidase alfa treatment included anaphylaxis and acute cardiorespiratory failure.
- The most common adverse reactions requiring intervention in clinical trials were hypersensitivity reactions, occurring in 20 of 39 (51%) patients treated with alglucosidase alfa, and included rash, pyrexia, urticaria, flushing, decreased oxygen saturation, cough, tachypnea, tachycardia, hypertension/increased blood pressure, pallor, rigors, vomiting, cyanosis, agitation, and tremor.
- These reactions were more likely to occur with higher infusion rates.
- Some patients who were pre-treated with antihistamines, antipyretics and/or corticosteroids still experienced hypersensitivity reactions.
- An open-label, single-center trial was conducted in 18 treatment-naïve infantile-onset Pompe disease patients who were treated exclusively with alglucosidase alfa.
- Adverse reactions observed in these patients were similar to infantile-onset Pompe disease patients who received alglucosidase alfa in other clinical trials.
- Additional hypersensitivity reactions observed in infantile-onset Pompe disease patients treated in other clinical trials and expanded access programs with alglucosidase alfa included livedo reticularis, irritability, retching, increased lacrimation, ventricular extrasystoles, nodal rhythm, rales, respiratory tract irritation, and cold sweat.
- Safety was also evaluated in 99 patients (51 male, 48 females) with Pompe disease in an ongoing, open-label, prospective study in patients 12 months of age and older who were previously treated with the 160 L scale of alglucosidase alfa and switched to the 4000 L scale of alglucosidase alfa.
- Patients were aged 1 to 18 years with a median duration of treatment of 437 days (range 13 to 466 days).
- No new safety findings were observed following the switch to 4000 L scale of alglucosidase alfa.
Clinical Trials in Late-Onset Pompe Disease
- Assessment of adverse reactions in patients with late-onset Pompe disease is based on the exposure of 90 patients (45 male, 45 female), aged 10 to 70 years, to 20 mg/kg alglucosidase alfa or placebo in a randomized, double-blind, placebo-controlled trial.
- The youngest alglucosidase alfa-treated patient was 16 years of age, and the youngest placebo-treated patient was 10 years of age.
- All patients were naïve to enzyme replacement therapy.
- Patients were randomized in a 2:1 ratio and received alglucosidase alfa or placebo every other week for 78 weeks (18 months).
- The study population included 34 males and 26 females (n=60) in the alglucosidase alfa group and 11 males and 19 females (n=30) in the placebo group.
- Two patients receiving alglucosidase alfa discontinued the trial due to anaphylactic reactions.
- Serious adverse reactions reported with alglucosidase alfa included anaphylaxis, which presented as angioedema, throat tightness and chest pain/discomfort.
- One patient with a history of Wolff-Parkinson-White syndrome experienced a serious adverse reaction of supraventricular tachycardia.
- The most common adverse reactions (≥ 3%; 2 or more patients) observed in alglucosidase alfa-treated patients were hypersensitivity reactions and included anaphylaxis, headache, nausea, urticaria, dizziness, chest discomfort, vomiting, hyperhidrosis, flushing/feeling hot, increased blood pressure, paresthesia, pyrexia, local swelling, diarrhea, pruritus, rash, and throat tightness.
- Delayed-onset reactions, defined as adverse reactions occurring 2 - 48 hours after completion of alglucosidase alfa infusion, that were observed in ≥ 3% more patients in the alglucosidase alfa-treated group compared to patients in the placebo-treated group in the controlled trial, included hyperhidrosis.
- Additional delayed-onset reactions occurring in alglucosidase alfa-treated patients included fatigue, myalgia, and nausea.
- Patients should be counseled about the possibility of delayed-onset hypersensitivity reactions and given proper follow-up instructions.

- In clinical trials, anaphylaxis and hypersensitivity reactions were managed with infusion interruption, decreased infusion rate, administration of antihistamines, corticosteroids, intravenous fluids, and/or oxygen, when clinically indicated.
- In some cases of anaphylactic reactions, epinephrine was administered.
- Patients who have experienced anaphylaxis or hypersensitivity reactions should be treated with caution when they are re-administered alglucosidase alfa.
Immunogenicity
- As with all therapeutic proteins, there is potential for immunogenicity.
