D-Aminolevulinic acid
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Rabin Bista, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
D-Aminolevulinic acid is a dermatological agent that is FDA approved for the treatment of minimally to moderately thick actinic keratoses of the face or scalp. Common adverse reactions include Disorder of skin pigmentation, Edema, Erythema , Pruritus, Scaly skin..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Indications
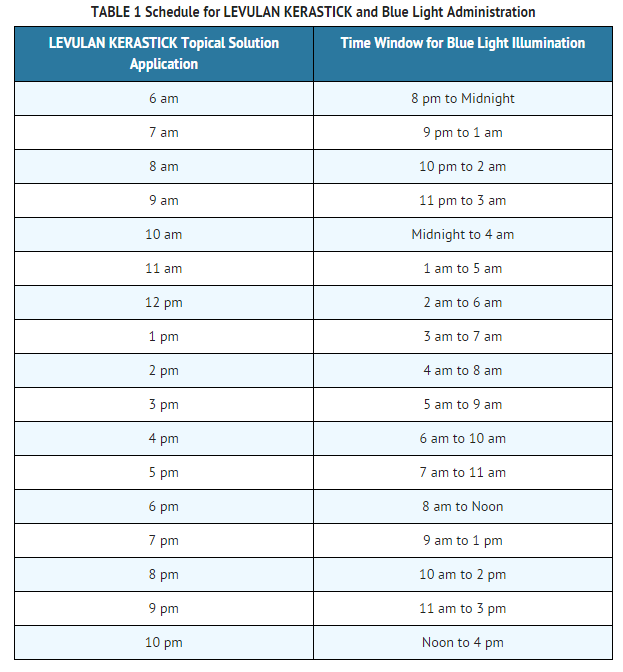
- The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy (PDT) Illuminator is indicated for the treatment of minimally to moderately thick actinic keratoses of the face or scalp.
Dosage
- LEVULAN KERASTICK for Topical Solution 20% is intended for direct application to individual lesions diagnosed as actinic keratoses and not to perilesional skin. This product is not intended for application by patients or unqualified medical personnel. Application should involve either scalp or face lesions, but not both simultaneously. The recommended treatment frequency is: one application of the LEVULAN KERASTICK Topical Solution and one dose of illumination per treatment site per 8-week treatment session. Each individual LEVULAN KERASTICK for Topical Solution should be used for only one patient. Photodynamic therapy for actinic keratoses with LEVULAN KERASTICK for Topical Solution is a two stage process involving a) application of the product to the target lesions with LEVULAN KERASTICK Topical Solution, followed 14 to 18 hours later by b) illumination with blue light using the BLU-U Blue Light Photodynamic Therapy Illuminator. The second visit, for illumination, must take place in the 14-18 hour window following application. Patients in clinical trials usually received application in the late afternoon, with illumination the following morning.
- Treated lesions that have not completely resolved after 8 weeks may be treated a second time with LEVULAN KERASTICK for Topical Solution Photodynamic Therapy.
- Step A - LEVULAN KERASTICK for Topical Solution Application: Actinic keratoses
- Preparation of lesions
- Actinic keratoses targeted for treatment should be clean and dry prior to application of LEVULAN KERASTICK Topical Solution.
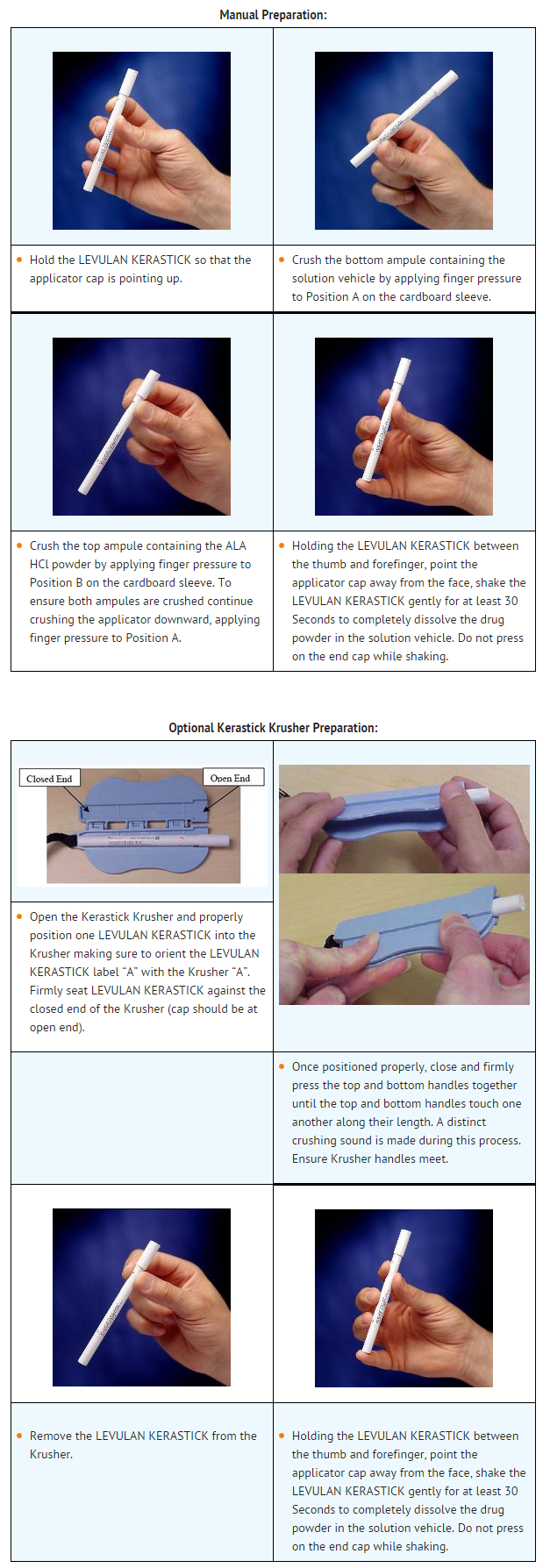
- Preparation of LEVULAN KERASTICK
- LEVULAN KERASTICK can be prepared either manually, or using the optional Kerastick Krusher. These methods are illustrated below.
