Bosutinib
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Aparna Vuppala, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Bosutinib is an antineoplastic agent that is FDA approved for the treatment of adult patients with chronic, accelerated, or blast phase Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) with resistance or intolerance to prior therapy. Common adverse reactions include diarrhea , nausea, thrombocytopenia, vomiting, abdominal pain, rash, anemia, pyrexia, fatigue and Serious adverse reactions include anaphylactic shock , myelosuppression, gastrointestinal toxicity (diarrhea), fluid retention, hepatotoxicity and rash..
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Chronic myelogenous leukemia
- Bosutinib is indicated for the treatment of adult patients with chronic, accelerated, or blast phase Philadelphia chromosome-positive (Ph+) chronic myelogenous leukemia (CML) with resistance or intolerance to prior therapy.
Recommended Dosing
- The recommended dose and schedule of Bosutinib is 500 mg orally once daily with food. Continue treatment with Bosutinib until disease progression or patient intolerance.
- If a dose is missed beyond 12 hours, the patient should skip the dose and take the usual prescribed dose on the following day.
Dose Escalation
- Consider dose escalation to 600 mg once daily with food in patients who do not reach complete hematological response (CHR) by week 8 or a complete cytogenetic response (CCyR) by week 12, who did not have Grade 3 or higher adverse reactions, and who are currently taking 500 mg daily.
Dose Adjustments for Non-Hematologic Adverse Reactions
- Elevated liver transaminases: If elevations in liver transaminases greater than 5×institutional upper limit of normal (ULN) occur, withhold Bosutinib until recovery to less than or equal to 2.5×ULN and resume at 400 mg once daily thereafter. If recovery takes longer than 4 weeks, discontinue Bosutinib. If transaminase elevations greater than or equal to 3×ULN occur concurrently with bilirubin elevations greater than 2×ULN and alkaline phosphatase less than 2×ULN (Hy's law case definition), discontinue Bosutinib.
- Diarrhea: For NCI CTCAE Grade 3-4 diarrhea (increase of greater than or equal to 7 stools/day over baseline/pretreatment), withhold Bosutinib until recovery to Grade less than or equal to 1. Bosutinib may be resumed at 400 mg once daily.
- For other clinically significant, moderate or severe non-hematological toxicity, withhold Bosutinib until the toxicity has resolved, then consider resuming Bosutinib at 400 mg once daily. If clinically appropriate, consider re-escalating the dose of Bosutinib to 500 mg once daily.
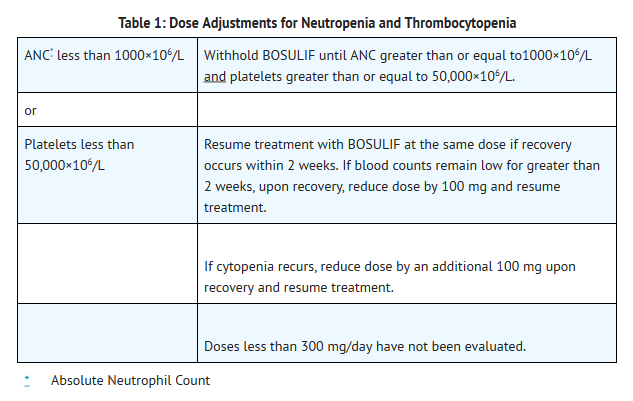
Dose Adjustments for Myelosuppression
- Dose reductions for severe or persistent neutropenia and thrombocytopenia are described below (Table 1).
Concomitant Use With CYP3A Inhibitors
- Avoid the concomitant use of strong or moderate CYP3A and/or P-gp inhibitors with Bosutinib as an increase in bosutinib plasma concentration is expected (strong CYP3A inhibitors include ritonavir, indinavir, nelfinavir, saquinavir, ketoconazole, boceprevir, telaprevir, itraconazole, voriconazole, posaconazole, clarithromycin, telithromycin, nefazodone and conivaptan. Moderate CYP3A inhibitors include fluconazole, darunavir, erythromycin, diltiazem, atazanavir, aprepitant, amprenavir, fosamprevir, crizotinib, imatinib, verapamil, grapefruit products and ciprofloxacin).
Concomitant Use With CYP3A Inducers
- Avoid the concomitant use of strong or moderate CYP3A inducers with Bosutinib as a large reduction in exposure is expected (strong CYP3A inducers include rifampin, phenytoin, carbamazepine, St. John's Wort, rifabutin and phenobarbital. Moderate CYP3A inducers include bosentan, nafcillin, efavirenz, modafinil and etravirine).
