Sodium nitrite
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Kiran Singh, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
WARNING: LIFE THREATENING HYPOTENSION AND METHEMOGLOBIN FORMATION
See full prescribing information for complete Boxed Warning.
* Sodium nitrite can cause serious adverse reactions and death in humans, even at doses less than twice the recommended therapeutic dose. Sodium nitrite causes hypotension and methemoglobin formation, which diminishes oxygen carrying capacity. Hypotension and methemoglobin formation can occur concurrently or separately. Because of these risks, sodium nitrite should be used to treat acute life-threatening cyanide poisoning and be used with caution in patients where the diagnosis of cyanide poisoning is uncertain.
|
Overview
Sodium nitrite is a cyanide antidote that is FDA approved for the treatment of acute cyanide poisoning. There is a Black Box Warning for this drug as shown here. Common adverse reactions include syncope, hypotension, tachycardia, methemoglobinemia, palpitations, dysrhythmia, methemoglobinemia, headache, dizziness, blurred vision.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
Sodium Nitrite Injection is indicated for sequential use with sodium thiosulfate for the treatment of acute cyanide poisoning that is judged to be life-threatening. When the diagnosis of cyanide poisoning is uncertain, the potentially life-threatening risks associated with Sodium Nitrite Injection should be carefully weighed against the potential benefits, especially if the patient is not in extremis.
Identifying Patients with Cyanide Poisoning
- Cyanide poisoning may result from inhalation, ingestion, or dermal exposure to various cyanide-containing compounds, including smoke from closed-space fires. Sources of cyanide poisoning include hydrogen cyanide and its salts, cyanogenic plants, aliphatic nitriles, and prolonged exposure to sodium nitroprusside.
- The presence and extent of cyanide poisoning are often initially unknown. There is no widely available, rapid, confirmatory cyanide blood test. Treatment decisions must be made on the basis of clinical history and signs and symptoms of cyanide intoxication. If clinical suspicion of cyanide poisoning is high, Sodium Nitrite Injection and Sodium thiosulfate injection should be administered without delay.
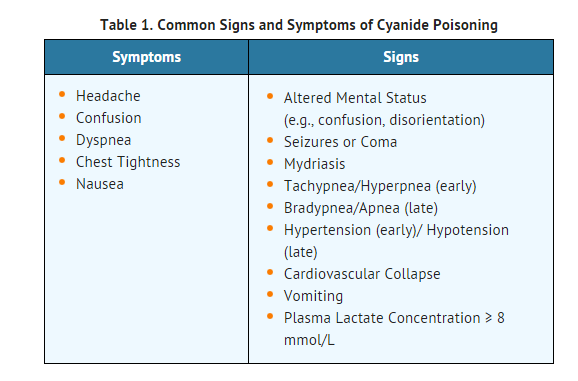
- In some settings, panic symptoms including tachypnea and vomiting may mimic early cyanide poisoning signs. The presence of altered mental status (e.g., confusion and disorientation) and/or mydriasis is suggestive of true cyanide poisoning although these signs can occur with other toxic exposures as well.
The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222.
Smoke Inhalation
- Not all smoke inhalation victims will have cyanide poisoning and may present with burns, trauma, and exposure to other toxic substances making a diagnosis of cyanide poisoning particularly difficult. Prior to administration of Sodium Nitrite Injection, smoke-inhalation victims should be assessed for the following:
- Exposure to fire or smoke in an enclosed area
- Presence of soot around the mouth, nose, or oropharynx
- Altered mental status
- Although hypotension is highly suggestive of cyanide poisoning, it is only present in a small percentage of cyanide-poisoned smoke inhalation victims. Also indicative of cyanide poisoning is a plasma lactate concentration greater than or equal to 10 mmol/L (a value higher than that typically listed in the table of signs and symptoms of isolated cyanide poisoning because carbon monoxide associated with smoke inhalation also contributes to lactic acidemia). If cyanide poisoning is suspected, treatment should not be delayed to obtain a plasma lactate concentration.
Use with Other Cyanide Antidotes
Caution should be exercised when administering cyanide antidotes, other than sodium thiosulfate, simultaneously with Sodium Nitrite Injection, as the safety of co-administration has not been established. If a decision is made to administer another cyanide antidote, other than sodium thiosulfate, with Sodium Nitrite Injection, these drugs should not be administered concurrently in the same IV line.