- Application of solution
- Following solution admixture, remove the cap from the LEVULAN KERASTICK Topical Solution. The dry applicator tip should be dabbed on a gauze pad until uniformly wet with solution. Apply the solution directly to the target lesions by dabbing gently with the wet applicator tip. Enough solution should be applied to uniformly wet the lesion surface, including the edges without excess running or dripping. The effect of LEVULAN KERASTICK Topical Solution on ocular tissues is unknown. LEVULAN KERASTICK Topical Solution should not be applied to the periorbital area or allowed to contact ocular or mucosal surfaces. Once the initial application has dried, apply again in the same manner. The LEVULAN KERASTICK Topical Solution must be used immediately following preparation (dissolution) due to the instability of the activated product. If the solution application is not completed within 2 hours of activation, the applicator should be discarded and a new LEVULAN KERASTICK for Topical Solution used.
- Photosensitization of the treated lesions will take place over the next 14-18 hours. The actinic keratoses should not be washed during this time. The patient should be advised to wear a wide-brimmed hat or other protective apparel to shade the treated actinic keratoses from sunlight or other bright light sources until BLU-U treatment. The patient should be advised to reduce light exposure if the sensations of stinging and/or burning are experienced.
- If for any reason the patient cannot be given BLU-U treatment during the prescribed time after LEVULAN KERASTICK Topical Solution application, he or she may nonetheless experience sensations of stinging and/or burning if the photosensitized actinic keratoses are exposed to sunlight or prolonged or intense light at that time. The patient should be advised to wear a wide-brimmed hat or other protective apparel to shade the treated actinic keratoses from sunlight or other bright light sources until at least 40 hours after the application of LEVULAN KERASTICK Topical Solution. The patient should be advised to reduce light exposure if the sensations of stinging and/or burning are experienced.
- Step B - Administration of BLU-U Treatment 14 to 18 hours after application
- LEVULAN KERASTICK for Topical Solution is not intended for use with any device other than the BLU-U Blue Light Photodynamic Therapy Illuminator. Use of LEVULAN KERASTICK Topical Solution without subsequent BLU-U illumination is not recommended.
- At the visit for light illumination, the actinic keratoses to be treated should be gently rinsed with water and patted dry. Photoactivation of actinic keratoses treated with LEVULAN KERASTICK Topical Solution is accomplished with BLU-U illumination from the BLU-U Blue Light Photodynamic Therapy Illuminator. A 1000 second (16 minutes 40 seconds) exposure is required to provide a 10 J/cm2 light dose. During light treatment, both patients and medical personnel should be provided with blue blocking protective eyewear, as specified in the BLU-U Operating Instructions. Please refer to the BLU-U Operating Instructions for further information on conducting the light treatment. Patients should be advised that transient stinging and/or burning at the target lesion sites occurs during the period of light exposure.
- If blue light treatment with the BLU-U Blue Light Photodynamic Therapy Illuminator is interrupted or stopped for any reason, it should not be restarted and the patient should be advised to protect the treated lesions from exposure to sunlight or prolonged or intense light for at least 40 hours after application of the LEVULAN KERASTICK Topical Solution.
- For patients with facial lesions:
- The BLU-U Blue Light Photodynamic Therapy Illuminator is positioned so that the base is slightly above the patient’s shoulder, parallel to the patient’s face.
- The BLU-U is positioned around the patient’s head so the entire surface area to be treated lies between 2” and 4” from the BLU-U surface:
- The patient’s nose should be no closer than 2” from the surface;
- The patient’s forehead and cheeks should be no further than 4” from the surface;
- The sides of the patient’s face and the patient’s ears should be no closer than 2” from the BLU-U surface.
- A Chin Rest, available from DUSA Pharmaceuticals, Inc., may be used to provide support for the patient’s head during treatment.
- For patients with scalp lesions:
- The knobs on either side of the BLU-U are loosened and the BLU-U is rotated to a horizontal position.
- The BLU-U is positioned around the patient’s head so the entire surface area to be treated lies between 2” and 4” from the BLU-U surface:
- The patient’s scalp should be no closer than 2” from the surface;
- The patient’s scalp should be no further than 4” from the surface;
- The sides of the patient’s face and the patient’s ears should be no closer than 2” from the BLU-U surface.
- A Chin Rest, available from DUSA Pharmaceuticals, Inc., may be used to provide support for the patient’s head during treatment.
DOSAGE FORMS AND STRENGTHS
- Solution containing 20% aminolevulinic acid hydrochloride (ALA HCl) by weight in a plastic applicator device.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of D-Aminolevulinic acid in adult patients.
Non–Guideline-Supported Use
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of D-Aminolevulinic acid in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of D-Aminolevulinic acid in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of D-Aminolevulinic acid in pediatric patients.
Contraindications
- The LEVULAN KERASTICK for Topical Solution plus blue light illumination using the BLU-U Blue Light Photodynamic Therapy Illuminator is contraindicated in patients with cutaneous photosensitivity at wavelengths of 400-450 nm, porphyria or known allergies to porphyrins, and in patients with known sensitivity to any of the components of the LEVULAN KERASTICK for Topical Solution.
