Action potential
| https://https://www.youtube.com/watch?v=BbUcWbtVjT4%7C350}} |

|
WikiDoc Resources for Action potential |
|
Articles |
|---|
|
Most recent articles on Action potential Most cited articles on Action potential |
|
Media |
|
Powerpoint slides on Action potential |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Action potential at Clinical Trials.gov Trial results on Action potential Clinical Trials on Action potential at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Action potential NICE Guidance on Action potential
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Action potential Discussion groups on Action potential Patient Handouts on Action potential Directions to Hospitals Treating Action potential Risk calculators and risk factors for Action potential
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Action potential |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
An action potential is a "spike" of electrical discharge that travels along the membrane of a cell. Action potentials are an essential feature of animal life, rapidly carrying information within and between tissues. They also occur in some plants. Action potentials can be created by many types of cells, but are used most extensively by the nervous system for communication between neurons and for transmitting information from neurons to other body tissues such as muscles and glands.
Action potentials are not the same in all cell types and can even vary in their properties at different locations in the same cell. For example, cardiac action potentials are significantly different from the action potentials in most neurons. This article is primarily concerned with the "typical" action potential of axons.
There is always a difference in electrostatic potential between the inside and outside of a cell, i.e. the cell is polarized. This membrane potential is the result of the distribution of ions across the cell membrane and the permeability of the membrane to these ions. The voltage of an inactive cell remains close to a resting potential with excess negative charge inside the cell. When the membrane of an excitable cell becomes depolarized beyond a threshold, the cell undergoes an action potential (it "fires"), often called a "spike" (see Threshold and initiation).
An action potential is a rapid change of the polarity of the voltage from negative to positive and then vice versa, the entire cycle lasting on the order of milliseconds. Each cycle — and therefore each action potential — has a rising phase, a falling phase, and finally an undershoot (see Phases). In specialized muscle cells of the heart, such as cardiac pacemaker cells, a plateau phase of intermediate voltage may precede the falling phase, extending the action potential duration into hundreds of milliseconds.
Action potentials are measured with the recording techniques of electrophysiology and more recently with neurochips containing EOSFETs. An oscilloscope recording the membrane potential from a single point on an axon shows each stage of the action potential as the wave passes. These phases trace an arc that resembles a distorted sine wave; its amplitude depends on whether the action potential wave has reached that point on the membrane or has passed it and if so, how long ago.
The action potential does not dwell in one location of the cell's membrane, but travels along the membrane (see Propagation). It can travel along an axon for long distances, for example to carry signals from the spinal cord to the muscles of the foot. In large animals, such as giraffes and whales, the distance traveled can be many meters. After traveling the whole length of the axon, the action potential reaches a synapse, where it stimulates the release of neurotransmitters. These neurotransmitters can immediately induce an action potential in the next neuron to propagate the signal, but the response is usually more complex.
Both the speed and complexity of action potentials vary between different types of cells, but their amplitudes tend to be roughly the same. Within any one cell, consecutive action potentials are typically indistinguishable. Neurons are thought to transmit information by generating sequences of action potentials called "spike trains". By varying both the rate as well as the precise timing of the action potentials they generate, neurons can change the information that they transmit.
Underlying mechanism
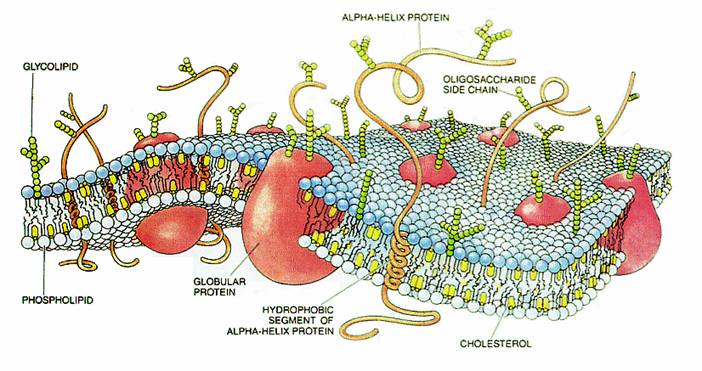
Resting potential
The resting potential is what would be maintained were there no action potentials, synaptic potentials, or other changes to the membrane potential. In neurons the resting potential is approximately -70 mV (the negative sign signifies excess negative charge inside the cell relative to the outside). The resting potential is mostly determined by the ion concentrations in the fluids on both sides of the cell membrane and the ion transport proteins in the cell membrane. The term resting is somewhat misleading, for the cell must constantly do work to maintain the resting potential.It takes cell more energy to maintain the resting potential as compared to transmission of nerve impulses. The establishment of this potential difference involves several factors, the most important of which are the transport of ions across the cell membrane and the selective permeability of the membrane to these ions.
