Ado-trastuzumab emtansine
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning: Hepatotoxicity, Cardiac Toxicity, Embryo-Fetal Toxicity
See full prescribing information for complete Boxed Warning.
Hepatotoxicity:
Cardiac Toxicity:
Embryo-Fetal Toxicity:
|
Overview
Ado-trastuzumab emtansine is an antineoplastic agent that is FDA approved for the treatment of patients with HER2-positive, metastatic breast cancer who previously received trastuzumab and a taxane, separately or in combination. Patients should have either received prior therapy for metastatic disease, or developed disease recurrence during or within six months of completing adjuvant therapy. There is a Black Box Warning for this drug as shown here. Common adverse reactions include fatigue, nausea, musculoskeletal pain, hemorrhage, thrombocytopenia, headache, increased transaminases, constipation and epistaxis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Recommended Doses and Schedules
- The recommended dose of Ado-trastuzumab emtansine is 3.6 mg/kg given as an intravenous infusion every 3 weeks (21-day cycle) until disease progression or unacceptable toxicity. Do not administer Ado-trastuzumab emtansine at doses greater than 3.6 mg/kg. Do not substitute Ado-trastuzumab emtansine for or with trastuzumab.
- Closely monitor the infusion site for possible subcutaneous infiltration during drug administration.
- Dosing regimen:
- First infusion: Administer infusion over 90 minutes. Patients should be observed during the infusion and for at least 90 minutes following the initial dose for fever, chills, or other infusion-related reactions.
- Subsequent infusions: Administer over 30 minutes if prior infusions were well tolerated. Patients should be observed during the infusion and for at least 30 minutes after infusion.
Dose Modifications
- Ado-trastuzumab emtansine dose should not be re-escalated after a dose reduction is made.
- If a planned dose is delayed or missed, it should be administered as soon as possible; do not wait until the next planned cycle. The schedule of administration should be adjusted to maintain a 3-week interval between doses. The infusion may be administered at the dose and rate the patient tolerated in the most recent infusion.
- The infusion rate of Ado-trastuzumab emtansine should be slowed or interrupted if the patient develops an infusion-related reaction. Permanently discontinue Ado-trastuzumab emtansine for life-threatening infusion-related reactions.
- Management of increased serum transaminases, hyperbilirubinemia, left ventricular dysfunction, thrombocytopenia, pulmonary toxicity or peripheral neuropathy may require temporary interruption, dose reduction or treatment discontinuation of Ado-trastuzumab emtansine as per guidelines provided in Tables 1 to 5.

Hepatotoxicity
- A reduction in the dose of Ado-trastuzumab emtansine is recommended in the case of hepatotoxicity exhibited as increases in serum transaminases and/or hyperbilirubinemia.

- Permanently discontinue Ado-trastuzumab emtansine treatment in patients with serum transaminases > 3 × ULN and concomitant total bilirubin > 2 × ULN.
- Permanently discontinue Ado-trastuzumab emtansine in patients diagnosed with nodular regenerative hyperplasia (NRH).
Left Ventricular Dysfunction

Thrombocytopenia
- A reduction in dose is recommended in the case of Grade 4 thrombocytopenia (platelets < 25,000/mm3).

Pulmonary Toxicity
- Ado-trastuzumab emtansine should be permanently discontinued in patients diagnosed with interstitial lung disease (ILD) or pneumonitis.
Peripheral Neuropathy
- Ado-trastuzumab emtansine should be temporarily discontinued in patients experiencing Grade 3 or 4 peripheral neuropathy until resolution to ≤ Grade 2.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ado-trastuzumab emtansine in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ado-trastuzumab emtansine in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ado-trastuzumab emtansine FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ado-trastuzumab emtansine in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ado-trastuzumab emtansine in pediatric patients.
Contraindications
- None
Warnings
|
Warning: Hepatotoxicity, Cardiac Toxicity, Embryo-Fetal Toxicity
See full prescribing information for complete Boxed Warning.
Hepatotoxicity:
Cardiac Toxicity:
Embryo-Fetal Toxicity:
|
Hepatotoxicity
- Hepatotoxicity, predominantly in the form of asymptomatic, transient increases in the concentrations of serum transaminases, has been observed in clinical trials with Ado-trastuzumab emtansine Serious hepatobiliary disorders, including at least two fatal cases of severe drug-induced liver injury and associated hepatic encephalopathy, have been reported in clinical trials with Ado-trastuzumab emtansine Some of the observed cases may have been confounded by comorbidities and/or concomitant medications with known hepatotoxic potential.
- Monitor serum transaminases and bilirubin prior to initiation of Ado-trastuzumab emtansine treatment and prior to each Ado-trastuzumab emtansine dose. Patients with known active hepatitis B virus or hepatitis C virus were excluded from Study 1. Reduce the dose or discontinue Ado-trastuzumab emtansine as appropriate in cases of increased serum transaminases and/or total bilirubin. Permanently discontinue Ado-trastuzumab emtansine treatment in patients with serum transaminases > 3 × ULN and concomitant total bilirubin > 2 × ULN. Ado-trastuzumab emtansine has not been studied in patients with serum transaminases > 2.5 × ULN or bilirubin > 1.5 × ULN prior to the initiation of treatment.
