Panitumumab: Difference between revisions
No edit summary |
No edit summary |
||
| Line 99: | Line 99: | ||
|drugInteractions=No formal drug-drug interaction studies have been conducted between Vectibix and [[oxaliplatin]] or [[fluoropyrimidine]]. | |drugInteractions=No formal drug-drug interaction studies have been conducted between Vectibix and [[oxaliplatin]] or [[fluoropyrimidine]]. | ||
|FDAPregCat=C | |FDAPregCat=C | ||
|useInPregnancyFDA=There are no studies of Vectibix in pregnant women. Reproduction studies in cynomolgus monkeys treated with 1.25 to 5 times the recommended human dose of panitumumab resulted in significant embryolethality and abortions; however, no other evidence of teratogenesis was noted in offspring | |useInPregnancyFDA=There are no studies of Vectibix in pregnant women. Reproduction studies in cynomolgus monkeys treated with 1.25 to 5 times the recommended human dose of panitumumab resulted in significant embryolethality and abortions; however, no other evidence of [[teratogenesis]] was noted in offspring. Vectibix should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | ||
Based on animal models, EGFR is involved in prenatal development and may be essential for normal organogenesis, proliferation, and differentiation in the developing embryo. Human IgG is known to cross the placental barrier; therefore, panitumumab may be transmitted from the mother to the developing fetus, and has the potential to cause fetal harm when administered to pregnant women. | Based on animal models, [[EGFR]] is involved in prenatal development and may be essential for normal organogenesis, proliferation, and differentiation in the developing embryo. Human [[IgG]] is known to cross the [[placental barrier]]; therefore, panitumumab may be transmitted from the mother to the developing fetus, and has the potential to cause fetal harm when administered to pregnant women. | ||
Women who become pregnant during Vectibix treatment are encouraged to enroll in Amgen’s Pregnancy Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll. | Women who become pregnant during Vectibix treatment are encouraged to enroll in Amgen’s Pregnancy Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll. | ||
|useInNursing=It is not known whether panitumumab is excreted into human milk; however, human IgG is excreted into human milk. Published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from Vectibix, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. If nursing is interrupted, based on the mean half-life of panitumumab, nursing should not be resumed earlier than 2 months following the last dose of Vectibix | |useInNursing=It is not known whether panitumumab is excreted into human milk; however, human [[IgG]] is excreted into human milk. Published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from Vectibix, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. If nursing is interrupted, based on the mean half-life of panitumumab, nursing should not be resumed earlier than 2 months following the last dose of Vectibix. | ||
Women who are nursing during Vectibix treatment are encouraged to enroll in Amgen’s Lactation Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll. | Women who are nursing during Vectibix treatment are encouraged to enroll in Amgen’s Lactation Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll. | ||
|useInPed=The safety and effectiveness of Vectibix have not been established in pediatric patients. The pharmacokinetic profile of Vectibix has not been studied in pediatric patients. | |useInPed=The safety and effectiveness of Vectibix have not been established in pediatric patients. The pharmacokinetic profile of Vectibix has not been studied in pediatric patients. | ||
|useInGeri= Of the 737 patients who received Vectibix monotherapy in Study 1 and 2, 36% were 65 and over while 8% were 75 and over. No overall differences in safety or efficacy were observed in elderly patients (≥ 65 years of age) treated with Vectibix monotherapy. | |useInGeri=Of the 737 patients who received Vectibix monotherapy in Study 1 and 2, 36% were 65 and over while 8% were 75 and over. No overall differences in safety or efficacy were observed in elderly patients (≥ 65 years of age) treated with Vectibix monotherapy. | ||
Of the 322 patients in Study 3 who received Vectibix plus FOLFOX, 128 (40%) were 65 and over while 8% were 75 and over. Patients older than 65 years of age experienced an increased incidence of serious adverse events (52% vs 36%) and an increased incidence of serious diarrhea (15% vs 5%) as compared to younger patients. | Of the 322 patients in Study 3 who received Vectibix plus FOLFOX, 128 (40%) were 65 and over while 8% were 75 and over. Patients older than 65 years of age experienced an increased incidence of serious adverse events (52% vs 36%) and an increased incidence of serious [[diarrhea]] (15% vs 5%) as compared to younger patients. | ||
|overdose=Doses up to approximately twice the recommended therapeutic dose (12 mg/kg) resulted in adverse reactions of [[skin toxicity]], [[diarrhea]], [[dehydration]], and [[fatigue]]. | |overdose=Doses up to approximately twice the recommended therapeutic dose (12 mg/kg) resulted in adverse reactions of [[skin toxicity]], [[diarrhea]], [[dehydration]], and [[fatigue]]. | ||
|drugBox={{Drugbox2 | |drugBox={{Drugbox2 | ||
| Line 163: | Line 163: | ||