Hepatic Impairment
- In patients with pre-existing mild, moderate, and severe hepatic impairment, the recommended dose of Bosutinib is 200 mg daily. A daily dose of 200 mg in patients with hepatic impairment is predicted to result in an area under the concentration curve (AUC) similar to the AUC seen in patients with normal hepatic function receiving 500 mg daily. However, there are no clinical data for efficacy at the dose of 200 mg once daily in patients with hepatic impairment and CML.
Renal Impairment
- In patients with pre-existing severe renal impairment (CLcr less than 30 mL/min), the recommended dose of Bosutinib is 300 mg daily. A daily dose of 300 mg in patients with severe renal impairment is predicted to result in an area under the concentration curve (AUC) similar to the AUC seen in patients with normal renal function receiving 500 mg daily. However, there are no clinical data for efficacy at the dose of 300 mg once daily in patients with severe renal impairment and CML.
Off-Label Use and Dosage (Adult)
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Bosutinib in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
The safety and efficacy of Bosutinib in patients less than 18 years of age have not been established.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
The safety and efficacy of Bosutinib in patients less than 18 years of age have not been established.
Non–Guideline-Supported Use
The safety and efficacy of Bosutinib in patients less than 18 years of age have not been established.
Contraindications
- Hypersensitivity to Bosutinib. In the Bosutinib clinical trials, anaphylactic shock occurred in less than 0.2% of treated patients.
Warnings
Gastrointestinal Toxicity
- Diarrhea, nausea, vomiting, and abdominal pain occur with Bosutinib treatment. Monitor and manage patients using standards of care, including antidiarrheals, antiemetics, and/or fluid replacement. In the single-arm Phase 1/2 clinical trial, the median time to onset for diarrhea (all grades) was 2 days and the median duration per event was 1 day. Among the patients who experienced diarrhea, the median number of episodes of diarrhea per patient during treatment with Bosutinib was 3 (range 1–221). To manage gastrointestinal toxicity, withhold, dose reduce, or discontinue Bosutinib as necessary.
Myelosuppression
- Thrombocytopenia, anemia and neutropenia occur with Bosutinib treatment. Patients with CML who are receiving Bosutinib should have a complete blood count performed weekly for the first month and then monthly thereafter, or as clinically indicated. To manage myelosuppression, withhold, dose reduce, or discontinue Bosutinib as necessary.
Hepatic Toxicity
- One case consistent with drug induced liver injury (defined as concurrent elevations in ALT or AST greater than or equal to 3×ULN with total bilirubin greater than 2×ULN and alkaline phosphatase less than 2×ULN) occurred in a trial of Bosutinib in combination with letrozole. The patient recovered fully following discontinuation of Bosutinib. This case represented 1 out of 1209 patients in Bosutinib clinical trials.
- In the 546 patients from the safety population, the incidence of ALT elevation was 17% and AST elevation was 14 %. Twenty percent of the patients experienced an increase in either ALT or AST. Most cases of transaminase elevations occurred early in treatment; of patients who experienced transaminase elevations of any grade, more than 80% experienced their first event within the first 3 months. The median time to onset of increased ALT and AST was 30 and 33 days, respectively, and the median duration for each was 21 days.
- Perform monthly hepatic enzyme tests for the first three months of treatment with Bosutinib and as clinically indicated. In patients with transaminase elevations, monitor liver enzymes more frequently. Withhold, dose reduce, or discontinue Bosutinib as necessary.
Fluid Retention
Fluid retention occurs with Bosutinib and may manifest as pericardial effusion, pleural effusion, pulmonary edema, and/or peripheral edema.
- In the single-arm Phase 1/2 clinical trial in 546 patients with CML treated with prior therapy, severe fluid retention was reported in 14 patients (3%). Specifically, 9 patients had a Grade 3 or 4 pleural effusion, 3 patients experienced both Grade 3 or Grade 4 pleural and pericardial effusions, 1 patient experienced Grade 3 peripheral and pulmonary edema, and 1 patient had a Grade 3 edema.
- Monitor and manage patients using standards of care. Interrupt, dose reduce or discontinue Bosutinib as necessary.
Adverse Reactions
Clinical Trials Experience
- The following adverse reactions are discussed in greater detail in other sections of the labeling:
- Gastrointestinal toxicity.
- Myelosuppression.
- Hepatic toxicity.
- Fluid retention.