The active transport of potassium and sodium ions into and out of the cell, respectively, is accomplished by a number of sodium-potassium pumps scattered across the cell membrane. Each pump transports two ions of potassium into the cell for every three ions of sodium pumped out. This establishes a particular distribution of positively charged ions across the cell membrane, with more sodium present outside the cell than inside, and more potassium inside the cell than outside. In some situations, the electrogenic sodium-potassium pumps make a significant contribution to the resting membrane potential, but in most cells there are potassium leak channels that dominate the value of the resting potential.
Sodium and potassium ions diffuse through open ion channels under the influence of their electrochemical gradients. At the resting potential, the net movement of sodium into the cell equals the net movement of potassium out of the cell. However, the resting cell membrane is approximately 75 times more permeable to potassium than to sodium because potassium leak channels are always open. As a result, the cell's resting membrane potential is closer to the equilibrium potential of potassium (=EK=−80 mV) than the equilibrium potential of sodium (=ENa=+60 mV).
Like the resting potential, action potentials depend upon the permeability of the cell membrane to sodium and potassium ions. Transient changes in conductance for different ions cause the changes in membrane potential necessary to initiate, sustain, and terminate action potentials.
Phases
The sequence of events that underlie the action potential are outlined below:
Resting potential
At resting potential some potassium leak channels are open but the voltage-gated sodium channels are closed. Even though no net current flows, potassium, the major ion species, moves across the membrane, thus pulling the resting potential close to the K+ equilibrium potential.
Stimulation
A local membrane depolarization caused by an excitatory stimulus causes some voltage-gated sodium channels in the neuron cell surface membrane to open, allowing sodium ions to diffuse in through the channels along their electrochemical gradient. Because they are positively charged, they begin a reversal in the potential difference across the membrane from a positive-outside to a negative-inside. Initially, the inward movement of sodium ions is also favored by the negative-inside membrane potential. Overall the ions are under the influence of the driving force, the difference between the membrane potential and the equilibrium potential of sodium.
Depolarization ("Rising phase")
As sodium ions enter and the membrane potential becomes less negative, more sodium channels open, causing an even greater influx of sodium ions. This is an example of positive feedback. As more sodium channels open, the sodium current dominates over the potassium leak current and the membrane potential becomes positive inside. Recent experiments on cortical neurons suggest that sodium channels open cooperatively,[1] allowing for a much faster uptake than is possible for Hodgkin-Huxley–type dynamics.
Peak
By the time the membrane potential has reached a peak value of around +40 mV, time-dependent inactivation gates on the sodium channels have already started to close, reducing and finally preventing further influx of sodium ions. While this occurs, the voltage-sensitive activation gates on the voltage-gated potassium channels begin to open.
Repolarization ("Falling phase")
As voltage-gated potassium channels open, there is a large outward movement of potassium ions driven by the potassium concentration gradient and initially favored by the positive-inside electrical gradient. As potassium ions diffuse out, this movement of positive charge causes a reversal of the membrane potential to negative-inside and repolarization of the neuron back towards the large negative-inside resting potential.
