Pralidoxime
 | |
| Clinical data | |
|---|---|
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
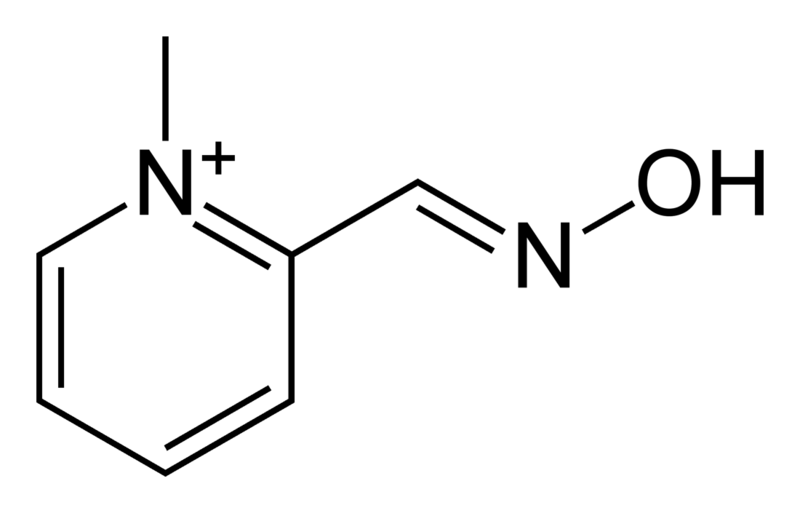
| Formula | C7H9N2O+ |
| Molar mass | 137.159 g/mol |
|
WikiDoc Resources for Pralidoxime |
|
Articles |
|---|
|
Most recent articles on Pralidoxime Most cited articles on Pralidoxime |
|
Media |
|
Powerpoint slides on Pralidoxime |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Pralidoxime at Clinical Trials.gov Clinical Trials on Pralidoxime at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Pralidoxime
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Pralidoxime Discussion groups on Pralidoxime Patient Handouts on Pralidoxime Directions to Hospitals Treating Pralidoxime Risk calculators and risk factors for Pralidoxime
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Pralidoxime |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [1] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Pralidoxime belongs to a family of compounds called oximes that bind to organophosphate-inactivated acetylcholinesterase. It is used to combat poisoning by organophosphates or acetylcholinesterase inhibitors (nerve gas), in conjunction with atropine. Pralidoxime is most commonly in the form of Pralidoxime Chloride, also known as 2-PAM CL (or just 2-PAM by the military).
In a normal nicotinic synaptic junction, including motor end plates and preganglionic fibers, acetylcholine (ACh) is released from the presynaptic axon terminal into the synaptic cleft. The ACh then diffuses through the synaptic cleft and binds to nicotinic receptors on the postsynaptic membrane. This induces a subsequent action potential (AP) that continues through the postganglionic cell, or induces contraction in the motor end plate.
In order to prevent overstimulation or saturation of the synapse, or both, an enzyme known as acetylcholinesterase breaks down the neurotransmitter ACh. By removing the ACh, the synapse is brought to a state where it is ready for subsequent activation. Saturation of the synapse occurs when there is an excess of acetylcholine in the synaptic cleft, which inhibits further nerve transmission as the nicotinic receptors are full. Agents which inhibit acetylcholinesterase will lead to a build-up of ACh in the cleft.
Mechanism of action
Organophosphates inhibit cholinesterase by phosphorylation of the enzyme. Pralidoxime reactivates the cholinesterase by removing the phosphoryl group that is bound to the ester group. In this reaction both the organophosphate and the pralidoxime are mutually inactivated. These products undergo rapid metabolism, leading to the removal of the organophosphate.
Paradoxically, pralidoxime in doses above the optimal dose is itself an inhibitor of cholinesterase, and therefore can also produce the same symptoms as the toxins themselves. However, unlike organophosphates, pralidoxime binding is reversible, hence some protection is extended to the cholinesterase enzyme, this although has a negligible effect on the pharmacology of pralidoxime.
Dosage
Recommended dosages, according to online sources, seem to be:
- Adults: 30 mg/kg (Typically 1.5 - 2 g), administered either by intravenous therapy or intramuscular injection
- Children: 50 mg/kg
Interactions
When atropine and pralidoxime are used together, the signs of atropinization (flushing, mydriasis, tachycardia, dryness of the mouth and nose) may occur earlier than might be expected when atropine is used alone. This is especially true if the total dose of atropine has been large and the administration of pralidoxime has been delayed.
The following precautions should be kept in mind in the treatment of anticholinesterase poisoning, although they do not bear directly on the use of pralidoxime: since barbiturates are potentiated by the anticholinesterases, they should be used cautiously in the treatment of convulsions; morphine, theophylline, aminophylline, succinylcholine, reserpine, and phenothiazine-type tranquilizers should be avoided in patients with organophosphate poisoning.
Contraindications
There are no known absolute contraindications for the use of pralidoxime. Relative contraindications include known hypersensitivity to the drug and other situations in which the risk of its use clearly outweighs possible benefit.
See Also
External links
- Pages with script errors
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Antidotes