Dosing
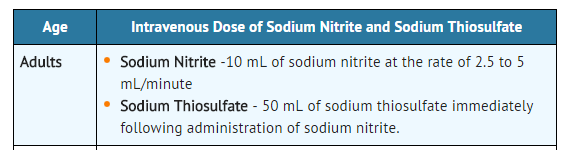
NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate.
In adult and pediatric patients with known anemia, it is recommended that the dosage of sodium nitrite should be reduced proportionately to the hemoglobin concentration.
All parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
DOSAGE FORMS AND STRENGTHS
Sodium Nitrite Injection consists of:
- One vial of sodium nitrite injection, USP 300 mg/10mL (30 mg/mL)
- Administration of the contents of one vial constitutes a single dose.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of sodium nitrite in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of sodium nitrite in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Indications
Sodium Nitrite Injection is indicated for sequential use with sodium thiosulfate for the treatment of acute cyanide poisoning that is judged to be life-threatening. When the diagnosis of cyanide poisoning is uncertain, the potentially life-threatening risks associated with Sodium Nitrite Injection should be carefully weighed against the potential benefits, especially if the patient is not in extremis.
Identifying Patients with Cyanide Poisoning
- Cyanide poisoning may result from inhalation, ingestion, or dermal exposure to various cyanide-containing compounds, including smoke from closed-space fires. Sources of cyanide poisoning include hydrogen cyanide and its salts, cyanogenic plants, aliphatic nitriles, and prolonged exposure to sodium nitroprusside.
- The presence and extent of cyanide poisoning are often initially unknown. There is no widely available, rapid, confirmatory cyanide blood test. Treatment decisions must be made on the basis of clinical history and signs and symptoms of cyanide intoxication. If clinical suspicion of cyanide poisoning is high, Sodium Nitrite Injection and Sodium Thiosulfate Injection should be administered without delay.

- In some settings, panic symptoms including tachypnea and vomiting may mimic early cyanide poisoning signs. The presence of altered mental status (e.g., confusion and disorientation) and/or mydriasis is suggestive of true cyanide poisoning although these signs can occur with other toxic exposures as well.
The expert advice of a regional poison control center may be obtained by calling 1-800-222-1222.
Smoke Inhalation
- Not all smoke inhalation victims will have cyanide poisoning and may present with burns, trauma, and exposure to other toxic substances making a diagnosis of cyanide poisoning particularly difficult. Prior to administration of Sodium Nitrite Injection, smoke-inhalation victims should be assessed for the following:
- Exposure to fire or smoke in an enclosed area
- Presence of soot around the mouth, nose, or oropharynx
- Altered mental status
- Although hypotension is highly suggestive of cyanide poisoning, it is only present in a small percentage of cyanide-poisoned smoke inhalation victims. Also indicative of cyanide poisoning is a plasma lactate concentration greater than or equal to 10 mmol/L (a value higher than that typically listed in the table of signs and symptoms of isolated cyanide poisoning because carbon monoxide associated with smoke inhalation also contributes to lactic acidemia). If cyanide poisoning is suspected, treatment should not be delayed to obtain a plasma lactate concentration.
Use with Other Cyanide Antidotes
Caution should be exercised when administering cyanide antidotes, other than sodium thiosulfate, simultaneously with Sodium Nitrite Injection, as the safety of co-administration has not been established. If a decision is made to administer another cyanide antidote, other than sodium thiosulfate, with Sodium Nitrite Injection, these drugs should not be administered concurrently in the same IV line.
Dosing

NOTE: If signs of poisoning reappear, repeat treatment using one-half the original dose of both sodium nitrite and sodium thiosulfate.
In adult and pediatric patients with known anemia, it is recommended that the dosage of sodium nitrite should be reduced proportionately to the hemoglobin concentration.
All parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of sodium nitrite in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of sodium nitrite in pediatric patients.
Contraindications
- None
Warnings
|
WARNING: LIFE THREATENING HYPOTENSION AND METHEMOGLOBIN FORMATION
See full prescribing information for complete Boxed Warning.