- In clinical trials of Ado-trastuzumab emtansine, cases of nodular regenerative hyperplasia (NRH) of the liver have been identified from liver biopsies (3 cases out of 884 treated patients, one of which was fatal). Two of these three cases of NRH were observed in the randomized trial (Study 1). NRH is a rare liver condition characterized by widespread benign transformation of hepatic parenchyma into small regenerative nodules; NRH may lead to non-cirrhotic portal hypertension. The diagnosis of NRH can be confirmed only by histopathology. NRH should be considered in all patients with clinical symptoms of portal hypertension and/or cirrhosis-like pattern seen on the computed tomography (CT) scan of the liver but with normal transaminases and no other manifestations of cirrhosis. Upon diagnosis of NRH, Ado-trastuzumab emtansine treatment must be permanently discontinued.
Left Ventricular Dysfunction
- Patients treated with Ado-trastuzumab emtansine are at increased risk of developing left ventricular dysfunction. A decrease of LVEF to < 40% has been observed in patients treated with Ado-trastuzumab emtansine. In the randomized trial (Study 1), left ventricular dysfunction occurred in 1.8% of patients in the Ado-trastuzumab emtansine-treated group and 3.3% of patients in the lapatinib plus capecitabine-treated group.
- Assess LVEF prior to initiation of Ado-trastuzumab emtansine and at regular intervals (e.g. every three months) during treatment to ensure the LVEF is within the institution's normal limits. Treatment with Ado-trastuzumab emtansine has not been studied in patients with LVEF < 50% prior to initiation of treatment. If, at routine monitoring, LVEF is < 40%, or is 40% to 45% with a 10% or greater absolute decrease below the pretreatment value, withhold Ado-trastuzumab emtansine and repeat LVEF assessment within approximately 3 weeks. Permanently discontinue Ado-trastuzumab emtansine if the LVEF has not improved or has declined further. Patients with a history of symptomatic congestive heart failure (CHF), serious cardiac arrhythmia, or history of myocardial infarction or unstable angina within 6 months were excluded from Study 1.
Embryo-Fetal Toxicity
- Ado-trastuzumab emtansine can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of Ado-trastuzumab emtansine in pregnant women and no reproductive and developmental toxicology studies have been conducted with ado-trastuzumab emtansine. Nevertheless, treatment with trastuzumab, the antibody component of Ado-trastuzumab emtansine, during pregnancy in the postmarketing setting has resulted in oligohydramnios, some associated with fatal pulmonary hypoplasia, skeletal abnormalities and neonatal death. DM1, the cytotoxic component of Ado-trastuzumab emtansine, can be expected to cause embryo-fetal toxicity based on its mechanism of action.
- If Ado-trastuzumab emtansine is used during pregnancy, or if the patient becomes pregnant while receiving Ado-trastuzumab emtansine, apprise the patient of the potential hazard to the fetus.
- Verify pregnancy status prior to the initiation of Ado-trastuzumab emtansine. Advise patients of the risks of embryo-fetal death and birth defects and the need for contraception during and after treatment. Advise patients to contact their healthcare provider immediately if they suspect they may be pregnant. If Ado-trastuzumab emtansine is administered during pregnancy or if a patient becomes pregnant while receiving Ado-trastuzumab emtansine, immediately report exposure to the Genentech Adverse Event Line at 1-888-835-2555. Encourage women who may be exposed during pregnancy to enroll in the MotHER Pregnancy Registry by contacting 1-800-690-6720.
Pulmonary Toxicity
- Cases of interstitial lung disease (ILD), including pneumonitis, some leading to acute respiratory distress syndrome or fatal outcome have been reported in clinical trials with Ado-trastuzumab emtansine. Pneumonitis at an incidence of 0.8% (7 out of 884 treated patients) has been reported, with one case of grade 3 pneumonitis. Signs and symptoms include dyspnea, cough, fatigue, and pulmonary infiltrates. These events may or may not occur as sequelae of infusion reactions. In the randomized trial (Study 1), the overall frequency of pneumonitis was 1.2%.
- Permanently discontinue treatment with Ado-trastuzumab emtansine in patients diagnosed with ILD or pneumonitis.
- Patients with dyspnea at rest due to complications of advanced malignancy and co-morbidities may be at increased risk of pulmonary toxicity.
Infusion-Related Reactions, Hypersensitivity Reactions
- Treatment with Ado-trastuzumab emtansine has not been studied in patients who had trastuzumab permanently discontinued due to infusion-related reactions (IRR) and/or hypersensitivity; treatment with Ado-trastuzumab emtansine is not recommended for these patients.
- Infusion-related reactions, characterized by one or more of the following symptoms – flushing, chills, pyrexia, dyspnea, hypotension, wheezing, bronchospasm, and tachycardia have been reported in clinical trials of Ado-trastuzumab emtansine In the randomized trial (Study 1), the overall frequency of IRRs in patients treated with Ado-trastuzumab emtansine was 1.4%. In most patients, these reactions resolved over the course of several hours to a day after the infusion was terminated. Ado-trastuzumab emtansine treatment should be interrupted in patients with severe IRR. Ado-trastuzumab emtansine treatment should be permanently discontinued in the event of a life-threatening IRR. Patients should be observed closely for IRR reactions, especially during the first infusion.