- The data reflect the percentage of patients whose test results were considered positive for antibodies to alglucosidase alfa using an enzyme-linked immunosorbent assay (ELISA) and confirmed by a radioimmunoprecipitation (RIP) assay for alglucosidase alfa-specific IgG antibodies.
- In the two clinical trials in infantile-onset patients, the majority of patients (34 of 38; 89%) tested positive for IgG antibodies to alglucosidase alfa.
- There is evidence to suggest that some patients who develop high sustained titers of anti-alglucosidase alfa antibodies may experience reduced clinical efficacy to alglucosidase alfa treatment.
- Some IgG-positive patients in clinical trials who were retrospectively evaluated for the presence of inhibitory antibodies tested positive for inhibition of enzyme activity and/or uptake in in vitro assays.
- Furthermore, CRIM-negative infants have shown reduced clinical effect in the presence of high sustained IgG antibody titers with inhibitory activity.
- Alglucosidase alfa-treated patients who experience a decrease in motor function should be tested for the presence of inhibitory antibodies that neutralize enzyme uptake or activity.
- In the randomized, double-blind, placebo-controlled trial in late-onset patients, all alglucosidase alfa-treated patients with available samples (N=59, 100%) developed IgG antibodies to alglucosidase alfa.
- Most patients who developed IgG antibodies did so within the first 3 months of exposure (median time to seroconversion was 4 weeks).
- There was no apparent association between mean or peak IgG antibody titers and the occurrence of adverse reactions.
- None of the 59 evaluable patients tested positive for inhibition of enzyme activity.
- Antibody titers for cellular uptake inhibition were present in 18 of 59 (31%) patients by Week 78.
- All other patients tested negative for inhibition of cellular uptake.
- Patients who tested positive for uptake inhibition tended to have higher IgG titers than patients who tested negative for uptake inhibition.
- Among the 32 patients with evaluable pharmacokinetic (PK) samples, 5 patients tested positive for uptake inhibition.
- The clinical relevance of this in vitro inhibition is not fully understood.
- The clearance values for 4 of these 5 patients were approximately 1.2- to 1.8-fold greater in the presence of inhibitory antibodies (Week 52) as compared to in the absence of inhibitory antibodies (Week 0).
- Some patients in the clinical studies or in the postmarketing setting have undergone testing for alglucosidase alfa-specific IgE antibodies. Testing was performed in patients who experienced moderate to severe or recurrent hypersensitivity reactions, for which mast-cell activation was suspected.
- Some of the patients who tested positive for alglucosidase alfa-specific IgE antibodies experienced anaphylactic reactions.
- Some patients who tested positive for alglucosidase alfa-specific IgE antibodies and experienced hypersensitivity reactions were able to be rechallenged with alglucosidase alfa using a slower infusion rate at lower starting doses and have continued to receive treatment under close clinical supervision.
- Since patients who develop IgE antibodies to alglucosidase alfa appear to be at a higher risk for developing anaphylaxis and hypersensitivity reactions, these patients should be monitored more closely during administration of alglucosidase alfa.
- The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease.
- For these reasons, comparison of the incidence of antibodies to alglucosidase alfa with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
- The following adverse reactions have been identified during post approval use of alglucosidase alfa. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- In postmarketing experience with alglucosidase alfa, serious adverse reactions have been reported, including anaphylaxis.
- Acute cardiorespiratory failure, possibly associated with fluid overload, has been reported in infantile-onset Pompe disease patients with pre-existing hypertrophic cardiomyopathy.
- Recurrent reactions consisting of flu-like illness or a combination of events such as pyrexia, chills, myalgia, arthralgia, pain, or fatigue occurring after completion of infusions and lasting usually for 1 - 3 days have been observed in some patients treated with alglucosidase alfa.
- The majority of patients were able to be rechallenged with alglucosidase alfa using lower doses and/or pretreatment with anti-inflammatory drugs and/or corticosteroids and were able to continue treatment under close clinical supervision.
- In addition to the hypersensitivity reactions reported in clinical trials, the following hypersensitivity reactions have been reported in at least 2 patients and included: anaphylactic shock, respiratory failure, respiratory arrest, cardiac arrest, hypoxia, dyspnea, wheezing, convulsions, peripheral coldness, restlessness, nervousness, back pain, stridor, pharyngeal edema, abdominal pain, apnea, muscle spasm, and conjunctivitis.
- In addition, one case of hyperparathyroidism has been reported.