Warnings
Photosensitivity
- During the time period between the application of LEVULAN KERASTICK Topical Solution and exposure to activating light from the BLU-U Blue Light Photodynamic Therapy Illuminator, the treatment site will become photosensitive. After LEVULAN KERASTICK Topical Solution application, patients should avoid exposure of the photosensitive treatment sites to sunlight or bright indoor light (e.g., examination lamps, operating room lamps, tanning beds, or lights at close proximity) during the period prior to blue light treatment. Exposure may result in a stinging and/or burning sensation and may cause erythema and/or edema of the lesions. Before exposure to sunlight, patients should, therefore, protect treated lesions from the sun by wearing a wide-brimmed hat or similar head covering of light-opaque material. Sunscreens will not protect against photosensitivity reactions caused by visible light. It has not been determined if perspiration can spread the LEVULAN KERASTICK Topical Solution outside the treatment site to eye or surrounding skin.
- Application of LEVULAN KERASTICK Topical Solution to perilesional areas of photodamaged skin of the face or scalp may result in photosensitization. Upon exposure to activating light from the BLU-U Blue Light Photodynamic Therapy Illuminator, such photosensitized skin may produce a stinging and/or burning sensation and may become erythematous and/or edematous in a manner similar to that of actinic keratoses treated with LEVULAN KERASTICK Photodynamic Therapy. Because of the potential for skin to become photosensitized, the LEVULAN KERASTICK should be used by a qualified health professional to apply drug only to actinic keratoses and not perilesional skin.
- If for any reason the patient cannot return for blue light treatment during the prescribed period after application of LEVULAN KERASTICK Topical Solution (14 to 18 hours), the patient should call the doctor. The patient should also continue to avoid exposure of the photosensitized lesions to sunlight or prolonged or intense light for at least 40 hours. If stinging and/or burning is noted, exposure to light should be reduced.
Irritation
- The LEVULAN KERASTICK Topical Solution contains alcohol and is intended for topical use only. Do not apply to the eyes or to mucous membranes. Excessive irritation may be experienced if this product is applied under occlusion.
Coagulation Defects
- The LEVULAN KERASTICK for Topical Solution has not been tested on patients with inherited or acquired coagulation defects
Adverse Reactions
Clinical Trials Experience
- In Phase 3 studies, no non-cutaneous adverse events were found to be consistently associated with LEVULAN KERASTICK Topical Solution application followed by blue light exposure.
Photodynamic Therapy Response
- The constellation of transient local symptoms of stinging and/or burning, itching, erythema and edema as a result of LEVULAN KERASTICK Topical Solution plus BLU-U treatment was observed in all clinical studies of LEVULAN KERASTICK for Topical Solution Photodynamic Therapy for actinic keratoses treatment. Stinging and/or burning subsided between 1 minute and 24 hours after the BLU-U Blue Light Photodynamic Therapy Illuminator was turned off, and appeared qualitatively similar to that perceived by patients with erythropoietic protoporphyria upon exposure to sunlight. There was no clear drug dose or light dose dependent change in the incidence or severity of stinging and/or burning.
- In two Phase 3 trials, the sensation of stinging and/or burning appeared to reach a plateau at 6 minutes into the treatment. Severe stinging and/or burning at one or more lesions being treated was reported by at least 50% of the patients at some time during treatment. The majority of patients reported that all lesions treated exhibited at least slight stinging and/or burning. Less than 3% of patients discontinued light treatment due to stinging and/or burning.
- In the Phase 3 trials, the most common changes in lesion appearance after LEVULAN KERASTICK for Topical Solution Photodynamic Therapy were erythema and edema. In 99% of active treatment patients, some or all lesions were erythematous shortly after treatment, while in 79% of vehicle treatment patients, some or all lesions were erythematous. In 35% of active treatment patients, some or all lesions were edematous, while no vehicle-treated patients had edematous lesions. Both erythema and edema resolved to baseline or improved by 4 weeks after therapy. LEVULAN KERASTICK Topical Solution application to photodamaged perilesional skin resulted in photosensitization of photodamaged skin and in a photodynamic response.
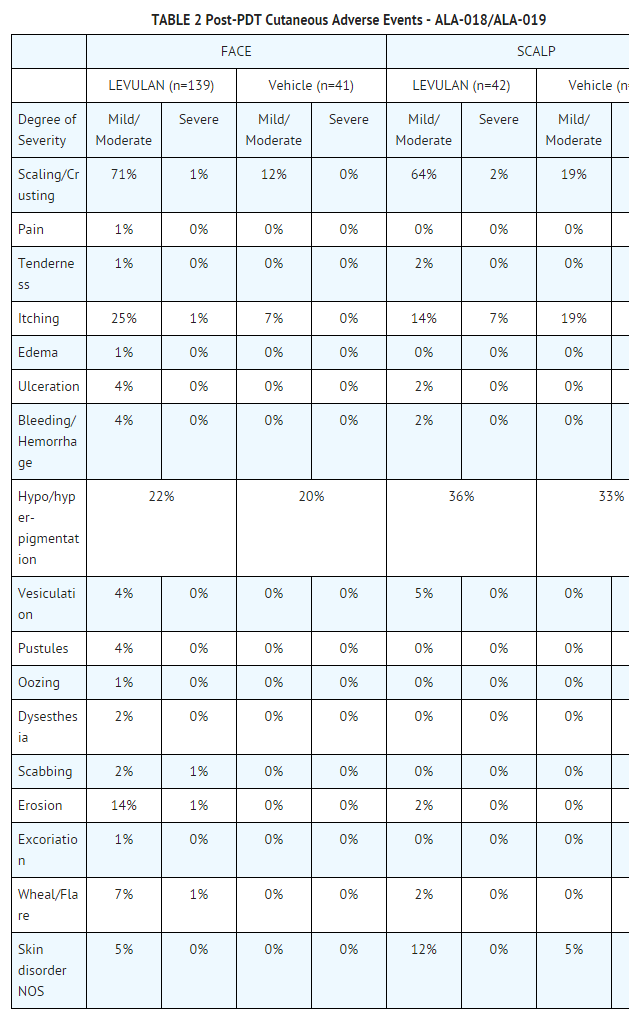
- Other Localized Cutaneous Adverse Experiences: TABLE 2 depicts the incidence and severity of cutaneous adverse events in Phase 3 studies, stratified by anatomic site treated.