Hyperpolarization ("Undershoot")
Closing of voltage-gated potassium channels is both voltage- and time-dependent. As potassium exits the cell, the resulting membrane repolarization initiates the closing of voltage-gated potassium channels. These channels do not close immediately in response to a change in membrane potential; rather, voltage-gated potassium channels (also called delayed rectifier potassium channels) have a delayed response, such that potassium continues to flow out of the cell even after the membrane has fully repolarized. Thus the membrane potential dips below the normal resting membrane potential of the cell for a brief moment; this dip of hyperpolarization is known as the undershoot.
Refractory Period
During the next ~ 1 msec, the Na+ and K+ Channels cannot be opened by a stimulus. The Na+/K+ Pump actively pumps Na+ out of the neuron and K+ into the neuron. This reestablishes the initial ion distribution of the resting neuron (the voltage returns to the resting potential due to leak currents, however, not due to the pump's action). The refractory period is important because it ensures unidirectional (one way) propagation of the action potential.
Threshold and initiation
Action potentials are triggered when an initial depolarization reaches the threshold. This threshold potential varies, but generally is about 15 millivolts more positive than the cell's resting membrane potential, occurring when the inward sodium current exceeds the outward potassium current. The net influx of positive charges carried by sodium ions depolarizes the membrane potential, leading to the further opening of voltage-gated sodium channels. These channels support greater inward current causing further depolarization, creating a positive-feedback cycle that drives the membrane potential to a very depolarized level.
The action potential threshold can be shifted by changing the balance between sodium and potassium currents. For example, if some of the sodium channels are in an inactivated state, then a given level of depolarization will open fewer sodium channels and a greater depolarization will be needed to trigger an action potential. This is the basis for the refractory period (see Refractory period).
Action potentials are largely dictated by the interplay between sodium and potassium ions (although there are minor contributions from other ions such as calcium and chloride), and are often modeled using hypothetical cells containing only two transmembrane ion channels (a voltage-gated sodium channel and a non-voltage-gated potassium channel). The origin of the action potential threshold may be studied using I/V curves (right) that plot currents through ion channels against the cell's membrane potential. (Note that the illustrated I/V is an "instantaneous" current voltage relationship. It represents the peak current through channels at a given voltage before any inactivation has taken place (i.e. ~ 1 ms after stepping to that voltage) for the Na current. The most positive voltages in this plot are only attainable by the cell through artificial means - i.e. voltages imposed by the voltage-clamp apparatus).

Four significant points in the I/V curve are indicated by arrows in the figure:
- The green arrow indicates the resting potential of the cell and also the value of the equilibrium potential for potassium (Ek). As the K+ channel is the only one open at these negative voltages, the cell will rest at Ek.
- The yellow arrow indicates the equilibrium potential for Na+ (ENa). In this two-ion system, ENa is the natural limit of membrane potential beyond which a cell cannot pass. Current values illustrated in this graph that exceed ENa are measured by artificially pushing the cell's voltage past its natural limit. Note however, that ENa could only be reached if the potassium current were absent.
- The blue arrow indicates the maximum voltage that the peak of the action potential can approach. This is the actual natural maximum membrane potential that this cell can reach. It cannot reach ENa because of the counteracting influence of the potassium current.
- The red arrow indicates the action potential threshold. This is where Isum becomes net-inward. Note that this is a zero-current crossing, but with a negative slope. Any such "negative slope crossing" of the zero current level in an I/V plot is an unstable point. At any voltage negative to this crossing, the current is outward and so a cell will tend to return to its resting potential. At any voltage positive of this crossing, the current is inward and will tend to depolarize the cell. This depolarization leads to more inward current, thus the sodium current become regenerative. The point at which the green line reaches its most negative value is the point where all sodium channels are open. Depolarizations beyond that point thus decrease the sodium current as the driving force decreases as the membrane potential approaches ENa.
The action potential threshold is often confused with the "threshold" of sodium channel opening. This is incorrect, because sodium channels have no threshold. Instead, they open in response to depolarization in a stochastic manner. Depolarization does not so much open the channel as increases the probability of it being open. Even at hyperpolarized potentials, a sodium channel will open very occasionally. In addition, the threshold of an action potential is not the voltage at which sodium current becomes significant; it is the point where it exceeds the potassium current.