- Systemic and cutaneous immune-mediated reactions, including proteinuria and nephrotic syndrome secondary to membranous glomerulonephritis, and necrotizing skin lesions have been reported in postmarketing safety experience with alglucosidase alfa.
Drug Interactions
No drug interaction or in vitro metabolism studies were performed.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There is a registry for Pompe disease patients that monitors the outcomes of women and their offspring exposed to alglucosidase alfa during pregnancy.
Risk Summary
- There are no studies of alglucosidase alfa in pregnant women.
- In animal reproduction studies, no effects on embryo-fetal development were observed in mice or rabbits given daily administration of alglucosidase alfa up to 0.4 or 0.5 times the human steady-state AUC (area under the plasma concentration-time curve), respectively, at the recommended human bi-weekly dose during the period of organogenesis.
- An increase in pup mortality was observed when alglucosidase alfa was administered every other day in mice during the period of organogenesis through lactation at a dose 0.4 times the human steady-state AUC at the recommended human bi-weekly dose.
- Alglucosidase alfa should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Animal Data
- All reproductive studies included pre-treatment with diphenhydramine to prevent or minimize hypersensitivity reactions.
- The effects of alglucosidase alfa were evaluated based on comparison to a control group treated with diphenhydramine alone.
- Daily intravenous (IV) administration of alglucosidase alfa up to 40 mg/kg in mice and rabbits (0.4 and 0.5 times the human steady-state AUC, respectively, at the recommended bi-weekly dose) during the period of organogenesis had no effects on embryo-fetal development.
- Administration of 40 mg/kg IV every other day in mice (0.4 times the human steady-state AUC at the recommended bi-weekly dose) during the period of organogenesis through lactation produced an increase in mortality of offspring during the lactation period.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Alglucosidase alfa in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Alglucosidase alfa during labor and delivery.
Nursing Mothers
- Alglucosidase alfa is present in human milk.
- In one case report, the enzymatic activity of alglucosidase alfa was detected in the breast milk of a lactating woman up to 24 hours after the end of intravenous alglucosidase alfa administration.
- To minimize infant exposure to alglucosidase alfa, a nursing mother may temporarily pump and discard breast milk produced during the 24 hours after administration of alglucosidase alfa.
- Exercise caution when administering alglucosidase alfa to a nursing mother.
Pediatric Use
- The safety and effectiveness of alglucosidase alfa have been established in pediatric patients with Pompe disease.
- The safety and effectiveness of alglucosidase alfa were assessed in 57 treatment-naïve infantile-onset Pompe disease patients, aged 0.2 month to 3.5 years at first infusion, in three separate clinical trials.
- The safety and effectiveness of alglucosidase alfa were assessed in pediatric patients with late (non-infantile) onset Pompe disease in a randomized, double-blind, placebo-controlled study in 90 patients, including 2 patients 16 years of age or less.
- Anaphylaxis, hypersensitivity reactions, and acute cardiorespiratory failure have occurred in pediatric patients. Additionally, cardiac arrhythmia and sudden cardiac death have occurred in pediatric patients during general anesthesia for central venous catheter placement.
Geriatic Use
The randomized, double-blind, placebo-controlled study of alglucosidase alfa did not include sufficient numbers (n=4) of patients aged 65 years and over to determine whether they respond differently from younger patients.
Gender
There is no FDA guidance on the use of Alglucosidase alfa with respect to specific gender populations.
Race
There is no FDA guidance on the use of Alglucosidase alfa with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Alglucosidase alfa in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Alglucosidase alfa in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Alglucosidase alfa in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Alglucosidase alfa in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Instructions for Use
- Alglucosidase alfa does not contain any preservatives. Vials are single-use only. Discard any unused product.
- The total volume of infusion is determined by the patient's body weight and should be administered over approximately 4 hours.
- Infusions should be administered in a step-wise manner using an infusion pump.
- The initial infusion rate should be no more than 1 mg/kg/hr.
- The infusion rate may be increased by 2 mg/kg/hr every 30 minutes, after patient tolerance to the infusion rate is established, until a maximum rate of 7 mg/kg/hr is reached.
- Vital signs should be obtained at the end of each step.
- If the patient is stable, alglucosidase alfa may be administered at the maximum rate of 7 mg/kg/hr until the infusion is completed.
- The infusion rate may be slowed or temporarily stopped in the event of mild to moderate hypersensitivity reactions.