- One case of a serious, allergic/anaphylactic-like reaction has been observed in clinical trials of single-agent Ado-trastuzumab emtansine Medications to treat such reactions, as well as emergency equipment, should be available for immediate use.
Hemorrhage
- Cases of hemorrhagic events, including central nervous system, respiratory, and gastrointestinal hemorrhage, have been reported in clinical trials with Ado-trastuzumab emtansine Some of these bleeding events resulted in fatal outcomes. In the randomized trial (Study 1), the overall frequency of hemorrhage was 32.2% in the Ado-trastuzumab emtansine-treated group and 16.4% in the lapatinib plus capecitabine-treated group. The incidence of ≥ Grade 3 hemorrhage was 1.8% in the Ado-trastuzumab emtansine-treated group and 0.8% in the lapatinib plus capecitabine-treated group. Although, in some of the observed cases the patients were also receiving anti-coagulation therapy, antiplatelet therapy, or had thrombocytopenia, in others there were no known additional risk factors. Use caution with these agents and consider additional monitoring when concomitant use is medically necessary.
Thrombocytopenia
- Thrombocytopenia, or decreased platelet count, was reported in clinical trials of Ado-trastuzumab emtansine (103 of 884 treated patients with ≥ Grade 3; 283 of 884 treated patients with any Grade). The majority of these patients had Grade 1 or 2 events (< LLN to ≥ 50,000/mm3) with the nadir occurring by day 8 and generally improving to Grade 0 or 1 (≥ 75,000 /mm3) by the next scheduled dose. In clinical trials of Ado-trastuzumab emtansine, the incidence and severity of thrombocytopenia were higher in Asian patients.
- In the randomized trial (Study 1), the overall frequency of thrombocytopenia was 31.2% in the Ado-trastuzumab emtansine-treated group and 3.3% in the lapatinib plus capecitabine-treated group. The incidence of ≥ Grade 3 thrombocytopenia was 14.5% in the Ado-trastuzumab emtansine-treated group and 0.4% in the lapatinib plus capecitabine-treated group. In Asian patients, the incidence of ≥ Grade 3 thrombocytopenia was 45.1% in the Ado-trastuzumab emtansine-treated group and 1.3% in the lapatinib plus capecitabine-treated group.
- Monitor platelet counts prior to initiation of Ado-trastuzumab emtansine and prior to each Ado-trastuzumab emtansine dose. Ado-trastuzumab emtansine has not been studied in patients with platelet counts <100,000/mm3 prior to initiation of treatment. In the event of decreased platelet count to Grade 3 or greater (< 50,000/mm3) do not administer Ado-trastuzumab emtansine until platelet counts recover to Grade 1 (≥ 75,000/mm3). Patients with thrombocytopenia (< 100,000/mm3) and patients on anti-coagulant treatment should be closely monitored during treatment with Ado-trastuzumab emtansine.
Neurotoxicity
- Peripheral neuropathy, mainly as Grade 1 and predominantly sensory, was reported in clinical trials of Ado-trastuzumab emtansine (14 of 884 treated patients with ≥ Grade 3; 196 of 884 treated patients with any Grade). In the randomized trial (Study 1), the overall frequency of peripheral neuropathy was 21.2% in the Ado-trastuzumab emtansine-treated group and 13.5% in the lapatinib plus capecitabine-treated group. The incidence of ≥ Grade 3 peripheral neuropathy was 2.2% in the Ado-trastuzumab emtansine-treated group and 0.2% in the lapatinib plus capecitabine-treated group.
- Ado-trastuzumab emtansine should be temporarily discontinued in patients experiencing Grade 3 or 4 peripheral neuropathy until resolution to ≤ Grade 2. Patients should be clinically monitored on an ongoing basis for signs or symptoms of neurotoxicity.
HER2 Testing
- Detection of HER2 protein overexpression or gene amplification is necessary for selection of patients appropriate for Ado-trastuzumab emtansine therapy because these are the only patients studied for whom benefit has been shown. In the randomized study (Study 1), patients with breast cancer were required to have evidence of HER2 overexpression defined as 3+ IHC by Dako Herceptest™ or evidence of overexpression defined as FISH amplification ratio ≥ 2.0 by Dako HER2 FISH PharmDx™ test kit. Only limited data were available for patients whose breast cancer was positive by FISH and 0 or 1+ by IHC.
- Assessment of HER2 status should be performed by laboratories with demonstrated proficiency in the specific technology being utilized. Improper assay performance, including use of sub- optimally fixed tissue, failure to utilize specified reagents, deviation from specific assay instructions, and failure to include appropriate controls for assay validation, can lead to unreliable results.
Extravasation
- In Ado-trastuzumab emtansine clinical studies, reactions secondary to extravasation have been observed. These reactions, observed more frequently within 24 hours of infusion, were usually mild and comprised erythema, tenderness, skin irritation, pain, or swelling at the infusion site. Specific treatment for Ado-trastuzumab emtansine extravasation is unknown. The infusion site should be closely monitored for possible subcutaneous infiltration during drug administration.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- In clinical trials, Ado-trastuzumab emtansine has been evaluated as single-agent in 884 patients with HER2-positive metastatic breast cancer. The most common (frequency ≥ 25%) adverse drug reactions (ADRs) seen in 884 patients treated with Ado-trastuzumab emtansine were fatigue, nausea, musculoskeletal pain, hemorrhage, thrombocytopenia, headache, increased transaminases, constipation and epistaxis.