Following single-dose administrations of panitumumab as 1-hour infusions, the area under the concentration-time curve (AUC) increased in a greater than dose-proportional manner, and clearance (CL) of panitumumab decreased from 30.6 to 4.6 mL/day/kg as the dose increased from 0.75 to 9 mg/kg. However, at doses above 2 mg/kg, the AUC of panitumumab increased in an approximately dose-proportional manner. | Following single-dose administrations of panitumumab as 1-hour infusions, the area under the concentration-time curve (AUC) increased in a greater than dose-proportional manner, and clearance (CL) of panitumumab decreased from 30.6 to 4.6 mL/day/kg as the dose increased from 0.75 to 9 mg/kg. However, at doses above 2 mg/kg, the AUC of panitumumab increased in an approximately dose-proportional manner. | ||
Following the recommended dose regimen (6 mg/kg given once every 2 weeks as a 1-hour infusion), panitumumab concentrations reached steady-state levels by the third infusion with mean (± SD) peak and trough concentrations of 213 ± 59 and 39 ± 14 mcg/mL, respectively. The mean (± SD) AUC0-tau and CL were 1306 ± 374 mcg•day/mL and 4.9 ± 1.4 mL/kg/day, respectively. The elimination half-life was approximately 7.5 days (range: 3.6 to 10.9 days). | Following the recommended dose regimen (6 mg/kg given once every 2 weeks as a 1-hour infusion), panitumumab concentrations reached steady-state levels by the third infusion with mean (± SD) peak and trough concentrations of 213 ± 59 and 39 ± 14 mcg/mL, respectively. The mean (± SD) AUC0-tau and CL were 1306 ± 374 mcg•day/mL and 4.9 ± 1.4 mL/kg/day, respectively. The elimination [[half-life]] was approximately 7.5 days (range: 3.6 to 10.9 days). | ||
A population pharmacokinetic analysis was performed to explore the potential effects of selected covariates on panitumumab pharmacokinetics. Results suggest that age (21-88 years), gender, race (15% nonwhite), mild-to-moderate renal dysfunction, mild-to-moderate hepatic dysfunction, and EGFR membrane-staining intensity (1+, 2+, and 3+) in tumor cells had no apparent impact on the pharmacokinetics of panitumumab. | A population pharmacokinetic analysis was performed to explore the potential effects of selected covariates on panitumumab pharmacokinetics. Results suggest that age (21-88 years), gender, race (15% nonwhite), mild-to-moderate renal dysfunction, mild-to-moderate [[hepatic dysfunction]], and [[EGFR]] membrane-staining intensity (1+, 2+, and 3+) in tumor cells had no apparent impact on the pharmacokinetics of panitumumab. | ||
No formal pharmacokinetic studies of panitumumab have been conducted in patients with renal or hepatic impairment. | No formal pharmacokinetic studies of panitumumab have been conducted in patients with [[renal impairment]] or [[hepatic impairment]]. | ||
|nonClinToxic=====Carcinogenesis, Mutagenesis, Impairment of Fertility==== | |||
No carcinogenicity or mutagenicity studies of panitumumab have been conducted. It is not known if panitumumab can impair fertility in humans. Prolonged menstrual cycles and/or amenorrhea occurred in normally cycling, female cynomolgus monkeys treated weekly with 1.25 to 5 times the recommended human dose of panitumumab (based on body weight). Menstrual cycle irregularities in panitumumab-treated female monkeys were accompanied by both a decrease and delay in peak progesterone and 17β-estradiol levels. Normal menstrual cycling resumed in most animals after discontinuation of panitumumab treatment. A no-effect level for menstrual cycle irregularities and serum hormone levels was not identified. The effects of panitumumab on male fertility have not been studied. However, no adverse effects were observed microscopically in reproductive organs from male cynomolgus monkeys treated for 26 weeks with panitumumab at doses of up to approximately 5-fold the recommended human dose (based on body weight). | |||
13.2 Animal Toxicology and/or Pharmacology | |||
Weekly administration of panitumumab to cynomolgus monkeys for 4 to 26 weeks resulted in dermatologic findings, including dermatitis, pustule formation and exfoliative rash, and deaths secondary to bacterial infection and sepsis at doses of 1.25 to 5-fold higher (based on body weight) than the recommended human dose. | |||
13.3 Reproductive and Developmental Toxicology | |||
Pregnant cynomolgus monkeys were treated weekly with panitumumab during the period of organogenesis (gestation day [GD] 20-50). While no panitumumab was detected in serum of neonates from panitumumab-treated dams, anti-panitumumab antibody titers were present in 14 of 27 offspring delivered at GD 100. There were no fetal malformations or other evidence of teratogenesis noted in the offspring. However, significant increases in embryolethality and abortions occurred at doses of approximately 1.25 to 5 times the recommended human dose (based on body weight). | |||
|alcohol=Alcohol-Panitumumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | |alcohol=Alcohol-Panitumumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication. | ||
}} | }} | ||
Revision as of 17:20, 29 January 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [2]; Associate Editor(s)-in-Chief: Alberto Plate [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Warning: Dermatological Toxicity
See full prescribing information for complete Boxed Warning.