- In the event of anaphylaxis or severe hypersensitivity reaction, immediately discontinue administration of alglucosidase alfa, and initiate appropriate medical treatment.

Reconstitution, Dilution, and Administration
- Alglucosidase alfa should be reconstituted, diluted and administered by a healthcare professional.
- Use aseptic technique during preparation. Do not use filter needles during preparation.
- Determine the number of vials to be reconstituted based on the individual patient's weight and the recommended dose of 20 mg/kg.
- Patient weight (kg) × dose (mg/kg) = patient dose (in mg)
- Patient dose (in mg) divided by 50 mg/vial = number of vials to reconstitute. If the number of vials includes a fraction, round up to the next whole number.
- Example: Patient weight (68 kg) × dose (20 mg/kg) = patient dose (1,360 mg)
- 1,360 mg divided by 50 mg/vial = 27.2 vials; therefore, 28 vials should be reconstituted.
- Remove the required number of vials from the refrigerator and allow them to reach room temperature prior to reconstitution (approximately 30 minutes).
- Reconstitute each alglucosidase alfa vial by slowly injecting 10.3 mL of sterile water for Injection, USP to the inside wall of each vial. Each vial will yield a concentration of 5 mg/mL.
- The total extractable dose per vial is 50 mg per 10 mL.
- Avoid forceful impact of the water for injection on the powder and avoid foaming.
- This is done by slow drop-wise addition of the water for injection down the inside of the vial and not directly onto the lyophilized cake.
- Tilt and roll each vial gently. Do not invert, swirl, or shake.
- The reconstituted alglucosidase alfa solution should be protected from light.
- Perform an immediate visual inspection on the reconstituted vials for particulate matter and discoloration.
- If upon immediate inspection opaque particles are observed or if the solution is discolored do not use.
- The reconstituted solution may occasionally contain some alglucosidase alfa particles (typically less than 10 in a vial) in the form of thin white strands or translucent fibers subsequent to the initial inspection. This may also happen following dilution for infusion. These particles have been shown to contain alglucosidase alfa and may appear after the initial reconstitution step and increase over time. Studies have shown that these particles are removed via in-line filtration without having a detectable effect on the purity or strength.
- Alglucosidase alfa should be diluted in 0.9% Sodium Chloride for Injection, USP, immediately after reconstitution, to a final alglucosidase alfa concentration of 0.5 to 4 mg/mL.
- Slowly withdraw the reconstituted solution from each vial. Avoid foaming in the syringe.
- Remove airspace from the infusion bag to minimize particle formation due to the sensitivity of alglucosidase alfa to air-liquid interfaces.
- Add the reconstituted alglucosidase alfa solution slowly and directly into the sodium chloride solution. Do not add directly into airspace that may remain within the infusion bag. Avoid foaming in the infusion bag.
- Gently invert or massage the infusion bag to mix. Do not shake.
- Administer alglucosidase alfa using an in-line low protein binding 0.2 µm filter.
- Do not infuse alglucosidase alfa in the same intravenous line with other products.
- The reconstituted and diluted solution should be administered without delay. If immediate use is not possible, the reconstituted and diluted solution is stable for up to 24 hours at 2°C to 8°C (36°F to 46°F).
- Storage of the reconstituted solution at room temperature is not recommended.
- The reconstituted and diluted alglucosidase alfa solution should be protected from light.
- Do not freeze or shake.
- Alglucosidase alfa does not contain any preservatives. Vials are single-use only.
- Discard any unused product.
Monitoring
- improvement in the signs and symptoms of Pompe disease is indicative of efficacy
- IgG antibody formation; every 3 months for 2 years, and annually thereafter
- IgG titers or IgE antibodies; in patients develop hypersensitivity reactions, other immune-mediated responses, or lose clinical response; contact Genzyme Corporation (1-800-745-4447) for information on testing and to obtain a sample collection box
- liver enzymes; baseline and periodically
- urinalysis; periodically
- vital signs; each time infusion rate is stepped up (approximately every 30 minutes during infusion)
- infusion reactions; particularly in patients with acute illness or compromised cardiac and/or respiratory function, and in patients who develop IgE antibodies; prolonged observation may be necessary
- systemic immune complex-mediated reactions involving skin and other organs
IV Compatibility
- Alglucosidase alfa is supplied as sterile, nonpyrogenic, white to off-white, lyophilized cake or powder for reconstitution with 10.3 mL Sterile Water for Injection, USP.