* Sodium nitrite can cause serious adverse reactions and death in humans, even at doses less than twice the recommended therapeutic dose. Sodium nitrite causes hypotension and methemoglobin formation, which diminishes oxygen carrying capacity. Hypotension and methemoglobin formation can occur concurrently or separately. Because of these risks, sodium nitrite should be used to treat acute life-threatening cyanide poisoning and be used with caution in patients where the diagnosis of cyanide poisoning is uncertain.
|
Hypotension
Sodium nitrite has been associated with severe hypotension, methemoglobinemia, and death at doses less than twice recommended therapeutic doses. Hypotension may occur concurrently or separately. Sodium nitrite should be used to treat life-threatening cyanide poisoning. When the diagnosis of cyanide poisoning is uncertain and/or the patient is not in extremis, special consideration should be given to administration of sodium nitrite if the patient is known or suspected to have diminished oxygen or cardiovascular reserve (e.g., smoke inhalation victims, pre-existing anemia, substantial blood loss, cardiac or respiratory compromise) or to be at higher risk of developing methemoglobinemia (e.g., congenital methemoglobin reductase deficiency).
Methemoglobinemia
Supportive care alone may be sufficient treatment without administration of antidotes for many cases of cyanide intoxication, particularly in conscious patients without signs of severe toxicity. Patients should be closely monitored to ensure adequate perfusion and oxygenation during treatment with sodium nitrite.
Methemoglobin levels should be monitored and oxygen administered during treatment with sodium nitrite whenever possible. When sodium nitrite is administered to humans a wide range of methemoglobin concentrations occur. Methemoglobin concentrations as high as 58% have been reported after two 300-mg doses of sodium nitrite administered to an adult. Sodium nitrite should be used with caution in the presence of other drugs that may cause methemoglobinemia such as procaine and nitroprusside. Sodium nitrite should be used with caution in patients who may be particularly susceptible to injury from vasodilation and its related hemodynamic sequelae. Hemodynamics should be monitored closely during and after administration of sodium nitrite, and infusion rates should be slowed if hypotension occurs.
Anemia
Sodium nitrite should be used with caution in patients with known anemia. Patients with anemia will form more methemoglobin (as a percentage of total hemoglobin) than persons with normal red blood cell (RBC) volumes. Optimally, these patients should receive a sodium nitrite dose that is reduced in proportion to their oxygen carrying capacity.
Smoke Inhalation Injury
Sodium nitrite should be used with caution in persons with smoke inhalation injury or carbon monoxide poisoning because of the potential for worsening hypoxia due to methemoglobin formation.
Neonates and Infants
Neonates and infants may be more susceptible than adults and older pediatric patients to severe methemoglobinemia when sodium nitrite is administered. Reduced dosing guidelines should be followed in pediatric patients.
G6PD Deficiency
Because patients with G6PD deficiency are at increased risk of a hemolytic crisis with sodium nitrite administration, alternative therapeutic approaches should be considered in these patients. Patients with known or suspected G6PD deficiency should be monitored for an acute drop in hematocrit. Exchange transfusion may be needed for patients with G6PD deficiency who receive sodium nitrite.
Use with Other Drugs
Sodium nitrite should be used with caution in the presence of concomitant antihypertensive medications, diuretics or volume depletion due to diuretics, or drugs known to increase vascular nitric oxide, such as PDE5 inhibitors.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of sodium nitrite in the drug label.
Postmarketing Experience
- There have been no controlled clinical trials conducted to systematically assess the adverse events profile of sodium nitrite.
- The medical literature has reported the following adverse events in association with sodium nitrite administration. These adverse events were not reported in the context of controlled trials or with consistent monitoring and reporting methodologies for adverse events. Therefore, frequency of occurrence of these adverse events cannot be assessed.
Cardiovascular system: syncope, hypotension, tachycardia, methemoglobinemia, palpitations, dysrhythmia
Hematological: methemoglobinemia
Central nervous system: headache, dizziness, blurred vision, seizures, confusion, coma
Gastrointestinal system: nausea, vomiting, abdominal pain
Respiratory system: tachypnea, dyspnea
Body as a Whole: anxiety, diaphoresis, lightheadedness, injection site tingling, cyanosis, acidosis, fatigue, weakness, urticaria, generalized numbness and tingling
- Severe hypotension, methemoglobinemia, cardiac dysrhythmias, coma and death have been reported in patients without life-threatening cyanide poisoning but who were treated with injection of sodium nitrite at doses less than twice those recommended for the treatment of cyanide poisoning.
Drug Interactions
- Formal drug interaction studies have not been conducted with Sodium Nitrite Injection.
Use in Specific Populations
Pregnancy
Pregnancy
Teratogenic Effects. Pregnancy Category C.