- The ADRs described in TABLE 6 were identified in patients with HER2-positive metastatic breast cancer treated in a randomized trial (Study 1). Patients were randomized to receive Ado-trastuzumab emtansine or lapatinib plus capecitabine. The median duration of study treatment was 7.6 months for patients in the Ado-trastuzumab emtansine-treated group and 5.5 months and 5.3 months for patients treated with lapatinib and capecitabine, respectively. Two hundred and eleven (43.1%) patients experienced ≥ Grade 3 adverse events in the Ado-trastuzumab emtansine-treated group compared with 289 (59.2%) patients in the lapatinib plus capecitabine-treated group. Dose adjustments for Ado-trastuzumab emtansine were permitted. Thirty-two patients (6.5%) discontinued Ado-trastuzumab emtansine due to an adverse event, compared with 41 patients (8.4%) who discontinued lapatinib, and 51 patients (10.5%) who discontinued capecitabine due to an adverse event. The most common adverse events leading to Ado-trastuzumab emtansine withdrawal were thrombocytopenia and increased transaminases. Eighty patients (16.3%) treated with Ado-trastuzumab emtansine had adverse events leading to dose reductions. The most frequent adverse events leading to dose reduction of Ado-trastuzumab emtansine (in ≥ 1% of patients) included thrombocytopenia, increased transaminases, and peripheral neuropathy. Adverse events that led to dose delays occurred in 116 (23.7%) of Ado-trastuzumab emtansine treated patients. The most frequent adverse events leading to a dose delay of Ado-trastuzumab emtansine (in ≥ 1% of patients) were neutropenia, thrombocytopenia, leukopenia, fatigue, increased transaminases and [pyrexia]].
- TABLE 6 reports the ADRs that occurred in patients in the Ado-trastuzumab emtansine-treated group (n=490) of the randomized trial (Study 1). Selected laboratory abnormalities are shown in TABLE 7. The most common ADRs seen with Ado-trastuzumab emtansine in the randomized trial (frequency > 25%) were nausea, fatigue, musculoskeletal pain, hemorrhage, thrombocytopenia, increased transaminases, headache, and constipation. The most common NCI–CTCAE (version 3) ≥ Grade 3 ADRs (frequency >2%) were thrombocytopenia, increased transaminases, anemia, hypokalemia, peripheral neuropathy and fatigue.

- Hepatic failure has been observed in two patients (0.2%) with HER2-positive metastatic breast cancer in clinical trials (n=884) with Ado-trastuzumab emtansine as single-agent.
Immunogenicity
- As with all therapeutic proteins, there is the potential for an immune response to Ado-trastuzumab emtansine A total of 836 patients from six clinical studies were tested at multiple time points for anti-therapeutic antibody (ATA) responses to Ado-trastuzumab emtansine. Following Ado-trastuzumab emtansine dosing, 5.3% (44/836) of patients tested positive for anti-Ado-trastuzumab emtansine antibodies at one or more post-dose time points. The presence of Ado-trastuzumab emtansine in patient serum at the time of ATA sampling may interfere with the ability of this assay to detect anti-Ado-trastuzumab emtansine antibodies. As a result, data may not accurately reflect the true incidence of anti-Ado-trastuzumab emtansine antibody development. In addition, neutralizing activity of anti-Ado-trastuzumab emtansine antibodies has not been assessed.
- Immunogenicity data are highly dependent on the sensitivity and specificity of the test methods used. Additionally, the observed incidence of a positive result in a test method may be influenced by several factors, including sample handling, timing of sample collection, drug interference, concomitant medication and the underlying disease. Therefore, comparison of the incidence of antibodies to Ado-trastuzumab emtansine with the incidence of antibodies to other products may be misleading. Clinical significance of anti-Ado-trastuzumab emtansine antibodies is not yet known.
Postmarketing Experience
There is limited information regarding Ado-trastuzumab emtansine Postmarketing Experience in the drug label.
Drug Interactions
- No formal drug-drug interaction studies with Ado-trastuzumab emtansine have been conducted. In vitro studies indicate that DM1, the cytotoxic component of Ado-trastuzumab emtansine is metabolized mainly by CYP3A4 and to a lesser extent by CYP3A5. Concomitant use of strong CYP3A4 inhibitors (e.g., ketoconazole, itraconazole, clarithromycin, atazanavir, indinavir, nefazodone, nelfinavir, ritonavir, saquinavir, telithromycin, and voriconazole) with Ado-trastuzumab emtansine should be avoided due to the potential for an increase in DM1 exposure and toxicity. Consider an alternate medication with no or minimal potential to inhibit CYP3A4. If concomitant use of strong CYP3A4 inhibitors is unavoidable, consider delaying Ado-trastuzumab emtansine treatment until the strong CYP3A4 inhibitors have cleared from the circulation (approximately 3 elimination half-lives of the inhibitors) when possible. If a strong CYP3A4 inhibitor is coadministered and Ado-trastuzumab emtansine treatment cannot be delayed, patients should be closely monitored for adverse reactions.