- Each 50 mg vial contains 52.5 mg alglucosidase alfa, 210 mg mannitol, 0.5 mg polysorbate 80, 9.9 mg sodium phosphate dibasic heptahydrate, 31.2 mg sodium phosphate monobasic monohydrate.
- Following reconstitution as directed, each vial contains 10.5 mL reconstituted solution and a total extractable volume of 10 mL at 5 mg/mL alglucosidase alfa.
- Alglucosidase alfa does not contain preservatives; each vial is for single use only.
Overdosage
There is limited information regarding Alglucosidase alfa overdosage. If you suspect drug poisoning or overdose, please contact the National Poison Help hotline (1-800-222-1222) immediately.
Pharmacology
Alglucosidase alfa
| |
| Systematic (IUPAC) name | |
| Human glucosidase, prepro-α-[199-arginine,223-histidine] [1] | |
| Identifiers | |
| CAS number | |
| ATC code | A16 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 105338 [1] |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
FDA approved for children |
| Routes | Intravenous |
Mechanism of Action
Pompe disease (acid maltase deficiency, glycogen storage disease type II, GSD II, glycogenosis type II) is an inherited disorder of glycogen metabolism caused by the absence or marked deficiency of the lysosomal enzyme GAA.
- Alglucosidase alfa provides an exogenous source of GAA.
- Binding to mannose-6-phosphate receptors on the cell surface has been shown to occur via carbohydrate groups on the GAA molecule, after which it is internalized and transported into lysosomes, where it undergoes proteolytic cleavage that results in increased enzymatic activity.
- It then exerts enzymatic activity in cleaving glycogen.
Structure
There is limited information regarding Alglucosidase alfa Structure in the drug label.
Pharmacodynamics
Clinical pharmacodynamic studies have not been conducted for alglucosidase alfa.
Pharmacokinetics
- The pharmacokinetics of alglucosidase alfa were evaluated in 13 patients with infantile-onset Pompe disease, aged 1 month to 7 months, who received 20 mg/kg (approximately as a 4-hour infusion) or 40 mg/kg (approximately as a 6.5-hour infusion) of alglucosidase alfa every 2 weeks.
- The measurement of alglucosidase alfa plasma concentration was based on an activity assay using an artificial substrate.
- Systemic exposure was approximately dose proportional between the 20 and 40 mg/kg doses.
- Based on the pharmacokinetic blood samples collected for 12 hours after a 4-hour intravenous infusion of 20 mg/kg (n=5), the estimated mean AUC was 811 mcg∙hr/mL with 17% coefficient of variation [CV], Cmax was 162 mcg/mL with 19% CV, clearance was 25 mL/hr/kg with 16% CV, and half-life was 2.3 hours with 17% CV.
- The pharmacokinetics of alglucosidase alfa were also evaluated in a separate trial of 14 patients with infantile-onset Pompe disease, aged 6 months to 3.5 years, who received 20 mg/kg of alglucosidase alfa as a 4-hour infusion every 2 weeks.
- The pharmacokinetic parameters were similar to those observed for the infantile-onset Pompe disease patients aged 1 month to 7 months who received the 20 mg/kg dose.
- Nineteen of 21 patients who received treatment with alglucosidase alfa and had pharmacokinetics and antibody titer data available at Week 12 developed antibodies to alglucosidase alfa.
- Five patients with antibody titers ≥ 12,800 at Week 12 had an average increase in clearance of 50% (range 5% to 90%) from Week 1 to Week 12.
- The other 14 patients with antibody titers < 12,800 at Week 12 had similar average clearance values at Week 1 and Week 12.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Long-term studies in animals to evaluate carcinogenic potential or studies to evaluate mutagenic potential have not been performed with alglucosidase alfa.
- Intravenous administration of alglucosidase alfa every other day in mice at doses up to 40 mg/kg (0.4 times the human AUC at the recommended bi-weekly dose) had no effect on fertility and reproductive performance.
Clinical Studies
Clinical Trials in Infantile-Onset Pompe Disease
- The safety and efficacy of alglucosidase alfa were assessed in 57 treatment-naïve infantile-onset Pompe disease patients, aged 0.2 month to 3.5 years at first infusion, in three separate clinical trials.