- Adverse Experiences Reported by Body System: In the Phase 3 studies, 7 patients experienced a serious adverse event. All were deemed remotely or not related to treatment. No clinically significant patterns of clinical laboratory changes were observed for standard serum chemical or hematologic parameters in any of the controlled clinical trials.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of D-Aminolevulinic acid in the drug label.
Drug Interactions
- There have been no formal studies of the interaction of LEVULAN KERASTICK Topical Solution with any other drugs, and no drug-specific interactions were noted during any of the controlled clinical trials. It is, however, possible that concomitant use of other known photosensitizing agents such as griseofulvin, thiazide diuretics, sulfonylureas, phenothiazines, sulfonamides and tetracyclines might increase the photosensitivity reaction of actinic keratoses treated with LEVULAN KERASTICK for Topical Solution.
Use in Specific Populations
Pregnancy
- Animal reproduction studies have not been conducted with aminolevulinic acid (ALA HCl). It is also not known whether LEVULAN KERASTICK Topical Solution can cause fetal harm when administered to a pregnant woman or can affect reproductive capacity. LEVULAN KERASTICK for Topical Solution should be given to a pregnant woman only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of D-Aminolevulinic acid in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of D-Aminolevulinic acid during labor and delivery.
Nursing Mothers
- The levels of ALA or its metabolites in the milk of subjects treated with LEVULAN KERASTICK for Topical Solution have not been measured. Because many drugs are excreted in human milk, caution should be exercised when LEVULAN KERASTICK for Topical Solution is administered to a nursing woman.
Pediatric Use
- The safety and effectiveness in pediatric patients below the age of 18 have not been established.
Geriatic Use
- Of the 243 subjects in controlled clinical trials of LEVULAN KERASTICK for Topical Solution, 64% (156/243) were 65 years old and over, while 23% (55/243) were 75 years old and over. No overall differences in safety or effectiveness were observed between these subjects and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of D-Aminolevulinic acid with respect to specific gender populations.
Race
There is no FDA guidance on the use of D-Aminolevulinic acid with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of D-Aminolevulinic acid in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of D-Aminolevulinic acid in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of D-Aminolevulinic acid in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of D-Aminolevulinic acid in patients who are immunocompromised.
Administration and Monitoring
Administration
- Topical
Monitoring
There is limited information regarding Monitoring of D-Aminolevulinic acid in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of D-Aminolevulinic acid in the drug label.
Overdosage
LEVULAN KERASTICK Topical Solution Overdose
- LEVULAN KERASTICK Topical Solution overdose has not been reported. In the unlikely event that the drug is ingested, monitoring and supportive care are recommended. The patient should be advised to avoid incidental exposure to intense light sources for at least 40 hours after ingestion. The consequences of exceeding the recommended topical dosage are unknown.
BLU-U Light Overdose
- There is no information on overdose of blue light from the BLU-U Blue Light Photodynamic Therapy Illuminator following LEVULAN KERASTICK Topical Solution application.
Pharmacology
| |
δ-Aminolevulinic acid
| |
| Systematic (IUPAC) name | |
| 5-amino-4-oxo-pentanoic acid | |
| Identifiers | |
| CAS number | |
| ATC code | L01 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 131.13 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C |
| Legal status |
Rx only |
| Routes | ? |
Mechanism of Action
- Photosensitization following application of LEVULAN KERASTICK Topical Solution occurs through the metabolic conversion of aminolevulinic acid to protoporphyrin IX (PpIX), a photosensitizer, which accumulates in the skin to which LEVULAN KERASTICK Topical Solution has been applied. When exposed to light of appropriate wavelength and energy, the accumulated photoactive porphyrins produce a photodynamic reaction, resulting in a cytotoxic process dependent upon the simultaneous presence of oxygen. The absorption of light results in an excited state of porphyrin molecules, and subsequent spin transfer from photoreactive porphyrins to molecular oxygen generates singlet oxygen, which can further react to form superoxide and hydroxyl radicals. LEVULAN KERASTICK Photodynamic Therapy of actinic keratoses is the combination of photosensitization by topical application of LEVULAN KERASTICK Topical Solution to the lesions and subsequent illumination with the BLU-U Blue Light Photodynamic Therapy Illuminator (BLU-U).