Condition Name: Dermatologic Toxicity: Dermatologic toxicities occurred in 90% of patients and were severe (NCI-CTC grade 3 and higher) in 15% of patients receiving Vectibix monotherapy.
|
Overview
Panitumumab is an Immunological Agent and Monoclonal Antibody that is FDA approved for the treatment of metastatic colorectal cancer with wild-type KRAS (exon 2 in codons 12 or 13) as first-line therapy in combination with FOLFOX, monotherapy following disease progression after prior treatment with fluoropyrimidine, oxaliplatin, and irinotecan-containing chemotherapy. There is a Black Box Warning for this drug as shown here. Common adverse reactions include rash with variable presentations, paronychia, fatigue, nausea, and diarrhea, stomatitis, mucosal inflammation, asthenia, paronychia, anorexia, hypomagnesemia, hypokalemia, acneiform dermatitis, pruritus, and dry skin.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
There is limited information regarding Panitumumab FDA-Labeled Indications and Dosage (Adult) in the drug label.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Panitumumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Panitumumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Panitumumab FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Panitumumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Panitumumab in pediatric patients.
Contraindications
None.
Warnings
|
Warning: Dermatological Toxicity
See full prescribing information for complete Boxed Warning.
Condition Name: Dermatologic Toxicity: Dermatologic toxicities occurred in 90% of patients and were severe (NCI-CTC grade 3 and higher) in 15% of patients receiving Vectibix monotherapy.
|
Dermatologic and Soft Tissue Toxicity
In Study 1, dermatologic toxicities occurred in 90% of patients and were severe (NCI-CTC grade 3 and higher) in 15% of patients with mCRC receiving Vectibix. The clinical manifestations included, but were not limited to, acneiform dermatitis, pruritus, erythema, rash, skin exfoliation, paronychia, dry skin, and skin fissures.
Monitor patients who develop dermatologic or soft tissue toxicities while receiving Vectibix for the development of inflammatory or infectious sequelae. Life-threatening and fatal infectious complications including necrotizing fasciitis, abscesses, and sepsis have been observed in patients treated with Vectibix. Life-threatening and fatal bullous mucocutaneous disease with blisters, erosions, and skin sloughing has also been observed in patients treated with Vectibix. It could not be determined whether these mucocutaneous adverse reactions were directly related to EGFR inhibition or to idiosyncratic immune-related effects (eg, Stevens-Johnson syndrome or toxic epidermal necrolysis). Withhold or discontinue Vectibix for dermatologic or soft tissue toxicity associated with severe or life-threatening inflammatory or infectious complications. Dose modifications for Vectibix concerning dermatologic toxicity are provided.
Increased Tumor Progression, Increased Mortality, or Lack of Benefit in Patients with RAS- and KRAS-Mutant mCRC
A predefined retrospective subset analysis of Study 3 further identified a shortening of progression-free survival (PFS) and overall survival (OS) in patients with RAS-mutant tumors who received Vectibix and FOLFOX versus FOLFOX alone. Determination of RAS-mutant tumor status should be performed by an experienced laboratory.
Determination of KRAS mutational status in colorectal tumors using an FDA-approved test indicated for this use is necessary for selection of patients for treatment with Vectibix. Vectibix is indicated only for the treatment of patients with KRAS wild-type mCRC. Vectibix is not indicated for the treatment of patients with colorectal cancer that harbor somatic mutations in codons 12 and 13 (exon 2) as determined by an FDA-approved test for this use. In Study 3, 221 patients with KRAS-mutant mCRC tumors receiving Vectibix in combination with FOLFOX experienced shorter OS compared to 219 patients receiving FOLFOX alone (HR = 1.16, 95% CI: 0.94-1.41). Perform the assessment for KRAS mutational status in colorectal cancer in laboratories with demonstrated proficiency in the specific technology being utilized. Improper assay performance can lead to unreliable test results. Refer to an FDA-approved test’s package insert for instructions on the identification of patients eligible for the treatment of Vectibix.