Study 1
Study 1 was an international, multicenter, open-label, clinical trial of 18 infantile-onset Pompe disease patients. This study was conducted between 2003 and 2005. Patients were randomized 1:1 to receive either 20 mg/kg or 40 mg/kg alglucosidase alfa every two weeks, with length of treatment ranging from 52 to 106 weeks. Enrollment was restricted to patients 7 months of age or younger at first infusion with clinical signs of Pompe disease and cardiac hypertrophy, and who did not require ventilatory support at study entry. Fourteen patients were Cross Reactive Immunologic Material (CRIM) positive and 4 patients were CRIM-negative.
- Efficacy was assessed by comparing the proportions of alglucosidase alfa-treated patients who died or needed invasive ventilator support at 18 months of age with the mortality experience of a historical cohort of untreated infantile-onset Pompe disease patients with similar age and disease severity.
- In the historical cohort, 61 untreated patients with infantile-onset Pompe disease diagnosed by age 6 months, born between 1982 and 2002, were identified by a retrospective review of medical charts.
- By 18 months of age, 15 of 18 (83%) alglucosidase alfa-treated patients were alive without invasive ventilatory support and 3 (17%) required invasive ventilator support, whereas only one of the 61 (2%) historical control patients was alive.
- No differences in outcome were observed between patients who received 20 mg/kg versus 40 mg/kg.
- Other outcome measures in this study included unblinded assessments of motor function by the Alberta Infant Motor Scale (AIMS), a measure of infant motor performance that assesses motor maturation of the infant through age 18 months.
- Although gains in motor function were noted in 13 patients, the motor function was substantially delayed compared to normal infants of comparable age in the majority of patients.
- Two of 9 patients who had initially demonstrated gains in motor function after 12 months of alglucosidase alfa treatment regressed despite continued treatment.
- Changes from baseline to Month 12 in left ventricular mass index (LVMI), a measure of pharmacodynamic effect, were evaluated by echocardiography. Fifteen patients who underwent both baseline and Month 12 echocardiograms demonstrated decreases from baseline in LVMI (mean decrease 118 g/m2, range 45 to 193 g/m2).
- However, the magnitude of the decrease in LVMI did not correlate with the clinical outcome measure of ventilator-free survival.
Study 2
Study 2 was an international, multicenter, non-randomized, open-label clinical trial that enrolled 21 infantile-onset patients aged 3 months to 3.5 years at first infusion.
- Eighteen patients were CRIM-positive and 3 patients were CRIM-negative.
- All patients received 20 mg/kg alglucosidase alfa every other week for up to 104 weeks.
- Five of 21 patients were receiving invasive ventilatory support at the time of first infusion.
- The primary outcome measure was the proportion of patients alive at the conclusion of treatment.
- At the 52–week interim analysis, 16 of 21 patients were alive.
- Sixteen patients were free of invasive ventilatory support at the time of first infusion; of these, 4 died, 2 required invasive ventilatory support, and 10 were free of invasive ventilatory support after 52 weeks of treatment.
- For the 5 patients who were receiving invasive ventilatory support at baseline, 1 died, and 4 remained on invasive ventilatory support at Week 52.
Study 3
Study 3 was an open-label, single-center trial in 18 infantile-onset Pompe disease patients who had a confirmed diagnosis of Pompe disease as identified through a newborn screening program.
- All patients were CRIM-positive.
- Patients were treated with alglucosidase alfa prior to 6 months of age (0.2 to 5.8 months at first infusion).
- Sixteen patients reached 18 months of age at the time of analysis, and all (100%) were alive without invasive ventilator support.
Clinical Trials in Late-Onset Pompe Disease
- The safety and efficacy of alglucosidase alfa were assessed in 90 patients with late-onset Pompe disease, aged 10 to 70 years, in a randomized, double-blind, placebo-controlled trial.
- The youngest alglucosidase alfa-treated patient was 16 years of age, and the youngest placebo-treated patient was 10 years of age. All patients were naïve to enzyme replacement therapy.
- Patients were allocated in a 2:1 ratio and received 20 mg/kg alglucosidase alfa (n=60) or placebo (n=30) every other week for 78 weeks (18 months).
- The study population included 34 males and 26 females (n=60) in the alglucosidase alfa group and 11 males and 19 females (n=30) in the placebo group.