Biologically in neurons, depolarization typically originates in the dendrites at synapses. In principle, however, an action potential may be initiated anywhere along a nerve fiber. In his discovery of "animal electricity," Luigi Galvani made a leg of a dead frog kick as in life by touching a sciatic nerve with his scalpel, to which he had inadvertently transferred a negative, static-electric charge, thus initiating an action potential.
Circuit model
Cell membranes that contain ion channels can be modeled as RC circuits to better understand the propagation of action potentials in biological membranes. In such a circuit, the resistor represents the membrane's ion channels, while the capacitor models the insulating lipid membrane. Variable resistors are used for voltage-gated ion channels, as their resistance changes with voltage. A fixed resistor represents the potassium leak channels that maintain the membrane's resting potential. The sodium and potassium gradients across the membrane are modeled as voltage sources (batteries).
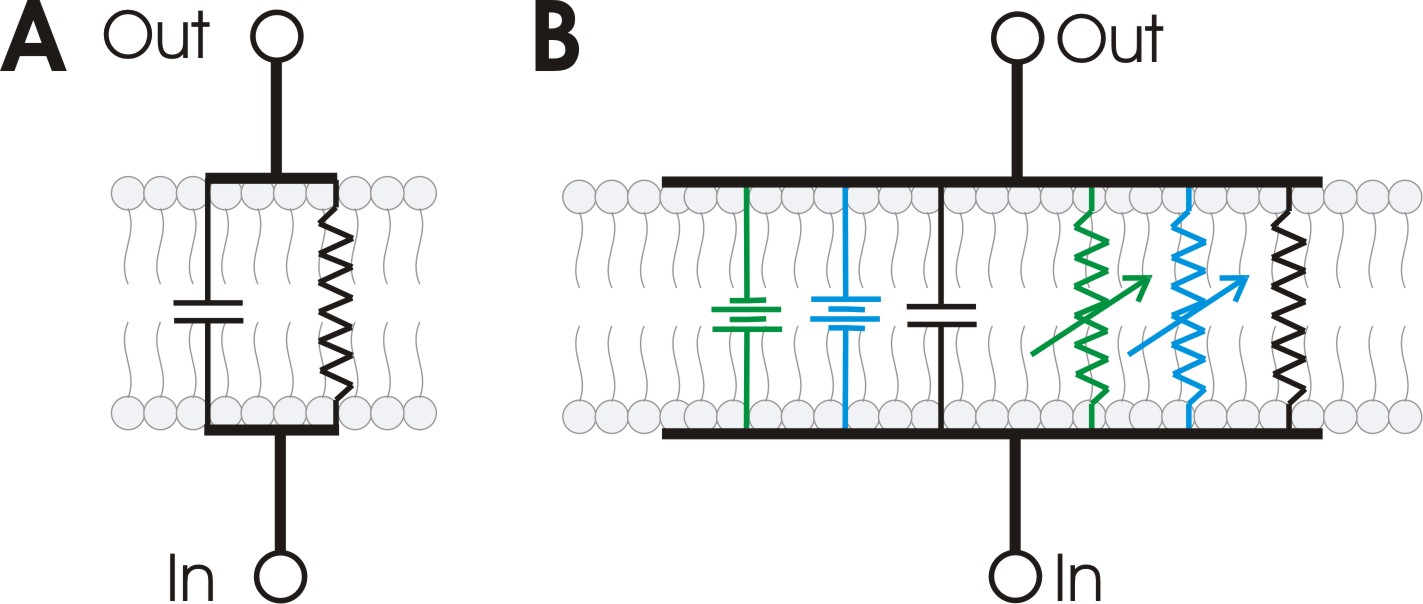
Propagation
In unmyelinated axons, action potentials propagate as an interaction between passively spreading membrane depolarization and voltage-gated sodium channels. When one patch of cell membrane is depolarized enough to open its voltage-gated sodium channels, sodium ions enter the cell by facilitated diffusion. Once inside, positively-charged sodium ions "nudge" adjacent ions down the axon by electrostatic repulsion (analogous to the principle behind Newton's cradle) and attract negative ions away from the adjacent membrane. As a result, a wave of positivity moves down the axon without any individual ion moving very far. Once the adjacent patch of membrane is depolarized, the voltage-gated sodium channels in that patch open, regenerating the cycle. The process repeats itself down the length of the axon, with an action potential regenerated at each segment of membrane.