Electrolyte Depletion/Monitoring
Progressively decreasing serum magnesium levels leading to severe (grade 3-4) hypomagnesemia occurred in up to 7% (in Study 2) of patients across clinical trials. Monitor patients for hypomagnesemia and hypocalcemia prior to initiating Vectibix treatment, periodically during Vectibix treatment, and for up to 8 weeks after the completion of treatment. Other electrolyte disturbances, including hypokalemia, have also been observed. Replete magnesium and other electrolytes as appropriate.
Infusion Reactions
In Study 1, 4% of patients experienced infusion reactions and 1% of patients experienced severe infusion reactions (NCI-CTC grade 3-4).
Infusion reactions, manifesting as fever, chills, dyspnea, bronchospasm, and hypotension, can occur following Vectibix administration. Fatal infusion reactions occurred in postmarketing experience. Terminate the infusion for severe infusion reactions.
Acute Renal Failure in Combination with Chemotherapy
Severe diarrhea and dehydration, leading to acute renal failure and other complications, have been observed in patients treated with Vectibix in combination with chemotherapy.
Pulmonary Fibrosis/Interstitial Lung Disease (ILD)
Fatal and nonfatal cases of interstitial lung disease (ILD) (1%) and pulmonary fibrosis have been observed in patients treated with Vectibix. Pulmonary fibrosis occurred in less than 1% (2/1467) of patients enrolled in clinical studies of Vectibix. In the event of acute onset or worsening of pulmonary symptoms, interrupt Vectibix therapy. Discontinue Vectibix therapy if ILD is confirmed.
In patients with a history of interstitial pneumonitis or pulmonary fibrosis, or evidence of interstitial pneumonitis or pulmonary fibrosis, the benefits of therapy with Vectibix versus the risk of pulmonary complications must be carefully considered.
Photosensitivity
Exposure to sunlight can exacerbate dermatologic toxicity. Advise patients to wear sunscreen and hats and limit sun exposure while receiving Vectibix.
Ocular Toxicities
Keratitis and ulcerative keratitis, known risk factors for corneal perforation, have been reported with Vectibix use. Monitor for evidence of keratitis or ulcerative keratitis. Interrupt or discontinue Vectibix therapy for acute or worsening keratitis.
Increased Mortality and Toxicity with Vectibix in Combination with Bevacizumab and Chemotherapy
In an interim analysis of an open-label, multicenter, randomized clinical trial in the first-line setting in patients with mCRC, the addition of Vectibix to the combination of bevacizumab and chemotherapy resulted in decreased OS and increased incidence of NCI-CTC grade 3-5 (87% vs 72%) adverse reactions. NCI-CTC grade 3-4 adverse reactions occurring at a higher rate in Vectibix-treated patients included rash/acneiform dermatitis (26% vs 1%), diarrhea (23% vs 12%), dehydration (16% vs 5%), primarily occurring in patients with diarrhea, hypokalemia (10% vs 4%), stomatitis/mucositis (4% vs < 1%), and hypomagnesemia (4% vs 0).
NCI-CTC grade 3-5 pulmonary embolism occurred at a higher rate in Vectibix-treated patients (7% vs 3%) and included fatal events in three (< 1%) Vectibix-treated patients.
As a result of the toxicities experienced, patients randomized to Vectibix, bevacizumab, and chemotherapy received a lower mean relative dose intensity of each chemotherapeutic agent (oxaliplatin, irinotecan, bolus 5-FU, and/or infusional 5-FU) over the first 24 weeks on study compared with those randomized to bevacizumab and chemotherapy.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates in the clinical trials of a drug cannot be directly compared to rates in clinical trials of another drug and may not reflect the rates observed in practice. The adverse reaction information from clinical studies does, however, provide a basis for identifying the adverse events that appear to be related to drug use and for approximating rates.
Safety data are presented from two clinical trials in which patients received Vectibix: Study 1, an open-label, multinational, randomized, controlled, monotherapy clinical trial (N = 463) evaluating Vectibix with best supportive care (BSC) versus BSC alone in patients with EGFR-expressing mCRC and Study 3, a randomized, controlled trial (N = 1183) in patients with mCRC that evaluated Vectibix in combination with FOLFOX chemotherapy versus FOLFOX chemotherapy alone. Safety data for Study 3 are limited to 656 patients with wild-type KRAS mCRC.
Vectibix Monotherapy
In Study 1, the most common adverse reactions (≥ 20%) with Vectibix were skin rash with variable presentations, paronychia, fatigue, nausea, and diarrhea.