- There are no adequate and well-controlled studies in pregnant women. Sodium Nitrite Injection should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Sodium nitrite has caused fetal death in humans as well as animals. There are no studies in humans that have directly evaluated the potential reproductive toxicity of sodium nitrite. There are two epidemiological studies conducted in Australia that report a statistically significant increase in the risk for congenital malformations, particularly in the CNS, associated with maternal consumption of water containing nitrate levels in excess of 5 ppm. Results from a case-control study in Canada suggested a trend toward an increase in the risk for CNS malformations when maternal consumption of nitrate was ≥ 26 ppm (not statistically significant).
- The potential reproductive toxicity of sodium nitrite exposure restricted to the prenatal period has been reported in guinea pigs, mice, and rats. There was no evidence of teratogenicity in guinea pigs, mice, or rats. However, sodium nitrite treatment of pregnant guinea pigs with 60 or 70 mg/kg/day resulted in abortion of the litters within 1-4 days of treatment. All animals treated subcutaneously with 70 mg/kg, sodium nitrite died within 60 minutes of treatment. Further studies demonstrated that a dose of 60 mg/kg resulted in measurable blood levels of methemoglobin in the dams and their fetuses for up to 6 hours post treatment. Maternal methemoglobin levels were higher than the levels in the offspring at all times measured. Based on a body surface area comparison, a 60 mg/kg dose in the guinea pig that resulted in death was only 1.7 times higher than the highest clinical dose of sodium nitrite that would be used to treat cyanide poisoning (based on a body surface area comparison).
- Studies testing prenatal and postnatal exposure have been reported in mice and rats. Treatment of pregnant rats via drinking water with sodium nitrite at concentrations of either 2000 or 3000 mg/L resulted in a dose-related increased mortality postpartum. This exposure regimen in the rat model would result in dosing of approximately 220 and 300 mg/kg/day (43 and 65 times the highest clinical dose of sodium nitrite that would be used to treat cyanide poisoning, based on a body surface area comparison).
- Sodium nitrite produces methemoglobin. Fetal hemoglobin is oxidized to methemoglobin more easily than adult hemoglobin. In addition, the fetus has lower levels of methemoglobin reductase than adults. Collectively, these data suggest that the human fetus would show greater sensitivity to methemoglobin resulting in nitrite-induced prenatal hypoxia leading to retarded development of certain neurotransmitter systems in the brain and long lasting dysfunction.
Nonteratogenic Effects:
- Behavioral and neurodevelopmental studies in rats suggest persistent effects of prenatal exposure to sodium nitrite that were detectable postnatally. Specifically, animals that were exposed prenatally to sodium nitrite demonstrated impaired discrimination learning behavior (both auditory and visual) and reduced long-term retention of the passive-avoidance response compared to control animals. Additional studies demonstrated a delay in the development of AchE and 5-HT positive fiber ingrowth into the hippocampal dentate gyrus and parietal neocortex during the first week of life of prenatal nitrite treated pups. These changes have been attributed to prenatal hypoxia following nitrite exposure.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of sodium nitrite in women who are pregnant.
Labor and Delivery
- Because fetal hemoglobin is more readily oxidized to methemoglobin and lower levels of methemoglobin appear to be fatal to the fetus compared to the adult, sodium nitrite should be used during labor and delivery only if the potential benefit justifies the potential risk to the fetus.
Nursing Mothers
- It is not known whether sodium nitrite is excreted in human milk. Because Sodium Nitrite Injection may be administered in life-threatening situations, breast-feeding is not a contraindication to its use. Because many drugs are excreted in human milk, caution should be exercised following Sodium Nitrite Injection administration to a nursing woman. There are no data to determine when breastfeeding may be safely restarted following administration of sodium nitrite. In studies conducted with Long-Evans rats, sodium nitrite administered in drinking water during pregnancy and lactation resulted in severe anemia, reduced growth and increased mortality in the offspring.
Pediatric Use
- There are case reports in the medical literature of sodium nitrite in conjunction with sodium thiosulfate being administered to pediatric patients with cyanide poisoning; however, there have been no clinical studies to evaluate the safety or efficacy of sodium nitrite in the pediatric population. As for adult patients, dosing recommendations for pediatric patients have been based on theoretical calculations of antidote detoxifying potential, extrapolation from animal experiments, and a small number of human case reports.
- Sodium nitrite must be used with caution in patients less than 6 months of age because they may be at higher risk of developing severe methemoglobinemia compared to older children and adults. The presence of fetal hemoglobin, which is oxidized to methemoglobin more easily than adult hemoglobin, and lower methemoglobin reductase levels compared to older children and adults may contribute to risk.