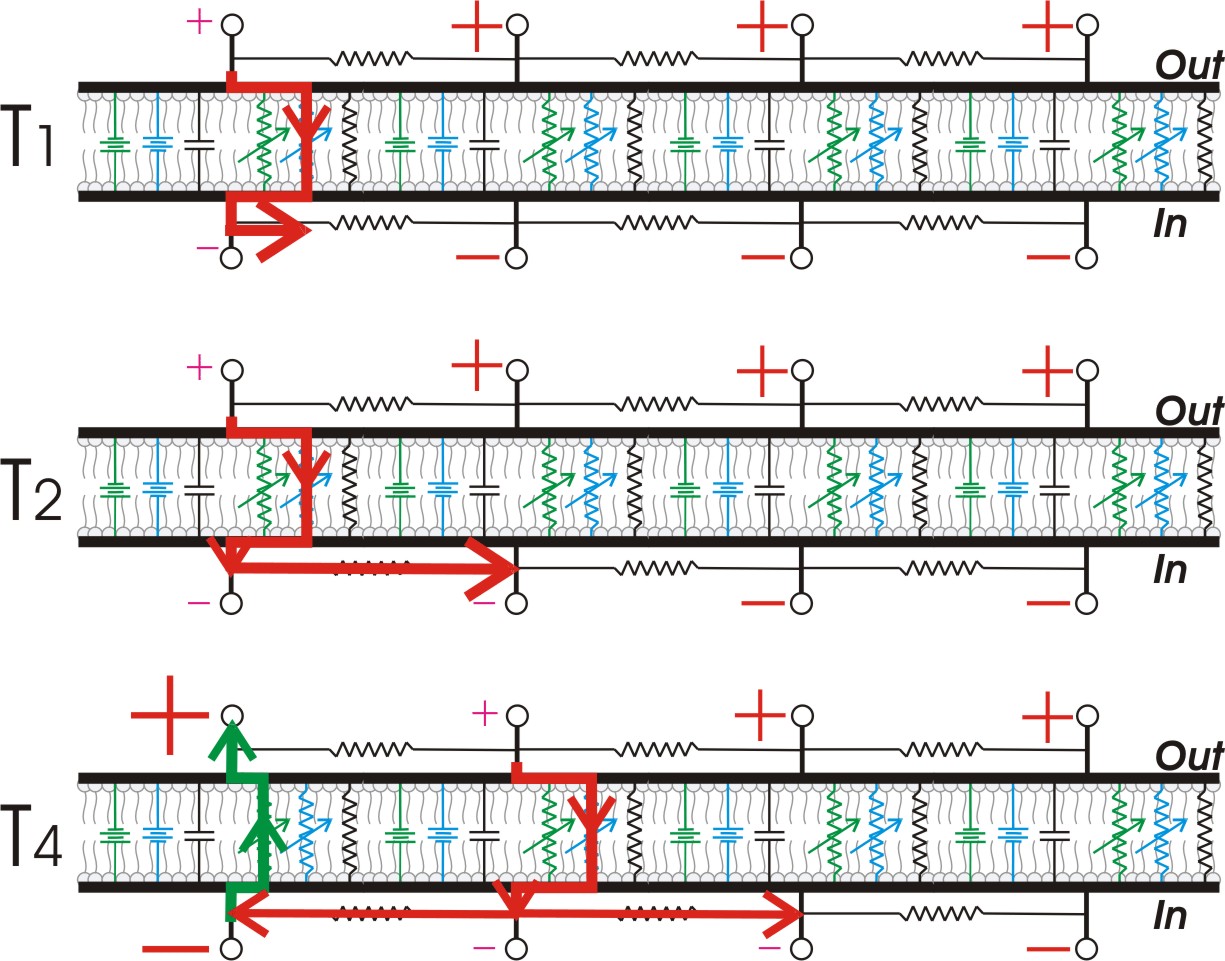
Speed of propagation
- See also: Time constant and Length constant
Action potentials propagate faster in axons of larger diameter, other things being equal. They typically travel from 10 – 100 m/s. The main reason is that the axial resistance of the axon lumen is lower with larger diameters, because of an increase in the ratio of cross-sectional area to membrane surface area. As the membrane surface area is the chief factor impeding action potential propagation in an unmyelinated axon, increasing this ratio is a particularly effective way of increasing conduction speed.