The most common (> 5%) serious adverse reactions in the Vectibix arm were general physical health deterioration and intestinal obstruction. The most frequently reported adverse reactions for Vectibix leading to withdrawal were general physical health deterioration (n = 2) and intestinal obstruction (n = 2).
For Study 1, the data described in Table 1 and in other sections below, except where noted, reflect exposure to Vectibix administered to patients with mCRC as a single agent at the recommended dose and schedule (6 mg/kg every 2 weeks).
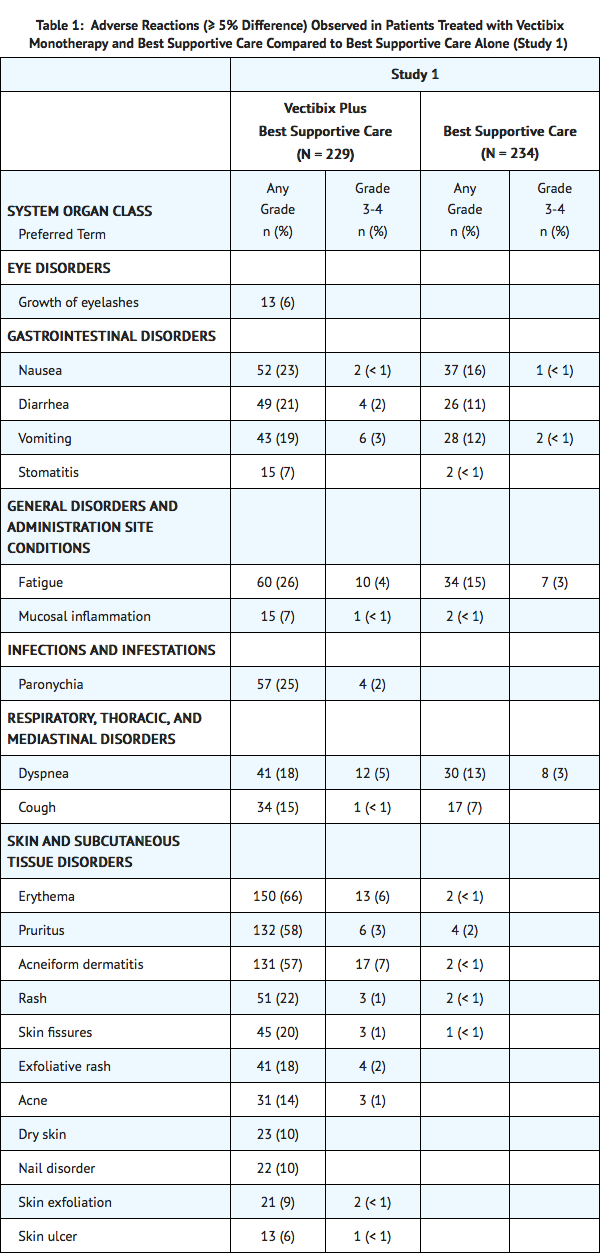
Adverse reactions in Study 1 that did not meet the threshold criteria for inclusion in Table 1 were conjunctivitis (4.8% vs < 1%), dry mouth (4.8% vs 0%), pyrexia (16.6% vs 13.2%), chills (3.1% vs < 1%), pustular rash (4.4% vs 0%), papular rash (1.7% vs 0%), dehydration (2.6% vs 1.7%), epistaxis (3.9% vs 0%), and pulmonary embolism (1.3% vs 0%).
In Study 1, dermatologic toxicities occurred in 90% of patients receiving Vectibix. Skin toxicity was severe (NCI-CTC grade 3 and higher) in 15% of patients. Ocular toxicities occurred in 16% of patients and included, but were not limited to, conjunctivitis (5%). One patient experienced an NCI-CTC grade 3 event of mucosal inflammation. The incidence of paronychia was 25% and was severe in 2% of patients.
In Study 1 (N = 229), median time to the development of dermatologic, nail, or ocular toxicity was 12 days after the first dose of Vectibix; the median time to most severe skin/ocular toxicity was 15 days after the first dose of Vectibix; and the median time to resolution after the last dose of Vectibix was 98 days. Severe toxicity necessitated dose interruption in 11% of Vectibix-treated patients.
Subsequent to the development of severe dermatologic toxicities, infectious complications, including sepsis, septic death, necrotizing fasciitis, and abscesses requiring incisions and drainage were reported.