- Mortality attributed to sodium nitrite was reported following administration of an adult dose (300 mg IV followed by a second dose of 150 mg) to a 17-month old child.
Geriatic Use
- Sodium nitrite is known to be substantially excreted by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Sodium nitrite with respect to specific gender populations.
Race
There is no FDA guidance on the use of sodium nitrite with respect to specific racial populations.
Renal Impairment
- Sodium nitrite is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Hepatic Impairment
There is no FDA guidance on the use of Sodium nitrite in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Sodium nitrite in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Sodium nitrite in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
Administration Recommendation
- Comprehensive treatment of acute cyanide intoxication requires support of vital functions. Administration of sodium nitrite, followed by sodium thiosulfate, should be considered adjunctive to appropriate supportive therapies. Airway, ventilatory and circulatory support, and oxygen administration should not be delayed to administer sodium nitrite and sodium thiosulfate.
- Sodium nitrite injection and sodium thiosulfate injection are administered by slow intravenous injection. They should be given as early as possible after a diagnosis of acute life-threatening cyanide poisoning has been established. Sodium nitrite should be administered first, followed immediately by sodium thiosulfate. Blood pressure must be monitored during infusion in both adults and children. The rate of infusion should be decreased if significant hypotension is noted.
Incompatibility Information
- Chemical incompatibility has been reported between sodium nitrite and hydroxocobalamin and these drugs should not be administered simultaneously through the same IV line. No chemical incompatibility has been reported between sodium thiosulfate and sodium nitrite, when administered sequentially through the same IV line.
Monitoring
Recommended Monitoring
- Patients should be monitored for at least 24-48 hours after Sodium Nitrite Injection administration for adequacy of oxygenation and perfusion and for recurrent signs and symptoms of cyanide toxicity. When possible, hemoglobin/hematocrit should be obtained when treatment is initiated. Measurements of oxygen saturation using standard pulse oximetry and calculated oxygen saturation values based on measured PO2 are unreliable in the presence of methemoglobinemia.
- Methemoglobin level: Administrations of sodium nitrite solely to achieve an arbitrary level of methemoglobinemia may be unnecessary and potentially hazardous. The therapeutic effects of sodium nitrite do not appear to be mediated by methemoglobin formation alone [see CLINICAL PHARMACOLOGY (12)] and clinical responses to sodium nitrite administration have been reported in association with methemoglobin levels of less than 10%. Administration of sodium nitrite beyond the initial dose should be guided primarily by clinical response to treatment (i.e., a second dose should be considered only if there is inadequate clinical response to the first dose). It is generally recommended that methemoglobin concentrations be closely monitored and kept below 30%. Serum methemoglobin levels should be monitored during treatment using co-oximetry, and administration of sodium nitrite should generally be discontinued when methemoglobin levels exceed 30%. Intravenous methylene blue and exchange transfusion have been reported in the literature as treatments for life-threatening methemoglobinemia.
IV Compatibility
There is limited information regarding IV Compatibility of Sodium nitrite in the drug label.
Overdosage
- Large doses of sodium nitrite result in severe hypotension and toxic levels of methemoglobin which may lead to cardiovascular collapse.
- Sodium nitrite administration has been reported to cause or significantly contribute to mortality in adults at oral doses as low as 1 g and intravenous doses as low as 600 mg. A death attributed to sodium nitrite has been reported following administration of an adult dose (300 mg IV followed by a second dose of 150 mg) to a 17-month old child.
- Cyanosis may become apparent at a methemoglobin level of 10-20%. Other clinical signs and symptoms of sodium nitrite toxicity (anxiety, dyspnea, nausea, and tachycardia) can be apparent at methemoglobin levels as low as 15%. More serious signs and symptoms, including cardiac dysrhythmias, circulatory failure, and central nervous system depression are seen as methemoglobin levels increase, and levels above 70% are usually fatal.
- Treatment of overdose involves supplemental oxygen and supportive measures such as exchange transfusion. Treatment of severe methemoglobinemia with intravenous methylene blue has been described in the medical literature; however, this may also cause release of cyanide bound to methemoglobin. Because hypotension appears to be mediated primarily by an increase in venous capacitance, measures to increase venous return may be most appropriate to treat hypotension.