An extreme example of an animal using axon diameter to speed action potential conduction is found in the Atlantic squid. The squid giant axon controls the muscle contraction associated with the squid's predator escape response. This axon can be more than 1 mm in diameter, and is presumably an adaptation to allow very fast activation of the escape behavior. The velocity of nerve impulses in these fibers is among the fastest in nature. Squids are notable examples of organisms with unmyelinated axons; the first tests to try to determine the mechanism by which impulses travel along axons, involving the detection of a potential difference between the inside and the surface of a neuron, were undertaken in the 1940s by Alan Hodgkin and Andrew Huxley using squid giant axons because of their relatively large axon diameter. Hodgkin and Huxley won their shares of the 1963 Nobel Prize in Physiology or Medicine for their work on the electrophysiology of nerve action potentials.
In the autonomic nervous system in mammals, postganglionic neurons are unmyelinated. The small diameter of these axons (about 2 µ) results in a propagatory speed of approximately 1 m/s, as opposed to approximately 18 m/s in myelinated nerve fibers of comparable diameter, thus highlighting the effect of myelination on the speed of transmission of impulses.
Saltatory conduction
In myelinated axons, saltatory conduction is the process by which an action potential appears to jump along the length of an axon, being regenerated only at uninsulated segments (the nodes of Ranvier). Saltatory conduction increases nerve conduction velocity without having to dramatically increase axon diameter.
Saltatory conduction has played an important role in the evolution of larger and more complex organisms whose nervous systems must rapidly transmit action potentials across greater distances. Without saltatory conduction, conduction velocity would need large increases in axon diameter, resulting in organisms with nervous systems too large for their bodies.
Detailed mechanism
The main impediment to conduction speed in unmyelinated axons is membrane capacitance. In an electric circuit, the capacitance of a capacitor can be decreased by decreasing the cross-sectional area of its plates, or by increasing the distance between plates. The nervous system uses myelin as its main strategy to decrease membrane capacitance. Myelin is an insulating sheath wrapped around axons by Schwann cells and oligodendrocytes, neuroglia that flatten their cytoplasm to form large sheets made up mostly of plasma membrane. These sheets wrap around the axon, moving the conducting plates (the intra- and extracellular fluid) farther apart to decrease membrane capacitance.
The resulting insulation allows the rapid (essentially instantaneous) conduction of ions through a myelinated segment of axon, but prevents the regeneration of action potentials through those segments. Action potentials are only regenerated at the unmyelinated nodes of Ranvier which are spaced intermittently between myelinated segments. An abundance of voltage-gated sodium channels on these bare segments (up to four orders of magnitude greater than their density in unmyelinated axons[2]) allows action potentials to be efficiently regenerated at the nodes of Ranvier.
As a result of myelination, the insulated portion of the axon behaves like a passive wire: it conducts action potentials rapidly because its membrane capacitance is low, and minimizes the degradation of action potentials because its membrane resistance is high. When this passively propagated signal reaches a node of Ranvier, it initiates an action potential, which subsequently travels passively to the next node where the cycle repeats.