Vectibix in Combination with FOLFOX Chemotherapy
The most commonly reported adverse reactions (≥ 20%) in patients with wild-type KRAS mCRC receiving Vectibix (6 mg/kg every 2 weeks) and FOLFOX therapy (N = 322) in Study 3 were diarrhea, stomatitis, mucosal inflammation, asthenia, paronychia, anorexia, hypomagnesemia, hypokalemia, rash, acneiform dermatitis, pruritus, and dry skin (Table 2). Serious adverse reactions (≥ 2% difference between treatment arms) in Vectibix-treated patients with wild-type KRAS mCRC were diarrhea and dehydration. The commonly reported adverse reactions (≥ 1%) leading to discontinuation in patients with wild-type KRAS mCRC receiving Vectibix were rash, paresthesia, fatigue, diarrhea, acneiform dermatitis, and hypersensitivity. One grade 5 adverse reaction, hypokalemia, occurred in a patient who received Vectibix.

Adverse reactions that did not meet the threshold criteria for inclusion in Table 2 were abdominal pain (28% vs 23%), localized infection (3.7% vs < 1%), cellulitis (2.5% vs 0%), hypocalcemia (5.6% vs 2.1%), and deep vein thrombosis (5.3% vs 3.1%).
Infusion Reactions
Infusional toxicity manifesting as fever, chills, dyspnea, bronchospasm or hypotension was assessed within 24 hours of an infusion during the clinical study. Vital signs and temperature were measured within 30 minutes prior to initiation and upon completion of the Vectibix infusion. The use of premedication was not standardized in the clinical trials. Thus, the utility of premedication in preventing the first or subsequent episodes of infusional toxicity is unknown. Across clinical trials of Vectibix monotherapy, 3% (24/725) experienced infusion reactions of which < 1% (3/725) were severe (NCI-CTC grade 3-4). In one patient, Vectibix was permanently discontinued for a serious infusion reaction.
Immunogenicity
As with all therapeutic proteins, there is potential for immunogenicity. The immunogenicity of Vectibix has been evaluated using two different screening immunoassays for the detection of binding anti-panitumumab antibodies: an acid dissociation bridging enzyme-linked immunosorbent assay (ELISA) detecting high-affinity antibodies and a Biacore® biosensor immunoassay detecting both high- and low-affinity antibodies. For patients whose sera tested positive in screening immunoassays, an in vitro biological assay was performed to detect neutralizing antibodies.
- Monotherapy: The incidence of binding anti-panitumumab antibodies (excluding preexisting and transient positive patients) was 0.4% (5/1123) as detected by the acid dissociation ELISA and 3.2% (36/1123) as detected by the Biacore® assay. The incidence of neutralizing anti-panitumumab antibodies (excluding preexisting and transient positive patients) was 0.8% (9/1123). There was no evidence of altered pharmacokinetic or safety profiles in patients who developed antibodies to Vectibix.
- In combination with chemotherapy: The incidence of binding anti-panitumumab antibodies (excluding preexisting positive patients) was 0.9% (12/1297) as detected by the acid dissociation ELISA and 0.7% (9/1296) as detected by the Biacore® assay. The incidence of neutralizing anti-panitumumab antibodies (excluding preexisting positive patients) was 0.2% (2/1297). No evidence of an altered safety profile was found in patients who developed antibodies to Vectibix.
The detection of antibody formation is highly dependent on the sensitivity and specificity of the assay. Additionally, the observed incidence of antibody (including neutralizing antibody) positivity in an assay may be influenced by several factors, including assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to panitumumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Vectibix. Because these reactions are reported in a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Skin and subcutaneous tissue disorders: Skin necrosis, angioedema, life-threatening and fatal bullous mucocutaneous disease.
- Immune system disorders: Infusion reaction.
- Eye disorders: Keratitis/ulcerative keratitis.
Drug Interactions
No formal drug-drug interaction studies have been conducted between Vectibix and oxaliplatin or fluoropyrimidine.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C There are no studies of Vectibix in pregnant women. Reproduction studies in cynomolgus monkeys treated with 1.25 to 5 times the recommended human dose of panitumumab resulted in significant embryolethality and abortions; however, no other evidence of teratogenesis was noted in offspring. Vectibix should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Based on animal models, EGFR is involved in prenatal development and may be essential for normal organogenesis, proliferation, and differentiation in the developing embryo. Human IgG is known to cross the placental barrier; therefore, panitumumab may be transmitted from the mother to the developing fetus, and has the potential to cause fetal harm when administered to pregnant women.
Women who become pregnant during Vectibix treatment are encouraged to enroll in Amgen’s Pregnancy Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Panitumumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Panitumumab during labor and delivery.