Pharmacology
Template:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox entryTemplate:Chembox ECNumberTemplate:Chembox E numberTemplate:Chembox RTECSTemplate:Chembox UNNumberTemplate:Chembox AppearanceTemplate:Chembox DensityTemplate:Chembox MeltingPtTemplate:Chembox SolubilityInWaterTemplate:Chembox SolubilityTemplate:Chembox pKaTemplate:Chembox RefractIndexTemplate:Chembox StructureTemplate:Chembox ThermochemistryTemplate:Chembox EUClassTemplate:Chembox RPhrasesTemplate:Chembox SPhrasesTemplate:Chembox NFPATemplate:Chembox AutoignitionPtTemplate:Chembox Lethal amounts (set)Template:Chembox OtherAnionsTemplate:Chembox OtherCationsTemplate:Chembox Supplement| Template:Chembox header2 | Sodium nitrite | |
|---|---|
| Identifiers | |
3D model (JSmol)
|
|
| ChEBI | |
| ChEMBL | |
| ChemSpider | |
| ECHA InfoCard | Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). Lua error in Module:Wikidata at line 879: attempt to index field 'wikibase' (a nil value). |
PubChem CID
|
|
| UNII | |
| |
| |
| Properties | |
| NaNO2 | |
| Molar mass | 68.9953 g/mol |
| Hazards | |
| Related compounds | |
| Template:Chembox header2 | Except where noted otherwise, data are given for materials in their standard state (at 25 °C, 100 kPa) Infobox disclaimer and references | |
Mechanism of Action
- Exposure to a high dose of cyanide can result in death within minutes due to the inhibition of cytochrome oxidase resulting in arrest of cellular respiration. Specifically, cyanide binds rapidly with cytochrome a3, a component of the cytochrome c oxidase complex in mitochondria. Inhibition of cytochrome a3 prevents the cell from using oxygen and forces anaerobic metabolism, resulting in lactate production, cellular hypoxia and metabolic acidosis. In massive acute cyanide poisoning, the mechanism of toxicity may involve other enzyme systems as well.
- The synergy resulting from treatment of cyanide poisoning with the combination of sodium nitrite and sodium thiosulfate is the result of differences in their primary mechanisms of action as antidotes for cyanide poisoning.
Sodium Nitrite
- Sodium nitrite is thought to exert its therapeutic effect by reacting with hemoglobin to form methemoglobin, an oxidized form of hemoglobin incapable of oxygen transport but with high affinity for cyanide. Cyanide preferentially binds to methemoglobin over cytochrome a3, forming the nontoxic cyanomethemoglobin. Methemoglobin displaces cyanide from cytochrome oxidase, allowing resumption of aerobic metabolism. The chemical reaction is as follows:
- NaNO2 + Hemoglobin → Methemoglobin
- HCN + Methemoglobin → Cyanomethemoglobin
- Vasodilation has also been cited to account for at least part of the therapeutic effect of sodium nitrite. It has been suggested that sodium nitrite-induced methemoglobinemia may be more efficacious against cyanide poisoning than comparable levels of methemoglobinemia induced by other oxidants. Also, sodium nitrite appears to retain some efficacy even when the formation of methemoglobin is inhibited by methylene blue.
Sodium Thiosulfate
- The primary route of endogenous cyanide detoxification is by enzymatic transulfuration to thiocyanate (SCN-), which is relatively nontoxic and readily excreted in the urine. Sodium thiosulfate is thought to serve as a sulfur donor in the reaction catalyzed by the enzyme rhodanese, thus enhancing the endogenous detoxification of cyanide in the following chemical reaction:
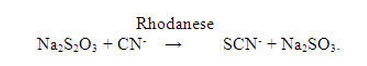
Structure
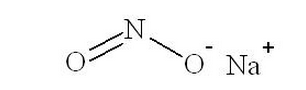
- Sodium nitrite has the chemical name nitrous acid sodium salt. The chemical formula is NaNO2 and the molecular weight is 69.0. The structural formula is:
- Structure of Sodium Nitrite
- Sodium Nitrite Injection is a cyanide antidote which contains one 10 mL glass vial of a 3% solution of sodium nitrite injection.
- Sodium nitrite injection is a sterile aqueous solution and is intended for intravenous injection. Each vial contains 300 mg of sodium nitrite in 10 mL solution (30 mg/mL). Sodium nitrite injection is a clear solution with a pH between 7.0 and 9.0.