Use in Specific Populations
Pregnancy
Risk Summary
- Ado-trastuzumab emtansine can cause fetal harm when administered to a pregnant woman. There are no adequate and well-controlled studies of Ado-trastuzumab emtansine in pregnant women. No reproductive and developmental toxicology studies have been conducted with ado-trastuzumab emtansine. Nevertheless, two components of Ado-trastuzumab emtansine (trastuzumab and DM1) are known or suspected to cause fetal harm or death when administered to a pregnant woman. If Ado-trastuzumab emtansine is administered during pregnancy, or if a patient becomes pregnant while receiving Ado-trastuzumab emtansine apprise the patient of the potential hazard to the fetus. Patients should be advised to use effective contraception during treatment with Ado-trastuzumab emtansine and for 6 months following the last dose of Ado-trastuzumab emtansine.
- If Ado-trastuzumab emtansine is administered during pregnancy or if a patient becomes pregnant while receiving Ado-trastuzumab emtansine immediately report exposure to the Genentech Adverse Event Line at 1-888-835-2555. Encourage women who may be exposed during pregnancy to enroll in the MotHER Pregnancy Registry by contacting 1-800-690-6720.
Human Data
- In the post-marketing setting, treatment with trastuzumab during pregnancy has resulted in cases of oligohydramnios, some associated with fatal pulmonary hypoplasia, skeletal abnormalities and neonatal death. These case reports described oligohydramnios in pregnant women who received trastuzumab either alone or in combination with chemotherapy. In some case reports, amniotic fluid index increased after trastuzumab was stopped. In one case, trastuzumab therapy resumed after the amniotic fluid index improved, and oligohydramnios recurred.
Animal Data
- There were no reproductive and developmental toxicology studies conducted with ado-trastuzumab emtansine. DM1, the cytotoxic component of Ado-trastuzumab emtansine, disrupts microtubule function. DM1 is toxic to rapidly dividing cells in animals and is genotoxic, suggesting it has the potential to cause embryotoxicity and teratogenicity. In studies where trastuzumab was administered to pregnant monkeys at doses up to 25 mg/kg (about 7 times the clinical dose), trastuzumab crossed the placental barrier during the early and late phases of gestation. The resulting concentrations of trastuzumab in fetal blood and amniotic fluid were approximately 33% and 25%, respectively, of those present in the maternal serum but were not associated with adverse findings.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ado-trastuzumab emtansine in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ado-trastuzumab emtansine during labor and delivery.
Nursing Mothers
- It is not known whether Ado-trastuzumab emtansine specifically, is excreted in human milk, but IgG is known to be excreted in human milk. In lactating monkeys, trastuzumab was excreted in small amounts (about 0.3% of maternal serum concentrations) in breast milk after post-partum doses of 25 mg/kg (about 7 times the clinical dose of Ado-trastuzumab emtansine . Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Ado-trastuzumab emtansine a decision should be made whether to discontinue nursing or discontinue Ado-trastuzumab emtansine, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness of Ado-trastuzumab emtansine have not been established in pediatric patients.
Geriatic Use
- Of 495 patients who were randomized to Ado-trastuzumab emtansine in the randomized trial (Study 1), 65 patients (13%) were ≥ 65 years of age and 11 patients (2%) were ≥ 75 years of age. In patients ≥ 65 years old (n=138 across both treatment arms) the hazard ratios for progression-free survival (PFS) and Overall Survival (OS) were 1.06 (95% CI: 0.68, 1.66) and 1.05 (95% CI: 0.58, 1.91), respectively.
Population pharmacokinetic analysis indicates that age does not have a clinically meaningful effect on the pharmacokinetics of ado-trastuzumab emtansine.
Gender
There is no FDA guidance on the use of Ado-trastuzumab emtansine with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ado-trastuzumab emtansine with respect to specific racial populations.
Renal Impairment
- No dedicated renal impairment trial for Ado-trastuzumab emtansine has been conducted. Based on the population pharmacokinetics, as well as analysis of Grade 3 or greater adverse drug reactions and dose modifications, dose adjustments of Ado-trastuzumab emtansine are not needed in patients with mild (creatinine clearance (CLcr) 60 to 89 mL/min) or moderate (CLcr 30 to 59 mL/min) renal impairment. No dose adjustment can be recommended for patients with severe renal impairment (CLcr less than 30 mL/min) because of the limited data available
Hepatic Impairment
- In vitro studies in human liver microsomes indicates that DM1 is metabolized by CYP3A4/CYP3A5. The influence of hepatic impairment on the pharmacokinetics of ado-trastuzumab emtansine conjugate has not been determined.
Females of Reproductive Potential and Males
- Ado-trastuzumab emtansine can cause embryo-fetal harm when administered during pregnancy. Counsel patients regarding pregnancy prevention and planning. Advise females of reproductive potential to use effective contraception while receiving Ado-trastuzumab emtansine and for 6 months following the last dose of Ado-trastuzumab emtansine.
- If Ado-trastuzumab emtansine is administered during pregnancy or if the patient becomes pregnant while receiving Ado-trastuzumab emtansine immediately report exposure to the Genentech Adverse Event Line at 1-888-835-2555. Encourage women who may be exposed during pregnancy to enroll in the MotHER Pregnancy Registry by contacting 1-800-690-6720.