Structure
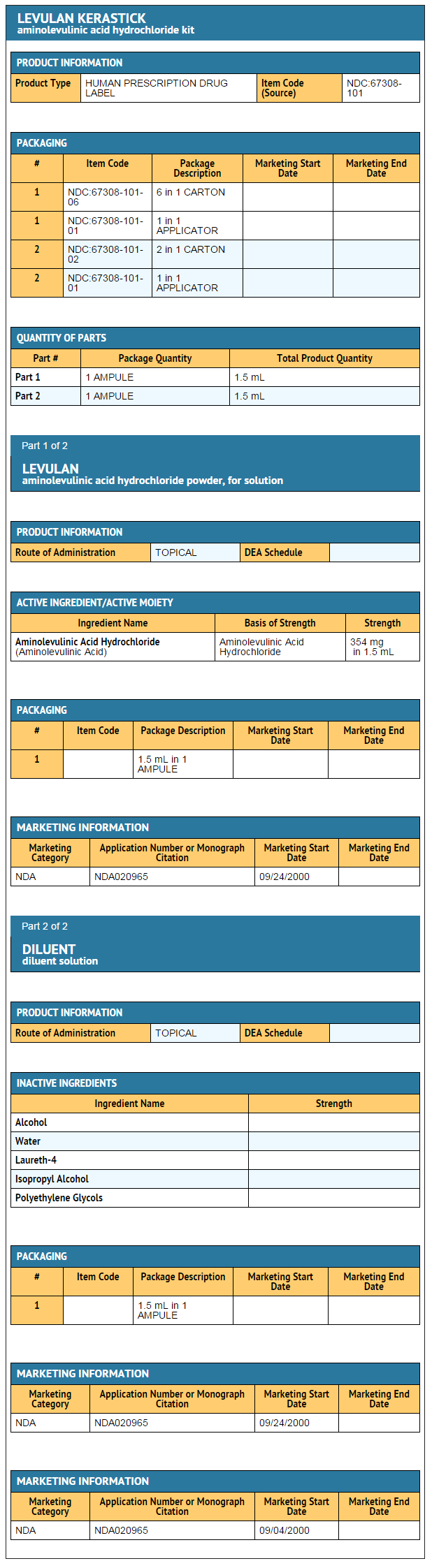
- LEVULAN® KERASTICK® (aminolevulinic acid HCl) for Topical Solution, 20%, a porphyrin precursor, contains the hydrochloride salt of aminolevulinic acid (ALA), an endogenous 5-carbon aminoketone.
- ALA HCl is a white to off-white, odorless crystalline solid that is very soluble in water, slightly soluble in methanol and ethanol, and practically insoluble in chloroform, hexane and mineral oil.
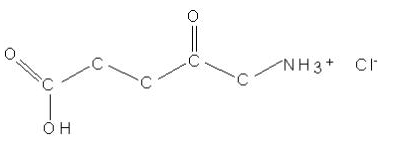
- The chemical name for ALA HCl is 5-amino-4-oxopentanoic acid hydrochloride (MW = 167.59). The structural formula is represented below:
- The LEVULAN KERASTICK for Topical Solution applicator is a two component system consisting of a plastic tube containing two sealed glass ampules and an applicator tip. One ampule contains 1.5 mL of solution vehicle comprising alcohol USP (ethanol content = 48% v/v), water, laureth-4, isopropyl alcohol, and polyethylene glycol. The other ampule contains 354 mg of ALA HCl as a dry solid. The applicator tube is enclosed in a protective cardboard sleeve and cap. The 20% topical solution is prepared just prior to the time of use by breaking the ampules and mixing the contents by shaking the LEVULAN KERASTICK applicator. The term “ALA HCl” refers to unformulated active ingredient, “LEVULAN KERASTICK for Topical Solution” refers to the drug product in its unmixed state, “LEVULAN KERASTICK Topical Solution” refers to the mixed drug product (in the applicator tube or after application), and “LEVULAN KERASTICK” refers to the applicator only.
Pharmacodynamics
- ALA does not exhibit fluorescence, while PpIX has a high fluorescence yield. Time-dependent changes in surface fluorescence have been used to determine PpIX accumulation and clearance in actinic keratoses and perilesional skin after application of LEVULAN KERASTICK Topical Solution in 12 patients. Peak fluorescence intensity was reached in 11 ± 1 hr in actinic keratoses and 12 ± 1 hr in perilesional skin. The mean clearance half-life of fluorescence for lesions was 30 ± 10 hr and 28 ± 6 hr for perilesional skin. The fluorescence in perilesional skin was similar to that in actinic keratoses. Therefore, LEVULAN KERASTICK Topical Solution should only be applied to the affected skin.
Pharmacokinetics
- In a human pharmacokinetic study (N=6) using a 128 mg dose of sterile intravenous ALA HCl and oral ALA HCl (equivalent to 100 mg ALA) in which plasma ALA and PpIX were measured, the mean half-life of ALA was 0.70 ± 0.18 h after the oral dose and 0.83 ± 0.05 h after the intravenous dose. The oral bioavailability of ALA was 50-60% with a mean Cmax of 4.65 ± 0.94 µg/mL. PpIX concentrations were low and were detectable only in 42% of the plasma samples. PpIX concentrations in plasma were quite low relative to ALA plasma concentrations, and were below the level of detection (10 ng/mL) after 10 to 12 hours.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- No carcinogenicity testing has been carried out using ALA. No evidence of mutagenic effects was seen in four studies conducted with ALA to evaluate this potential. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay (Ames mutagenicity assay), no increases in the number of revertants were observed with any of the tester strains. In the Salmonella-Escherichia coli/mammalian microsome reverse mutation assay in the presence of solar light radiation (Ames mutagenicity assay with light), ALA did not cause an increase in the number of revertants per plate of any of the tester strains in the presence or absence of simulated solar light. In the L5178Y TK± mouse lymphoma forward mutation assay, ALA was evaluated as negative with and without metabolic activation under the study conditions. PpIX formation was not demonstrated in any of these in vitro studies. In the in vivo mouse micronucleus assay, ALA was considered negative under the study exposure conditions. In contrast, at least one report in the literature has noted genotoxic effects in cultured rat hepatocytes after ALA exposure with PpIX formation. Other studies have documented oxidative DNA damage in vivo and in vitro as a result of ALA exposure.
- No assessment of effects of ALA HCl on fertility has been performed in laboratory animals. It is unknown what effects systemic exposure to ALA HCl might have on fertility or reproductive function.