Pharmacodynamics
Sodium Nitrite
- When 4 mg/kg sodium nitrite was administered intravenously to six healthy human volunteers, the mean peak methemoglobin concentration was 7%, achieved at 30-60 minutes after injection, consistent with reports in cyanide poisoning victims. Supine systolic and diastolic blood pressures dropped approximately 20% within 10 minutes, a drop which was sustained throughout the 40 minutes of testing. This was associated with a 20 beat per minute increase in pulse rate that returned to baseline in 10 minutes. Five of these subjects were unable to withstand orthostatic testing due to fainting. One additional subject, who received a 12 mg/kg dose of sodium nitrite, experienced severe cardiovascular effects and achieved a peak methemoglobin concentration of 30% at 60 minutes following injection.
- Oral doses of 120 to 180 mg of sodium nitrite administered to healthy volunteers caused minimal cardiovascular changes when subjects were maintained in the horizontal position. However, minutes after being placed in the upright position subjects exhibited tachycardia and hypotension with syncope.
- The half life for conversion of methemoglobin to normal hemoglobin in a cyanide poisoning victim who has been administered sodium nitrite is estimated to be 55 minutes.
Pharmacokinetics
Sodium Nitrite
- Sodium nitrite is a strong oxidant, and reacts rapidly with hemoglobin to form methemoglobin. The pharmacokinetics of free sodium nitrite in humans have not been well studied. It has been reported that approximately 40% of sodium nitrite is excreted unchanged in the urine while the remaining 60% is metabolized to ammonia and related small molecules.
Cyanide
- The apparent terminal elimination half life and volume of distribution of cyanide, in a patient treated for an acute cyanide poisoning with sodium nitrite and sodium thiosulfate administration, have been reported to be 19 hours and 0.41 L/kg, respectively. Additionally, an initial elimination half life of cyanide has been reported to be approximately 1-3 hours.
Thiocyanate
- After detoxification, in healthy subjects, thiocyanate is excreted mainly in the urine at a rate inversely proportional to creatinine clearance. In healthy subjects, the elimination half-life and volume of distribution of thiocyanate have been reported to be 2.7 days and 0.25 L/kg, respectively. However, in subjects with renal insufficiency the reported elimination half life is approximately 9 days.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- The potential benefit of an acute exposure to sodium nitrite as part of a cyanide antidote outweighs concerns raised by the equivocal findings in chronic rodent studies. Sodium nitrite (0, 750, 1500, or 3000 ppm equivalent to average daily doses of approximately 0, 35, 70, or 130 mg/kg for males and 0, 40, 80, or 150 mg/kg for females) was orally administered to rats (Fischer 344 strain) for 2 years via drinking water. There were no significant increases in the incidence of tumor in either male or female rats. Sodium nitrite (0, 750, 1500, or 3000 ppm equivalent to average daily doses of approximately 0, 60, 120, or 220 mg/kg for males and 0, 45, 90, or 165 mg/kg for females) was administered to B6C3F1 mice for 2 years via the drinking water. Equivocal results were obtained in female mice. Specifically, there was a positive trend toward an increase in the incidence of squamous cell papilloma or carcinoma in the forestomach of female mice. Although the incidence of hyperplasia of the glandular stomach epithelium was significantly greater in the high-dose male mice compared to controls, there were no significant increases in tumors in the male mice. Numerous reports in the published literature indicate that sodium nitrite may react in vivo with secondary amines to form carcinogenic nitrosamines in the stomach. Concurrent exposure to sodium nitrite and secondary amines in feed or drinking water resulted in an increase in the incidence of tumors in rodents.
Mutagenesis
- Sodium nitrite is mutagenic in S. typhimurium strains TA100, TA1530, TA1535 with and without metabolic activation; however, it was negative in strain TA98, TA102, DJ460 and E. coli strain WP2UVRA/PKM101. Sodium nitrite has been reported to be genotoxic to V79 hamster cells in vitro and in the mouse lymphoma assay, both assays conducted in the absence of metabolic activation. Sodium nitrite was negative in the in vitro chromosomal aberrations assay using human peripheral blood lymphocytes. Acute administration of sodium nitrite to male rats or male mice did not produce an increased incidence of micronuclei in bone marrow. Likewise, sodium nitrite administration to mice for 14-weeks did not result in an increase in the incidence of micronuclei in the peripheral blood.