Nursing Mothers
It is not known whether panitumumab is excreted into human milk; however, human IgG is excreted into human milk. Published data suggest that breast milk antibodies do not enter the neonatal and infant circulation in substantial amounts. Because many drugs are excreted into human milk and because of the potential for serious adverse reactions in nursing infants from Vectibix, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. If nursing is interrupted, based on the mean half-life of panitumumab, nursing should not be resumed earlier than 2 months following the last dose of Vectibix.
Women who are nursing during Vectibix treatment are encouraged to enroll in Amgen’s Lactation Surveillance Program. Patients or their physicians should call 1-800-77-AMGEN (1-800-772-6436) to enroll.
Pediatric Use
The safety and effectiveness of Vectibix have not been established in pediatric patients. The pharmacokinetic profile of Vectibix has not been studied in pediatric patients.
Geriatic Use
Of the 737 patients who received Vectibix monotherapy in Study 1 and 2, 36% were 65 and over while 8% were 75 and over. No overall differences in safety or efficacy were observed in elderly patients (≥ 65 years of age) treated with Vectibix monotherapy.
Of the 322 patients in Study 3 who received Vectibix plus FOLFOX, 128 (40%) were 65 and over while 8% were 75 and over. Patients older than 65 years of age experienced an increased incidence of serious adverse events (52% vs 36%) and an increased incidence of serious diarrhea (15% vs 5%) as compared to younger patients.
Gender
There is no FDA guidance on the use of Panitumumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Panitumumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Panitumumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Panitumumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Panitumumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Panitumumab in patients who are immunocompromised.
Administration and Monitoring
Administration
There is limited information regarding Panitumumab Administration in the drug label.
Monitoring
There is limited information regarding Panitumumab Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Panitumumab and IV administrations.
Overdosage
Doses up to approximately twice the recommended therapeutic dose (12 mg/kg) resulted in adverse reactions of skin toxicity, diarrhea, dehydration, and fatigue.
Pharmacology
Panitumumab?
| |
| Therapeutic monoclonal antibody | |
| Source | u |
| Target | EGFR |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | ? |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | ? |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ∼9.4 days (range: 4-11 days) |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status |
Template:Unicode Prescription only |
| Routes | intravenous |
Mechanism of Action
The EGFR is a transmembrane glycoprotein that is a member of a subfamily of type I receptor tyrosine kinases, including EGFR, HER2, HER3, and HER4. EGFR is constitutively expressed in normal epithelial tissues, including the skin and hair follicle. EGFR is overexpressed in certain human cancers, including colon and rectum cancers. Interaction of EGFR with its normal ligands (eg, EGF, transforming growth factor-alpha) leads to phosphorylation and activation of a series of intracellular proteins, which in turn regulate transcription of genes involved with cellular growth and survival, motility, and proliferation. Signal transduction through the EGFR results in activation of the wild-type KRAS protein. However, in cells with activating KRAS somatic mutations, the KRAS-mutant protein is continuously active and appears independent of EGFR regulation.
Panitumumab binds specifically to EGFR on both normal and tumor cells, and competitively inhibits the binding of ligands for EGFR. Nonclinical studies show that binding of panitumumab to the EGFR prevents ligand-induced receptor autophosphorylation and activation of receptor-associated kinases, resulting in inhibition of cell growth, induction of apoptosis, decreased proinflammatory cytokine and vascular growth factor production, and internalization of the EGFR. In vitro assays and in vivo animal studies demonstrate that panitumumab inhibits the growth and survival of selected human tumor cell lines expressing EGFR.
Structure
There is limited information regarding Panitumumab Structure in the drug label.
Pharmacodynamics
There is limited information regarding Panitumumab Pharmacodynamics in the drug label.
Pharmacokinetics
Panitumumab administered as a single agent exhibits nonlinear pharmacokinetics.
Following single-dose administrations of panitumumab as 1-hour infusions, the area under the concentration-time curve (AUC) increased in a greater than dose-proportional manner, and clearance (CL) of panitumumab decreased from 30.6 to 4.6 mL/day/kg as the dose increased from 0.75 to 9 mg/kg. However, at doses above 2 mg/kg, the AUC of panitumumab increased in an approximately dose-proportional manner.
Following the recommended dose regimen (6 mg/kg given once every 2 weeks as a 1-hour infusion), panitumumab concentrations reached steady-state levels by the third infusion with mean (± SD) peak and trough concentrations of 213 ± 59 and 39 ± 14 mcg/mL, respectively. The mean (± SD) AUC0-tau and CL were 1306 ± 374 mcg•day/mL and 4.9 ± 1.4 mL/kg/day, respectively. The elimination half-life was approximately 7.5 days (range: 3.6 to 10.9 days).