Clinical Studies
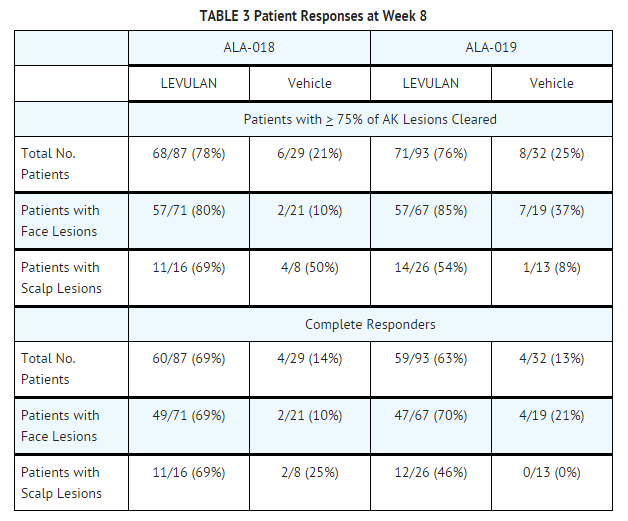
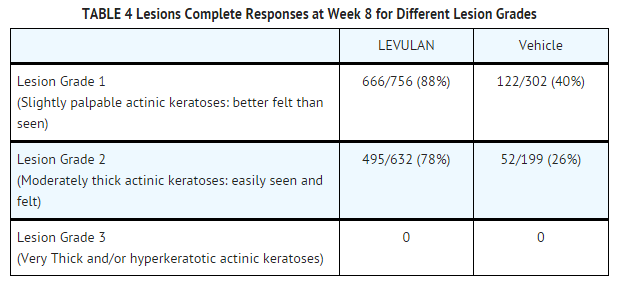
- LEVULAN KERASTICK for Topical Solution plus blue light at 6-10.9 J/cm2, has been used to treat actinic keratoses in 342 patients in seven clinical trials. Phase 3 studies were two, identically designed, multicenter, two-arm studies using LEVULAN KERASTICK for Topical Solution plus illumination from the BLU-U for 1000 seconds (16 min 40 sec) for a nominal exposure of 10 J/cm2. Patients were excluded from these studies who had a history of cutaneous photosensitization, porphyria, hypersensitivity to porphyrins, photodermatosis, or inherited or acquired coagulation defects. A minimum of 4 and a maximum of 15 clinically typical, discrete, [Grade 1 (Slightly palpable actinic keratoses: better felt than seen) or Grade 2, (Moderately thick actinic keratoses: easily seen and felt) see TABLE 4 for definition], target actinic keratoses were identified. Target lesions on the face or on the scalp, but not in both locations in the same patient, received treatment. The patients were randomized to receive treatment either with the LEVULAN KERASTICK Topical Solution plus BLU-U or vehicle plus BLU-U. Patients were randomized at a 3 to 1 LEVULAN to vehicle ratio. A total of 243 patients were enrolled in two Phase 3 studies (ALA-018, ALA-019). Lesions were designated as cleared (complete response) if the lesion had completely cleared and adherent scaling plaques of actinic keratoses were no longer evident on the surface of the treated skin when palpated. The percentage of patients in whom 75% or more of treated lesions were cleared, and the percentage of patients in whom 100% of treated lesions were cleared (Complete Responders), for each study at 8 weeks after treatment are shown in TABLE 3.
- Because clinical studies ALA-018 and ALA-019 had identical protocols, the combined results from the two trials are shown in the following tables. For actinic keratoses with a variety of thicknesses (excluding very thick, Grade 3 actinic keratoses which were not studied in the phase 3 trials), LEVULAN KERASTICK Topical Solution plus BLU-U is more effective than vehicle plus BLU-U, but as shown in TABLE 4, the percentage of lesions with complete responses at 8 weeks after treatment with LEVULAN KERASTICK Topical Solution plus blue light illumination was lower for those lesions that were thicker at baseline. Efficacy of LEVULAN KERASTICK Topical Solution plus BLU-U on higher grade lesions was not studied in the Phase 3 clinical efficacy trials.
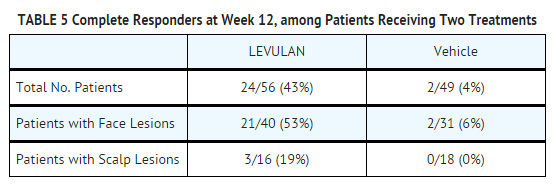
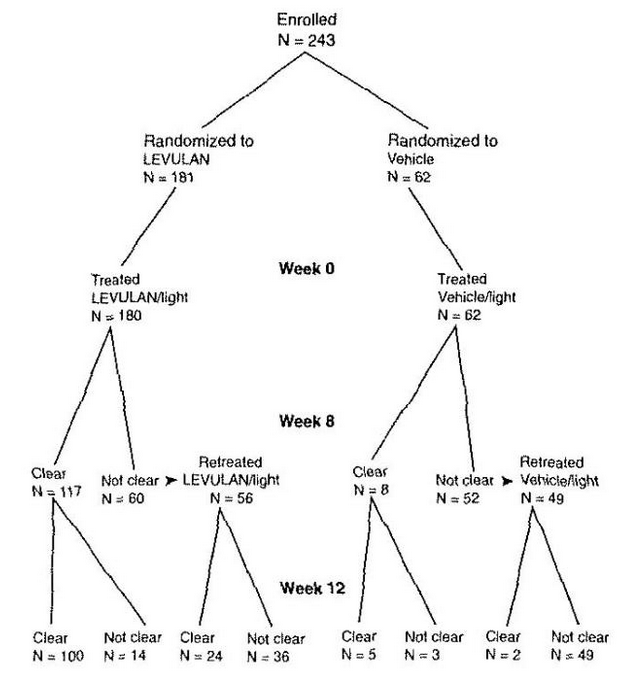
- Those patients who were not Complete Responders at week 8 had retreatment of the persistent target lesions at week 8. Among the patients undergoing retreatment, efficacy results seen at 12 weeks after the initial treatment, i.e., at 4 weeks after the second treatment, are shown in TABLE 5.