Immunocompromised Patients
There is no FDA guidance one the use of Ado-trastuzumab emtansine in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Ado-trastuzumab emtansine Administration in the drug label.
Monitoring
There is limited information regarding Ado-trastuzumab emtansine Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ado-trastuzumab emtansine and IV administrations.
Overdosage
- There is no known antidote for overdose of Ado-trastuzumab emtansine In clinical trials, overdose of Ado-trastuzumab emtansine has been reported at approximately two times the recommended dose which resulted in Grade 2 thrombocytopenia (resolved 4 days later) and one death. In the fatal case, the patient incorrectly received Ado-trastuzumab emtansine at 6 mg/kg and died approximately 3 weeks following the overdose; a cause of death and a causal relationship to Ado-trastuzumab emtansine were not established.
Pharmacology
| |
Ado-trastuzumab emtansine?
| |
| Therapeutic monoclonal antibody | |
| Source | zu/o |
| Target | ? |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| Chemical data | |
| Formula | C6448H9948N1720O2012S44·(C47H62ClN4O13S)n |
| Mol. mass | 148.5 kDa |
| Pharmacokinetic data | |
| Bioavailability | N/A |
| Protein binding | 93% (in vitro) |
| Metabolism | Hepatic (CYP3A4/3A5-mediated) |
| Half life | 4 days |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
D(US) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | Intravenous infusion |
Mechanism of Action
- Ado-trastuzumab emtansine is a HER2-targeted antibody-drug conjugate. The antibody is the humanized anti-HER2 IgG1, trastuzumab. The small molecule cytotoxin, DM1, is a microtubule inhibitor. Upon binding to sub-domain IV of the HER2 receptor, ado-trastuzumab emtansine undergoes receptor-mediated internalization and subsequent lysosomal degradation, resulting in intracellular release of DM1-containing cytotoxic catabolites. Binding of DM1 to tubulin disrupts microtubule networks in the cell, which results in cell cycle arrest and apoptotic cell death. In addition, in vitro studies have shown that similar to trastuzumab, ado-trastuzumab emtansine inhibits HER2 receptor signaling, mediates antibody-dependent cell-mediated cytotoxicity and inhibits shedding of the HER2 extracellular domain in human breast cancer cells that over express HER2.
Structure
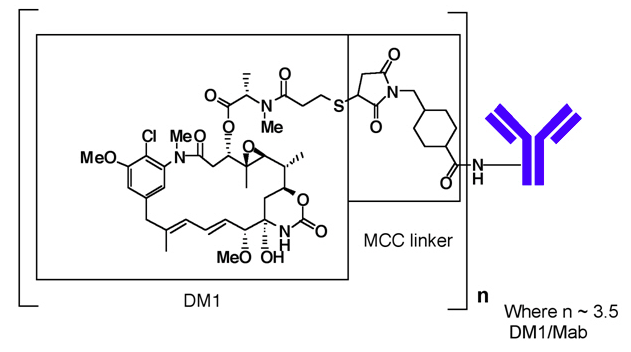
- Ado-trastuzumab emtansine (ado-trastuzumab emtansine) is a HER2-targeted antibody-drug conjugate (ADC) which contains the humanized anti-HER2 IgG1, trastuzumab, covalently linked to the microtubule inhibitory drug DM1 (a maytansine derivative) via the stable thioether linker MCC (4-[N-maleimidomethyl] cyclohexane-1-carboxylate). Emtansine refers to the MCC-DM1 complex.
- The antibody trastuzumab, is a well characterized recombinant monoclonal antibody product produced by mammalian (Chinese hamster ovary) cells, and the small molecule components (DM1 and MCC) are produced by chemical synthesis. Ado-trastuzumab emtansine contains an average of 3.5 DM1 molecules per antibody. Ado-trastuzumab emtansine has the following chemical structure:

Pharmacodynamics
Cardiac Electrophysiology
- The effect of multiple doses of Ado-trastuzumab emtansine (3.6 mg/kg every 3 weeks) on the QTc interval was evaluated in an open label, single arm study in 51 patients with HER2-positive metastatic breast cancer. No large changes in the mean QT interval (i.e., > 20 ms) were detected in the study.
Pharmacokinetics
The pharmacokinetics of Ado-trastuzumab emtansine was evaluated in a phase 1 study and in a population pharmacokinetic analysis for the ado-trastuzumab emtansine conjugate (ADC) using pooled data from 5 trials in patients with breast cancer. A linear two-compartment model with first-order elimination from the central compartment adequately describes the ADC concentration-time profile. In addition to ADC, the pharmacokinetics of total antibody (conjugated and unconjugated trastuzumab), DM1 were also determined. The pharmacokinetics of Ado-trastuzumab emtansine are summarized below.
Distribution
- Maximum concentrations (Cmax) of ADC and DM1 were observed close to the end of infusion. In Study 1, mean (SD) ADC and DM1 Cycle 1 Cmax following Ado-trastuzumab emtansine administration was 83.4 (16.5) mg/mL and 4.61 (1.61) ng/mL, respectively.
- In vitro, the mean binding of DM1 to human plasma proteins was 93%. In vitro, DM1 was a substrate of P-glycoprotein (P-gp).