Resilience to injury
The length of myelinated segments of axon is important to saltatory conduction. They should be as long as possible to maximize the length of fast passive conduction, but not so long that the decay of the passive signal is too great to reach threshold at the next node of Ranvier. In reality, myelinated segments are long enough for the passively propagated signal to travel for at least two nodes while retaining enough amplitude to fire an action potential at the second or third node. Thus, the safety factor of saltatory conduction is high, allowing transmission to bypass nodes in case of injury.
Role in disease
Some diseases degrade saltatory conduction and reduce the speed of action potential conductance. The most well-known of these diseases is multiple sclerosis, in which the breakdown of myelin impairs coordinated movement.
Refractory period
Where membrane has undergone an action potential, a refractory period follows. Thus, although the passive transmission of action potentials across myelinated segments would suggest that action potentials propagate in either direction, most action potentials travel unidirectionally because the node behind the propagating action potential is refractory.
This period arises primarily because of the time-dependent inactivation of sodium channels, as described by Hodgkin and Huxley in 1952. Immediately after an action potential, during the absolute refractory period, virtually all sodium channels are inactivated and thus it is impossible to fire another action potential in that segment of membrane.
With time, sodium channels are reactivated in a stochastic manner. As they become available, it becomes possible to fire an action potential, albeit one with a much higher threshold. This is the relative refractory period and together with the absolute refractory period, lasts approximately five milliseconds.
Termination and consequences
An action potential proceeding along a membrane is prevented from reversing its direction by the refractory period, and will eventually depolarize the entire cell. When the action potential reaches an area where all the cell membrane is already depolarized or still in the refractory period, the action potential can no longer propagate. Because an action potential propagates only along contiguous membrane, another mechanism is necessary to transmit action potentials between cells. Neurons communicate with each other at a chemical synapse. Other cell types, such as cardiac muscle cells, can communicate action potentials via electrical synapses.
The synapse is a very small gap between neurons that allows one-way communication. As the presynaptic neuron undergoes an action potential, voltage-sensitive calcium channels open and cause the release of neurotransmitters into the synapse. These chemical transmitters can initiate an action potential in the postsynaptic neuron, allowing communication between neurons. Some neurotransmitters inhibit action potentials, and the interaction of excitatory and inhibitory signals allows complex modulation of signals in the nervous system.
Evolutionary advantage
The action potential, as a method of long-distance communication, fits a particular biological need seen most readily when considering the transmission of information along a nerve axon. To move a signal from one end of an axon to the other, nature must contend with physics similar to those that govern the movement of electrical signals along a wire. Due to the resistance and capacitance of a wire, signals tend to degrade as they travel along that wire over a distance. These properties, known collectively as cable properties set the physical limits over which signals can travel. Thus, nonspiking neurons (which carry signals without action potentials) tend to be small. Proper function of the body requires that signals be delivered from one end of an axon to the other without loss. An action potential does not so much propagate along an axon, as it is newly regenerated by the membrane voltage and current at each stretch of membrane along its path. In other words, the nerve membrane recreates the action potential at its full amplitude as it travels down the axon, thus overcoming the limitations imposed by cable physics.
Plant action potentials
Many plants also exhibit action potentials that travel via their phloem to coordinate activity. The main difference between plant and animal action potentials is that plants primarily use potassium and calcium currents while animals typically use currents of potassium and sodium.
Alternative models
The model of electrical signal propagation in neurons employing voltage-gated ion channels described above is accepted by almost all scientists working in the field. However there are a few observations not easily reconciled with the model:
- A signal traveling along a neuron is accompanied by a slight local thickening of the membrane and a force acting outwards.[3]
- An action potential traveling along a neuron results in a slight increase in temperature followed by a decrease in temperature;[4] electrical charges traveling through a resistor however always produce heat.
One recent alternative, the soliton model, attempts to explain signals in neurons as pressure (or sound) solitons traveling along the membrane, accompanied by electrical field changes resulting from piezo-electric effects.