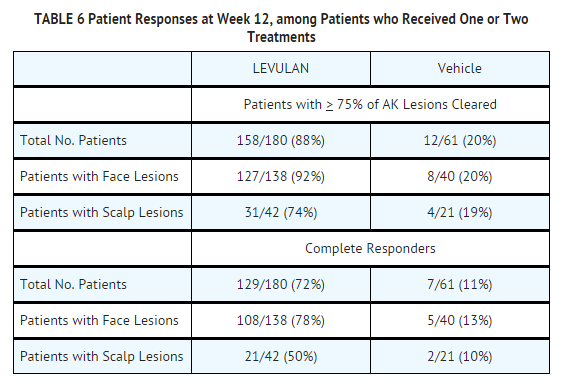
- The efficacy results seen at 12 weeks after treatment, which include the results at 12 weeks for those patients who received a single treatment as well as the results at 12 weeks for those patients who received a second treatment at week 8, are shown in TABLE 6.
- Among Complete Responders at week 8, 93% (in study ALA-018) and 83% (in study ALA-019) maintained complete response at week 12. Among patients with scalp lesions, the percentage of patients with 100% of AK lesions having complete response declined from week 8 (55%) to week 12 (50%), because there were more patients with scalp lesions with 100% of AK lesions cleared at week 8 who had a recurrence of a lesion by week 12 than there were patients with scalp lesions who had retreatment of persistent lesions at week 8 and who then achieved 100% of AK lesions cleared by week 12. Patients did not receive follow-up past 12 weeks after the initial treatment.
- Patient outcomes recorded in the two Phase 3 trials are depicted in the following flowchart, in which Complete Responders are designated clear. Seven patients in the active treatment arm and three patients in the vehicle treatment arm withdrew or were lost to follow-up. Three patients in the active treatment arm were treated at baseline but did not return for evaluation until week 12. One patient in the active treatment arm and two in the vehicle treatment arm who were not clear at week 8 did not receive retreatment.
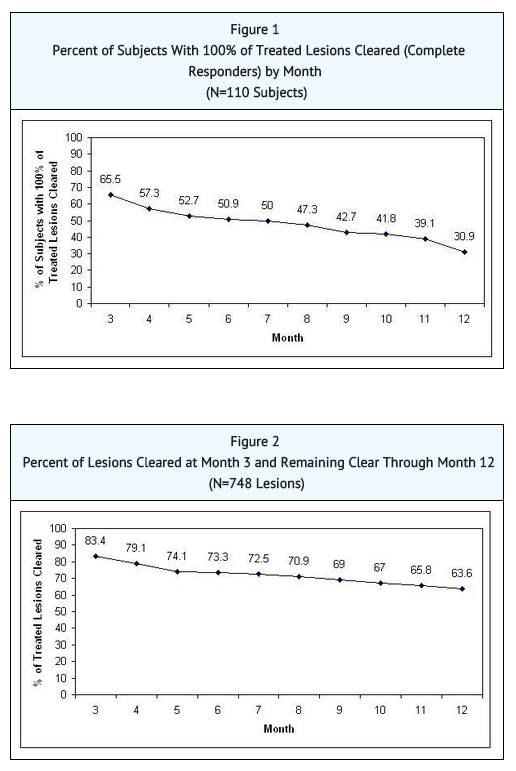
- An open-label study enrolled 110 subjects with 4 to 10 clinically typical, discrete actinic keratoses on the face or scalp, but not both locations. The target lesions were treated with the LEVULAN KERASTICK Topical Solution plus BLU-U. Any treated lesions that were not clear at Month 2 (Week 8) were re-treated with LEVULAN KERASTICK Topical Solution plus BLU-U. Subjects were followed monthly for 12 months. Lesions were designated as cleared if the lesion had completely cleared and adherent scaling plaques of actinic keratoses were no longer evident on the surface of the treated skin when palpated. The percentages of subjects in whom 100% of treated lesions were cleared (Complete Responders) by month, starting at Month 3 (Week 12), are shown in FIGURE 1. Of the 72 subjects with 100% of treated lesions cleared (Complete Responders) at Month 3, 53% had a recurrence by Month 12. A total of 748 individual lesions were treated in Study 3; 539 were treated once and 209 were treated twice. At Month 3, 624 lesions (83%) were cleared. From Month 3 through Month 12 of the study, 476 lesions (64%) remained clear. See FIGURE 2. Of the 624 treated lesions determined cleared at Month 3, 24% had recurred by Month 12, while 5% were lost to follow-up and their recurrence status is unknown.
How Supplied
- How Supplied
- The LEVULAN KERASTICK for Topical Solution, 20%, is a single-unit dosage form, supplied in packs of 6. Each LEVULAN KERASTICK for Topical Solution applicator consists of a plastic tube containing two sealed glass ampules and an applicator tip. One ampule contains 1.5 mL of solution vehicle. The other ampule contains 354 mg of aminolevulinic acid HCl. The applicator is covered with a protective cardboard sleeve and cap.
Product Package - NDC Number
- Carton of 6 LEVULAN KERASTICKS for Topical Solution, 20% 67308-101-06
Storage
- Store between 20° - 25 °C (68° - 77 °F); excursions permitted to 15°- 30 °C (59° - 86 °F). The LEVULAN KERASTICK Topical Solution should be used immediately following preparation (dissolution). Solution application must be completed within 2 hours of preparation. An applicator that has been prepared must be discarded 2 hours after mixing (dissolving) and a new LEVULAN KERASTICK for Topical Solution used, if needed.