A population pharmacokinetic analysis was performed to explore the potential effects of selected covariates on panitumumab pharmacokinetics. Results suggest that age (21-88 years), gender, race (15% nonwhite), mild-to-moderate renal dysfunction, mild-to-moderate hepatic dysfunction, and EGFR membrane-staining intensity (1+, 2+, and 3+) in tumor cells had no apparent impact on the pharmacokinetics of panitumumab.
No formal pharmacokinetic studies of panitumumab have been conducted in patients with renal impairment or hepatic impairment.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
No carcinogenicity or mutagenicity studies of panitumumab have been conducted. It is not known if panitumumab can impair fertility in humans. Prolonged menstrual cycles and/or amenorrhea occurred in normally cycling, female cynomolgus monkeys treated weekly with 1.25 to 5 times the recommended human dose of panitumumab (based on body weight). Menstrual cycle irregularities in panitumumab-treated female monkeys were accompanied by both a decrease and delay in peak progesterone and 17β-estradiol levels. Normal menstrual cycling resumed in most animals after discontinuation of panitumumab treatment. A no-effect level for menstrual cycle irregularities and serum hormone levels was not identified. The effects of panitumumab on male fertility have not been studied. However, no adverse effects were observed microscopically in reproductive organs from male cynomolgus monkeys treated for 26 weeks with panitumumab at doses of up to approximately 5-fold the recommended human dose (based on body weight).
13.2 Animal Toxicology and/or Pharmacology Weekly administration of panitumumab to cynomolgus monkeys for 4 to 26 weeks resulted in dermatologic findings, including dermatitis, pustule formation and exfoliative rash, and deaths secondary to bacterial infection and sepsis at doses of 1.25 to 5-fold higher (based on body weight) than the recommended human dose.
13.3 Reproductive and Developmental Toxicology Pregnant cynomolgus monkeys were treated weekly with panitumumab during the period of organogenesis (gestation day [GD] 20-50). While no panitumumab was detected in serum of neonates from panitumumab-treated dams, anti-panitumumab antibody titers were present in 14 of 27 offspring delivered at GD 100. There were no fetal malformations or other evidence of teratogenesis noted in the offspring. However, significant increases in embryolethality and abortions occurred at doses of approximately 1.25 to 5 times the recommended human dose (based on body weight).
Clinical Studies
There is limited information regarding Panitumumab Clinical Studies in the drug label.
How Supplied
There is limited information regarding Panitumumab How Supplied in the drug label.
Storage
There is limited information regarding Panitumumab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Panitumumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Panitumumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Panitumumab Patient Counseling Information in the drug label.
Precautions with Alcohol
Alcohol-Panitumumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
There is limited information regarding Panitumumab Brand Names in the drug label.
Look-Alike Drug Names
There is limited information regarding Panitumumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
|
WikiDoc Resources for Panitumumab |
|
Articles |
|---|
|
Most recent articles on Panitumumab Most cited articles on Panitumumab |
|
Media |
|
Powerpoint slides on Panitumumab |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Panitumumab at Clinical Trials.gov Clinical Trials on Panitumumab at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Panitumumab
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Panitumumab Discussion groups on Panitumumab Patient Handouts on Panitumumab Directions to Hospitals Treating Panitumumab Risk calculators and risk factors for Panitumumab
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Panitumumab |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Overview
Panitumumab (ABX-EGF) is a fully human monoclonal antibody specific to the EGF receptor (see illustration)[4]. It was FDA approved for the first time in September 2006, for the "the treatment of EGFR-expressing metastatic colorectal cancer with disease progression" despite prior treatment [1]. Panitumumab is manufactured by Amgen and marketed as Vectibix in the USA.
The compound works by binding to the extracellular domain of the EGFR (epidermal growth factor receptor) preventing its activation. This in turn, results in halting of the cascade of intracellular signals dependent on this receptor. [2]
Panitumumab is produced by immunization of transgenic mice (xenomouse), that are able to produce human immunoglobulin light and heavy chains. After immunization of these animals a specific clone of B cells that produced an antibody against EGFR was selected and immortalized in Chinese hamster ovary (CHO) cells. These cells are then used for the full scale manufacture of the antibody.
Although they both target the EGFR, panitumumab (IgG2) and cetuximab (IgG1) differ in their isotype and they might differ in their mechanism of action. Monoclonal antibodies of the IgG1 isotype may activate the complement pathway and mediate ADCC (antibody-dependent cellular cytotoxicity) better than their IgG2 counterparts, hence although they have not been documented, differences in the responses in treatments with these two antibodies might be expected. [5]
References
Further readings
Philippe Rougier, Emmanuel Mitry, Sophie Dominguez. Les Cancers Digestifs ; Springer , September 1, 2006, page=291 ; language=French