See also
- Cardiac action potential
- Ventricular action potential
- Membrane potential
- Depolarization
- Hyperpolarization
- Signals (biology)
- Time constant
- Length constant
- Bursting
References
General sources
- Bear, M.F., B.W. Connors, and M.A. Paradiso. 2001. Neuroscience: Exploring the Brain. Baltimore: Lippincott. ISBN 0781739446
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science, 4th ed. McGraw-Hill, New York (2000). ISBN 0-8385-7701-6
- Dale Purves, et al. Neuroscience, 2nd ed. 2001. Sinauer Associates, Inc. Ion Channels Underlying Action Potentials. ISBN 0878937250 Release of Transmitters from Synaptic Vesicles
- Kent, M., Advanced Biology. 2000. United Kingdom: Oxford University Press.
- Taylor, D.J., Green, N.P.O., & Stout, G.W. 2003. Biological Sciences, 3rd ed. United Kingdom : Cambridge University Press.
Primary sources
- Hodgkin AL, Huxley AF. Currents carried by sodium and potassium ions through the membrane of the giant axon of Loligo. J Physiol. 1952 Apr;116(4):449-72. PMID 14946713
- Hodgkin AL, Huxley AF. The components of membrane conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):473-96. PMID 14946714
- Hodgkin AL, Huxley AF. The dual effect of membrane potential on sodium conductance in the giant axon of Loligo. J Physiol. 1952 Apr;116(4):497 – 506. PMID 14946715
- Hodgkin AL, Huxley AF. A quantitative description of membrane current and its application to conduction and excitation in nerve. J Physiol. 1952 Aug;117(4):500-44. PMID 12991237
- Clay JR. Axonal excitability revisited. Prog Biophys Mol Biol. 2005 May;88(1):59 – 90. PMID 15561301
Specific citations
- ↑ "Unique features of action potential initiation in cortical neurons". 2006-04-20.
- ↑ "soma.npa.uiuc.edu/courses/bio303/Ch5b.html".
- ↑ Iwasa K, Tasaki I, Gibbons RC (1980). Swelling of nerve fibers associated with action potentials. Science Vol. 210. no. 4467, pp. 338 – 339
- ↑ Ritchie JM, Keynes RD (1985). The production and absorption of heat associated with electrical activity in nerve and electric organ. Q Rev Biophys. 1985 Nov;18(4):451-76.
External links
- Template:McGrawHillAnimation
- Electrochemistry of plant life, May, 2004 from Case Western Reserve University
- Demonstration of ion flow during action potential at Blackwell Publishing
- Action potential propagation in myelinated vs. unmyelinated axons at Blackwell Publishing
- Open-source software to simulate neuronal and cardiac action potentials at SourceForge
- Nernst/Goldman Equation Simulator at University of Arizona
- Electrophysiology and The Molecular Basis of Excitability at University of Chicago
- Action Potential Len Kravitz, University of New Mexico
- PSY 340 Brain and Behavior, 2.2a Neural Impulse (part 2) Vincent W. Hevern, Le Moyne College, Syracuse New York
- Action Potentials Dawn A. Tamarkin, Springfield Technical Community College, Springfield, Massechusets
- Action Potential Rachel McCready, Intensive Care Unit, London Health Services Centre
- Propagation of an Action Potential National Health Museum Resource Center, Washington DC
- Neurotransmission Steve Croker, University of Derby. Derby, England
- Neurotransmitter and neuron diagrams Edward I. Pollack, West Chester University of Pennsylvania
- Action Potential Conduction Encyclopædia Britannica
- The Action Potential John Kinnamon, University of Denver
- Excitable Cells John Kimball, Tufts University (retired)
- Resting and Action Membrane Potentials Teaching Resources Center, UC Davis. Animated tutorials
- Action Potential Frank Werblin, UC Berkely. Animated and interactive tutorials
- Action Potential Movement Through an Axon at learner.org
Template:Link FA ar:كمون الفعل de:Aktionspotential ko:활동전위 it:Potenziale d'azione he:דחף עצבי ms:Potensi aksi nl:Actiepotentiaal simple:Nerve impulse sl:Akcijski potencial fi:Toimintapotentiaali sv:Aktionspotential