Sulfonylurea
|
WikiDoc Resources for Sulfonylurea |
|
Articles |
|---|
|
Most recent articles on Sulfonylurea Most cited articles on Sulfonylurea |
|
Media |
|
Powerpoint slides on Sulfonylurea |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Sulfonylurea at Clinical Trials.gov Clinical Trials on Sulfonylurea at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Sulfonylurea
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Sulfonylurea Discussion groups on Sulfonylurea Patient Handouts on Sulfonylurea Directions to Hospitals Treating Sulfonylurea Risk calculators and risk factors for Sulfonylurea
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Sulfonylurea |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
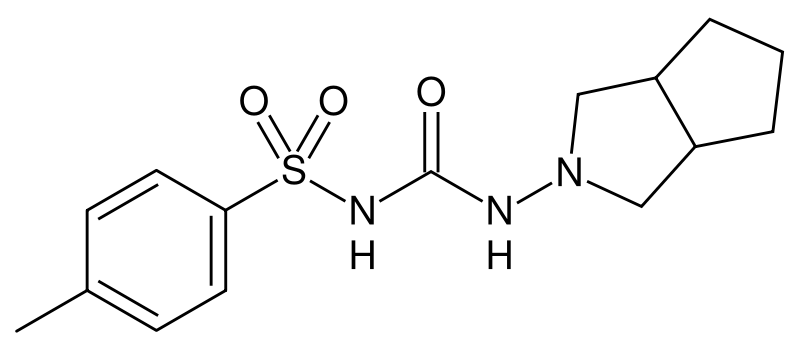
Sulfonylurea derivatives are a class of antidiabetic drugs that are used in the management of diabetes mellitus type 2 ("adult-onset"). They act by increasing insulin release from the beta cells in the pancreas.
Drugs in this class
First generation:
Second generation:
- Glipizide
- Gliclazide
- Glibenclamide (glyburide)
- Gliquidone
Third generation:
Chemistry
All sulfonylureas have a central phenyl ring with two branching chains
Pharmacology
Mechanism of action
Sulfonylureas bind to an ATP-dependent K+ (KATP) channel on the cell membrane of pancreatic beta cells. This inhibits a tonic, hyperpolarizing outflux of potassium, which causes the electric potential over the membrane to become more positive. This depolarization opens voltage-gated Ca2+ channels. The rise in intracellular calcium leads to increased fusion of insulin granulae with the cell membrane, and therefore increased secretion of (pro)insulin.
There is some evidence that sulfonylureas also sensitize β-cells to glucose, that they limit glucose production in the liver, that they decrease lipolysis (breakdown and release of fatty acids by adipose tissue) and decrease clearance of insulin by the liver.
The KATP channel in turn is a complex of the inward-rectifier potassium ion channel Kir6.2 and sulfonylurea receptor SUR1 which associate with a stoichiometry of Kir6.24/SUR14.
Pharmacokinetics
Various sulfonylureas have different pharmacokinetics. The choice depends on the propensity of the patient to develop hypoglycemia - long-acting sulfonylureas with active metabolites can induce hypoglycemia. They can, however, help achieve glycemic control when tolerated by the patient. The shorter-acting agents may not control blood sugar levels adequately.
Due to varying half-life, some drugs have to be taken twice (e.g. tolbutamide) or three times a day rather than once (e.g. glimepiride). The short-acting agents may have to be taken about 30 minutes before the meal, to ascertain maximum efficacy when the food leads to increased blood glucose levels.
Some sulfonylureas are metabolised by liver metabolic enzymes (cytochrome P450) and inducers of this enzyme system (such as the antibiotic rifampicin) can therefore increase the clearance of sulfonylureas. In addition, because some sulfonylureas are bound to plasma proteins, use of drugs that also bind to plasma proteins can release the sulfonylureas from their binding places, leading to increased clearance.
Uses
Sulfonylureas are used almost exclusively in diabetes mellitus type 2. Sulfonylureas are ineffective where there is absolute deficiency of insulin production such as in type 1 diabetes or post-pancreatectomy.
Although for many years sulfonylureas were the first drugs to be used in new cases of diabetes, in the 1990s it was discovered that obese patients might benefit more from metformin.
In about 10% of patients, sulfonylureas alone are ineffective in controlling blood glucose levels. Addition of metformin or a thiazolidinedione may be necessary, or (ultimately) insulin. Triple therapy of sulfonylureas, a biguanide (metformin) and a thiazolidinedione is generally discouraged, but some doctors prefer this combination over resorting to insulin.
Side-effects and cautions
Sulfonylureas, as opposed to metformin and the thiazolidinediones, can induce hypoglycemia when insulin production overshoots. It is treated with sugary food, or (in the case of hypoglycemic coma) with intravenous dextrose. The best way to prevent this side-effect is to choose the lowest possible dose that adequately controls glucose levels.
Like insulin, sulfonylureas can induce weight gain, mainly as a result of edema and reduction of the osmotic diuresis caused by hyperglycemia. Other side-effects are: abdominal upset, headache and hypersensitivity reactions.
Sulfonylureas are potentially teratogenic and cannot be used in pregnancy or in patients who intend to get pregnant. Impairment of liver or kidney function increase the risk of hypoglycemia, and are contraindications. As other anti-diabetic drugs cannot be used either under these circumstances, insulin therapy is the only option in pregnancy and hepatic and renal failure.
Second-generation sulfonylureas have increased potency by weight, compared to first-generation sulfonylureas. They have decreased side effects but are more expensive.
History
Sulfonylureas were discovered by the chemist Marcel Janbon and co-workers,[1] who were studying sulfonamide antibiotics and discovered that the compound sulfonylurea induced hypoglycemia in animals.[2]