Fertility
- Clinical studies to evaluate the potential effects of sodium nitrite intake on fertility of either males or females have not been reported. In contrast, multigenerational fertility and reproduction studies conducted by the National Toxicology Program did not detect any evidence of an effect of sodium nitrite (0.0, 0.06, 0.12, and 0.24% weight/volume) on either fertility or any reproductive parameter in Swiss CD-1 mice. This treatment protocol resulted in approximate doses of 125, 260, and 425 mg/kg/day. The highest exposure in this mouse study is 4.6 times greater than the highest clinical dose of sodium nitrite that would be used to treat cyanide poisoning (based on a body surface area comparison).
Animal Pharmacology
- Due to the extreme toxicity of cyanide, experimental evaluation of treatment efficacy has predominantly been completed in animal models. The efficacy of sodium thiosulfate treatment alone to counteract the toxicity of cyanide was initially reported in 1895 by Lang. The efficacy of amyl nitrite treatment in cyanide poisoning of the dog model was first reported in 1888 by Pedigo. Further studies in the dog model, which demonstrated the utility of sodium nitrite as a therapeutic intervention, were reported in 1929 by Mladoveanu and Gheorghiu. However, Hugs and Chen et al. independently reported upon the superior efficacy of the combination of sodium nitrite and sodium thiosulfate in 1932-1933. Treatment consisted of intravenously administered 22.5 mg/kg (half the lethal dose) sodium nitrite or 1 g/kg sodium thiosulfate alone or in sequence immediately after subcutaneous injection of sodium cyanide into dogs over a range of doses. Subsequent doses of 10 mg/kg sodium nitrite and/or 0.5 g/kg sodium thiosulfate were administered when clinical signs or symptoms of poisoning persisted or reappeared. Either therapy administered alone increased the dose of sodium cyanide required to cause death, and when administered together, sodium nitrite and sodium thiosulfate resulted in a synergistic effect in raising the lethal dose of sodium cyanide. The combined therapy appeared to have reduced efficacy when therapy was delayed until signs of poisoning (e.g. convulsions) appeared; however, other investigators have reported survival in dogs that were administered antidotal treatment after respiratory arrest had occurred.
- Animal studies conducted in other species (e.g., rat, guinea pig, sheep, pigeon and cat) have also supported a synergistic effect of intravenous sodium nitrite and sodium thiosulfate in the treatment of cyanide poisoning.
- While intravenous injection of sodium nitrite and sodium thiosulfate was effective in reversing the effects of lethal doses of cyanide in dogs, intramuscular injection of sodium nitrite, with or without sodium thiosulfate, was found not to be effective in the same setting.
Clinical Studies
- The human data supporting the use of sodium nitrite for cyanide poisoning consists primarily of published case reports. There are no randomized controlled clinical trials. Nearly all the human data describing the use of sodium thiosulfate report its use in conjunction with sodium nitrite. Dosing recommendations for humans have been based on theoretical calculations of antidote detoxifying potential, extrapolation from animal experiments, and a small number of human case reports.
- There have been no human studies to prospectively and systematically evaluate the safety of sodium nitrite in humans. Available human safety information is based largely on anecdotal case reports and case series of limited scope.
How Supplied
- Each Sodium Nitrite carton (NDC 60267-311-10) consists of the following:
- One 10 mL glass vial of sodium nitrite injection 30 mg/mL (containing 300 mg of sodium nitrite);
Storage
- Store at controlled room temperature between 20°C and 25°C (68°F to 77°F); excursions permitted from 15 to 30°C (59 to 86°F). Protect from direct light. Do not freeze.
(Note: Sodium Thiosulfate must be obtained separately.)
Images
Drug Images
{{#ask: Page Name::Sodium nitrite |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Sodium nitrite |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Sodium Nitrite Injection is indicated for acute cyanide poisoning that is judged to be life-threatening and in this setting, patients will likely be unresponsive or may have difficulty in comprehending counseling information.
Hypotension and Methemoglobin Formation
- When feasible, patients should be informed of the possibility of life-threatening hypotension and methemoglobin formation.
Monitoring
- Where feasible, patients should be informed of the need for close monitoring of blood pressure and oxygenation.
Precautions with Alcohol
- Alcohol-sodium nitrite interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Sodium Nitrite
Look-Alike Drug Names
There is limited information regarding Sodium nitrite Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Sodium nitrite
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Sodium nitrite |Label Name=Sodium nitrite ingredients and appearance.png
}}
{{#subobject:
|Label Page=Sodium nitrite |Label Name=Sodium nitrite fig01.jpg
}}