- Based on population pharmacokinetic analysis, the central volume of distribution of ADC was 3.13 L.
Metabolism
- In vitro studies indicate that DM1, the small molecule component of Ado-trastuzumab emtansine, undergoes metabolism by CYP3A4/CYP3A5. DM1 did not inhibit or induce major CYP450 enzymes in vitro. In human plasma, ado-trastuzumab emtansine catabolites MCC-DM1, Lys-MCC-DM1, and DM1 were detected at low levels.
Elimination
- Based on population pharmacokinetic analysis, following intravenous infusion of Ado-trastuzumab emtansine, the clearance of the ADC was 0.68 L/day and the elimination half-life (t1/2) was approximately 4 days. No accumulation of Ado-trastuzumab emtansine was observed after repeated dosing of intravenous infusion every 3 weeks.
- Based on population pharmacokinetic analysis (n=671), body weight, sum of longest diameter of target lesions by RECIST, HER2 extracellular domain (ECD) concentrations, AST, albumin, and baseline trastuzumab concentrations were identified as statistically significant covariates for ado-trastuzumab emtansine clearance. However, the magnitude of effect of these covariates on ado-trastuzumab emtansine exposure suggests that, with the exception of body weight, these covariates are unlikely to have a clinically meaningful effect on Ado-trastuzumab emtansine exposure. Therefore, the body weight based dose of 3.6 mg/kg every 3 weeks without correction for other covariates is considered appropriate.
Pharmacokinetics in Special Population
Effect of Renal Impairment
- Based on population pharmacokinetic analysis in 668 patients, including moderate (CLcr 30 - 59 mL/min, n=53) and mild (CLcr 60 - 89 mL/min, n=254) renal impairment, indicate that pharmacokinetics of the ADC is not affected by mild to moderate renal impairment as compared to normal renal function (CLcr ≥ 90 mL/min, n=361). Data from only one patient with severe renal impairment (CLcr < 30 mL/min) is available .
Effects of Age and Race
- Based on population pharmacokinetic analysis, age (< 65 (n=577); 65 - 75 (n=78); > 75 (n=16)) and race (Asian (n=73); non-Asian (n=598)) do not have a clinically meaningful effect on the pharmacokinetics of ado-trastuzumab emtansine.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity studies have not been conducted with ado-trastuzumab emtansine.
- DM1 was aneugenic or clastogenic in an in vivo single-dose rat bone marrow micronucleus assay at exposures that were comparable to mean maximum concentrations of DM1 measured in humans administered Ado-trastuzumab emtansine. DM1 was not mutagenic in an in vitro bacterial reverse mutation (Ames) assay.
- Based on results from animal toxicity studies, Ado-trastuzumab emtansine may impair fertility in humans. In a single-dose toxicity study of ado-trastuzumab emtansine in rats, degeneration of seminiferous tubules with hemorrhage in the testes associated with increased weights of testes and epididymides at a severely toxic dose level (60 mg/kg; about 4 times the clinical exposure based on AUC) were observed. The same dose in female rats resulted in signs of hemorrhage and necrosis of the corpus luteum in ovaries. In monkeys dosed with ado-trastuzumab emtansine once every three weeks for 12 weeks (four doses), at up to 30 mg/kg (about 7 times the clinical exposure based on AUC), there were decreases in the weights of epididymides, prostate, testes, seminal vesicles and uterus, although the interpretation of these effects is unclear due to the varied sexual maturity of enrolled animals.
Animal Toxicology and/or Pharmacology
- In monkeys, treatment with doses of ado-trastuzumab emtansine up to 30 mg/kg (about 7 times the clinical exposure based on AUC) caused dose dependent axonal degeneration in the sciatic nerve with hypertrophy or hyperplasia of the Schwann cells, and axonal degeneration of the dorsal funiculus in the spinal cord. Based on the mechanism of action of the cytotoxic component DM1, there is clinical potential for neurotoxicity.
Clinical Studies
Metastatic Breast Cancer
- The efficacy of Ado-trastuzumab emtansine was evaluated in a randomized, multicenter, open-label trial of 991 patients with HER2-positive, unresectable locally advanced or metastatic breast cancer. Prior taxane and trastuzumab-based therapy was required before trial enrollment. Patients with only prior adjuvant therapy were required to have disease recurrence during or within six months of completing adjuvant therapy. Breast tumor samples were required to show HER2 overexpression defined as 3+ IHC or FISH amplification ratio ≥ 2.0 determined at a central laboratory. Patients were randomly allocated (1:1) to receive lapatinib plus capecitabine or Ado-trastuzumab emtansine Randomization was stratified by world region (United States, Western Europe, other), number of prior chemotherapy regimens for unresectable locally advanced or metastatic disease (0–1, >1) and visceral versus non-visceral disease as determined by the investigators.
- Ado-trastuzumab emtansine was given intravenously at 3.6 mg/kg on Day 1 of a 21-day cycle. Lapatinib was administered at 1250 mg/day orally once per day of a 21-day cycle and capecitabine was administered at 1000 mg/m2 orally twice daily on Days 1–14 of a 21-day cycle. Patients were treated with Ado-trastuzumab emtansine or lapatinib plus capecitabine until progression of disease, withdrawal of consent, or unacceptable toxicity. At the time of the primary analysis, median time on study drug was 5.7 months (range: 0–28.4) for Ado-trastuzumab emtansine, 4.9 months (range: 0–30.8) for lapatinib, and 4.8 months (range: 0–30.4) for capecitabine.