Images
Drug Images
{{#ask: Page Name::D-Aminolevulinic acid |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
PACKAGE LABEL
NDC 67308-101-01
Levulan® Kerastick®
(aminolevulinic acid HCl) for Topical Solution, 20%
SINGLE USE APPLICATOR 1.5 ML
LOT NO.:
EXP. DATE:
DUSA®
Manufactured for:
DUSA Pharmaceuticals, Inc.
25 Upton Dr.
Wilmington, MA 01887
LAB-1411 REV.: E
Levulan® Kerastick®
(aminolevulinic acid HCl) for Topical Solution, 20%
6 SINGLE USE APPLICATORS, 1.5 mL EACH
DUSA®
For Topical Use Only
Not For Use In Eyes
FOR ADMINISTRATION BY HEALTH PROFESSIONAL ONLY
Rx Only
LOT No.:
EXP. DATE:
Each applicator contains: Active Ingredient: 354mg aminolevulinic acid HCl Inactive Ingredients: Alcohol, USP (ethanol content-48% v/v), water, laureth-4, isopropyl alcohol, and polyethylene glycol.
Usual Dose: For indications, dosage, precautions, etc., see enclosed package insert.
Store between 20°25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature]
Use and discard within two hours after activiation.
DUSA Pharmaceuticals, Inc.® 25 Upton Dr. Wilmington, MA 01887 1-877-533-3872 or 1-978-657-7500
US Patents: 5,079,262 5,211,938 5,422,093 5,954,703 6,710,066
6 SINGLE USE APPLICATORS, 1.5 mL EACH
Carefully follow the step by step directions provided in the package insert to prepare Levulan® Kerastick® for application. Please note the locations of the crush points "A" and "B" which are indicated by the red diamonds "♦" on each Kerastick®.
COM-0534 REV. F
NDC 67308-101-01
Levulan® Kerastick®
(aminolevulinic acid HCl) for Topical Solution, 20%
SINGLE USE APPLICATOR 1.5 ML
LOT NO.:
EXP. DATE:
SAMPLE: NOT FOR SALE
DUSA® Manufactured for:
DUSA Pharmaceuticals, Inc. 25 Upton Dr. Wilmington, MA 01887
LAB-1411 REV.: B
Levulan® Kerastick®
(aminolevulinic acid HCl) for Topical Solution, 20%
2 SINGLE USE APPLICATORS, 1.5 mL EACH
SAMPLE NOT FOR SALE
DUSA®
For Topical Use Only
Not For Use in Eyes
FOR ADMINISTRATION BY HEALTH PROFESSIONAL ONLY
Rx only
Carefully follow the step by step directions provided in the package to prepare Levulan® Kerastick® for application. Please note the locations of the crush points "A" and "B" which are indicated by the red diamonds "♦" on each Kerastick®.
CAT. NO.:
LOT NO.:
EXP. DATE:
DUSA Pharmaceuticals, Inc.®
25 Upton Dr.
Wilmington, MA 01887
1-877-533-3872 or
1-978-657-7500
US Patents: 5,079,262 5,954,703
LAB-1462AW REV. A
Each applicator contains:
Active Ingredient: 354mg aminolevulinic acid HCl
Inactive Ingredients: Alcohol, USP (ethanol content-48% v/v), water, laureth-4, isopropyl alcohol, and polyethylene glycol.
Usual Dose: For indications, dosage, precautions, etc., see enclosed package insert.Store between 20°25°C (68°-77°F); excursions permitted to 15°-30°C (59°-86°F)
[See USP Controlled Room Temperature]
Use and discard within two hours after activiation.
Ingredients and Appearance
{{#ask: Label Page::D-Aminolevulinic acid |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
LEVULAN KERASTICK Photodynamic Therapy for Actinic Keratoses
- The first step in LEVULAN KERASTICK Photodynamic Therapy (PDT) for actinic keratoses is application of the LEVULAN KERASTICK Topical Solution to actinic keratoses located on the patient’s face or scalp.
- After LEVULAN KERASTICK Topical Solution is applied to the actinic keratoses in the doctor’s office, the patient will be told to return the next day. During this time the actinic keratoses will become sensitive to light (photosensitive). Care should be taken to keep the treated actinic keratoses dry and out of bright light. After LEVULAN KERASTICK Topical Solution is applied, it is important for the patient to wear light-protective clothing, such as a wide-brimmed hat, when exposed to sunlight or sources of light.
- Fourteen to eighteen hours after application of LEVULAN KERASTICK Topical Solution the patient will return to the doctor’s office to receive blue light treatment, which is the second and final step in the treatment. Prior to blue light treatment, the actinic keratoses will be rinsed with tap water. The patient will be given goggles to wear as eye protection during the blue light treatment.
The blue light is of low intensity and will not heat the skin. However, during the light treatment, which lasts for approximately 17 minutes, the patient will experience sensations of tingling, stinging, prickling or burning of the treated lesions. These feelings of discomfort should improve at the end of the light treatment.
- Following treatment, the actinic keratoses and, to some degree, the surrounding skin, will redden, and swelling and scaling may also occur. However, these lesion changes are temporary and should completely resolve by 4 weeks after treatment.
Precautions with Alcohol
- Alcohol-D-Aminolevulinic acid interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Levulan Kerastick®[3]
Look-Alike Drug Names
There is limited information regarding D-Aminolevulinic acid Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Hürlimann AF, Hänggi G, Panizzon RG (1998). "Photodynamic therapy of superficial basal cell carcinomas using topical 5-aminolevulinic acid in a nanocolloid lotion". Dermatology. 197 (3): 248–54. PMID 9812030.
- ↑ Peng Q, Warloe T, Berg K, Moan J, Kongshaug M, Giercksky KE; et al. (1997). "5-Aminolevulinic acid-based photodynamic therapy. Clinical research and future challenges". Cancer. 79 (12): 2282–308. PMID 9191516.
- ↑ "Aminolevulinic acid".