- At baseline, all patients were ambulatory (some required assistive walking devices), did not require invasive ventilator support or non-invasive ventilation while awake and sitting upright, and had a forced vital capacity (FVC) between 30 and 79% of predicted in the sitting position.
- Patients who could not walk 40 meters in 6 minutes or were unable to perform appropriate pulmonary and muscle function testing were excluded from the study.
- A total of 81 of 90 patients completed the trial.
- Of the 9 patients who discontinued, 5 were in the alglucosidase alfa group and 4 were in the placebo group. Three patients discontinued the study due to an adverse event, two patients were in the alglucosidase alfa treatment group and one patient was in placebo group.
- At study entry, the mean % predicted FVC in the sitting position among all patients was about 55%.
- After 78 weeks, the mean % predicted FVC increased to 56.2% for alglucosidase alfa-treated patients and decreased to 52.8% for placebo-treated patients indicating an alglucosidase alfa treatment effect of 3.4% (95% confidence interval: [1.3% to 5.5%]; p=0.004).
- Stabilization of % predicted FVC in the alglucosidase alfa-treated patients was observed.

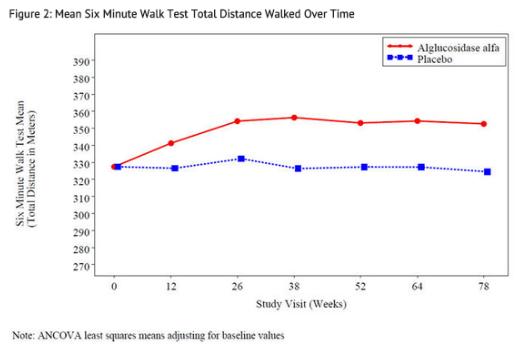
At study entry, the mean 6 minute walk test (6MWT) among all patients was about 330 meters. After 78 weeks, the mean 6MWT increased by 25 meters for alglucosidase alfa-treated patients and decreased by 3 meters for placebo-treated patients indicating an alglucosidase alfa treatment effect of 28 meters (95% confidence interval: [-1 to 52 meters]; p=0.06)
How Supplied
- 50 mg vials are supplied as a sterile, nonpyrogenic, white to off-white lyophilized cake or powder in single-use vials.
- NDC 58468-0160-1 (Carton of one single-use vial)
- NDC 58468-0160-2 (Carton of ten single-use vials)
Storage
- Store alglucosidase alfa under refrigeration between 2°C to 8°C (36°F to 46°F).
- Do not use alglucosidase alfa after the expiration date on the vial.
Images
Drug Images
{{#ask: Page Name::Alglucosidase alfa |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Alglucosidase alfa |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Anaphylaxis, Hypersensitivity and Immune-Mediated Reactions
- Advise the patients and caregivers that reactions related to administration and infusion may occur during and after alglucosidase alfa treatment, including life-threatening anaphylaxis, hypersensitivity reactions, and immune-mediated reactions.
- Patients who have experienced anaphylaxis or hypersensitivity reactions may require close observation during and after alglucosidase alfa administration.
- Inform patients of the signs and symptoms of anaphylaxis, hypersensitivity reactions, and immune-mediated reactions and have them seek medical care should signs and symptoms occur.
Risk of Acute Cardiorespiratory Failure
- Advise patients and caregivers that patients with underlying respiratory illness or compromised cardiac or respiratory function may be at risk of acute cardiorespiratory failure.
- Patients with compromised cardiac or respiratory function may require close observation during and after alglucosidase alfa administration.
Pompe Registry
- Inform patients and their caregivers that the Pompe Registry has been established in order to better understand the variability and progression of Pompe disease, and to continue to monitor and evaluate long-term treatment effects of alglucosidase alfa.
- The Pompe Registry will also monitor the effect of alglucosidase alfa on pregnant women and their offspring.
- Patients and their caregivers should be encouraged to participate in the Pompe Registry and advised that their participation is voluntary and may involve long-term follow-up.
Precautions with Alcohol
Alcohol-Alglucosidase alfa interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Lumizyme
- Myozyme
Look-Alike Drug Names
There is limited information regarding Alglucosidase alfa Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 1.2 American Medical Association (USAN). "Alglucosidase alfa" (Microsoft Word). Statement on a Nonproprietary Name Adopted by the USAN Council. Retrieved 18 December 2007.