- The co-primary efficacy endpoints of the study were progression-free survival (PFS) based on tumor response assessments by an independent review committee (IRC), and overall survival (OS). PFS was defined as the time from the date of randomization to the date of disease progression or death from any cause (whichever occurred earlier). Overall survival was defined as the time from the date of randomization to the date of death from any cause. Additional endpoints included PFS (based on investigator tumor response assessments), objective response rate (ORR), duration of response and time to symptom progression.
- Patient demographics and baseline tumor characteristics were balanced between treatment arms. All patients had metastatic disease at study entry. The median age was approximately 53 years (range 24-84 years), 74% were White, 18% were Asian and 5% were Black. All but 5 patients were women. Twenty-seven percent of patients were enrolled in United States, 32% in Europe and 16% in Asia. Tumor prognostic characteristics including hormone receptor status (positive: 55%, negative: 43%), presence of visceral disease (68%) and non-visceral disease only (33%) and the number of metastatic sites (< 3: 61%, ≥ 3: 37%) were similar in the study arms.
- The majority of patients (88%) had received prior systemic treatment in the metastatic setting. Twelve percent of patients had prior treatment only in the neoadjuvant or adjuvant setting and had disease relapse within 6 months of treatment. All but one patient received trastuzumab prior to study entry; approximately 85% of patients received prior trastuzumab in the metastatic setting. Over 99% percent of patients had received a taxane, and 61% of patients had received an anthracycline prior to study entry. Overall, patients received a median of 3 systemic agents in the metastatic setting. Among patients with hormone receptor-positive tumors, 44.4% received prior adjuvant hormonal therapy and 44.8% received hormonal therapy for locally advanced/metastatic disease.
- The randomized trial demonstrated a statistically significant improvement in IRC-assessed PFS in the Ado-trastuzumab emtansine-treated group compared with the lapatinib plus capecitabine-treated group [hazard ratio (HR) = 0.65, 95% CI: 0.55, 0.77, p < 0.0001], and an increase in median PFS of 3.2 months (median PFS of 9.6 months in the Ado-trastuzumab emtansine-treated group vs. 6.4 months in the lapatinib plus capecitabine group). See TABLE 8 and FIGURE 1. The results for investigator-assessed PFS were similar to those observed for IRC-assessed PFS.
- At the time of PFS analysis, 223 patients had died. More deaths occurred in the lapatinib plus capecitabine arm (26%) compared with the Ado-trastuzumab emtansine arm (19%), however the results of this interim OS analysis did not meet the pre-specified stopping boundary for statistical significance. At the time of the second interim OS analysis, 331 events had occurred. The co-primary endpoint of OS was met; OS was significantly improved in patients receiving Ado-trastuzumab emtansine (HR = 0.68, 95% CI: 0.55, 0.85, p = 0.0006). This result crossed the pre-specified efficacy stopping boundary (HR = 0.73 or p = 0.0037). The median duration of survival was 30.9 months in the Ado-trastuzumab emtansine arm vs. 25.1 months in the lapatinib plus capecitabine arm. See TABLE 8 and FIGURE 2.
- A treatment benefit with Ado-trastuzumab emtansine in terms of PFS and OS was observed in patient subgroups based on stratification factors, key baseline demographic and disease characteristics, and prior treatments. In the subgroup of patients with hormone receptor-negative disease (n=426), the hazard ratios for PFS and OS were 0.56 (95% CI: 0.44, 0.72) and 0.75 (95% CI: 0.54, 1.03), respectively. In the subgroup of patients with hormone receptor-positive disease (n=545), the hazard ratios for PFS and OS were 0.72 (95% CI: 0.58, 0.91) and 0.62 (95% CI: 0.46, 0.85), respectively. In the subgroup of patients with non-measurable disease (n=205), based on IRC assessments, the hazard ratios for PFS and OS were 0.91 (95% CI: 0.59, 1.42) and 0.96 (95% CI: 0.54, 1.68), respectively; in patients with measurable disease the hazard ratios were 0.62 (95% CI: 0.52, 0.75) and 0.65 (95% CI: 0.51, 0.82), respectively. The PFS and OS hazard ratios in patients who were younger than 65 years old (n=853) were 0.62 (95% CI: 0.52, 0.74) and 0.66 (95% CI: 0.52, 0.83), respectively. In patients ≥ 65 years old (n=138), the hazard ratios for PFS and OS were 1.06 (95% CI: 0.68, 1.66) and 1.05 (95% CI: 0.58, 1.91), respectively.

How Supplied
- Ado-trastuzumab emtansine (ado-trastuzumab emtansine) is supplied as:

Storage
- Store vials in a refrigerator at 2°C to 8°C (36°F to 46°F) until time of reconstitution. Do not freeze or shake.
Images
Drug Images
{{#ask: Page Name::Ado-trastuzumab emtansine |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Ado-trastuzumab emtansine |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Ado-trastuzumab emtansine Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Ado-trastuzumab emtansine interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Ado-trastuzumab emtansine Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.