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
- Serious adverse reactions reported include anaphylactic shock, myelosuppression, gastrointestinal toxicity (diarrhea), fluid retention, hepatotoxicity and rash.
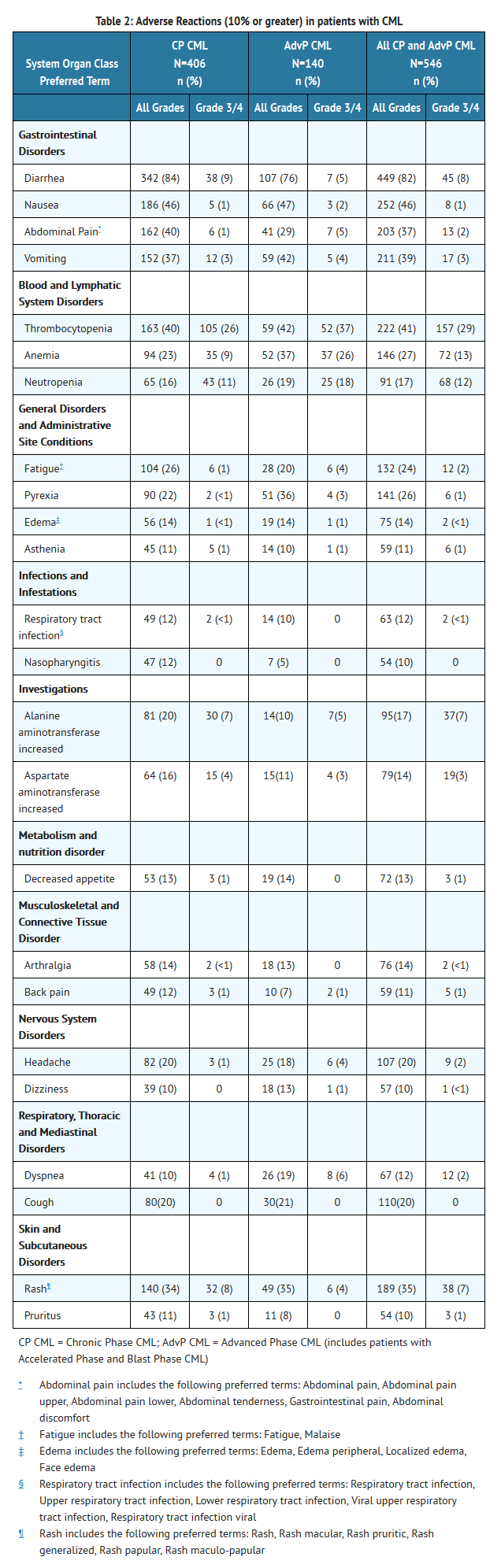
- Adverse reactions of any toxicity grade reported for greater than 20% of patients in the Phase 1/2 safety population (n=546) were diarrhea (82%), nausea (46%), thrombocytopenia (41%), vomiting (39%), abdominal pain (37%), rash (35%), anemia (27%), pyrexia (26%), and fatigue (24%).
Imatinib-Resistant or -Intolerant Ph+ Chronic Phase (CP), Accelerated Phase (AP), and Blast Phase (BP) CML
- The single-arm Phase 1/2 clinical trial enrolled patients with Ph+ chronic, accelerated, or blast phase chronic myelogenous leukemia (CML) and Ph+ acute lymphoblastic leukemia (ALL) with resistance or intolerance to prior therapy. The safety population (received at least 1 dose of Bosutinib) included 546 CML patients. Within the safety population there were 287 patients with CP CML previously treated with imatinib only who had a median duration of Bosutinib treatment of 24 months, and a median dose intensity of 484 mg/day. There were 119 patients with CP CML previously treated with both imatinib and at least 1 additional TKI who had a median duration of Bosutinib treatment of 9 months and a median dose intensity of 475 mg/day. There were 76 patients with AP CML, and 64 patients with BP CML. In the patients with AP CML and BP CML, the median duration of Bosutinib treatment was 10 months and 3 months, respectively. The median dose intensity was 483 mg/day, and 500 mg/day, in the AP CML and BP CML cohorts, respectively.
- Table 2 identifies adverse reactions greater than or equal to 10% for all grades and grades 3 or 4 for the Phase 1/2 CML safety population.
- In the single-arm Phase 1/2 clinical trial, one patient (0.2%) experienced QTcF interval of greater than 500 ms. Patients with uncontrolled or significant cardiovascular disease including QT interval prolongation were excluded by protocol.
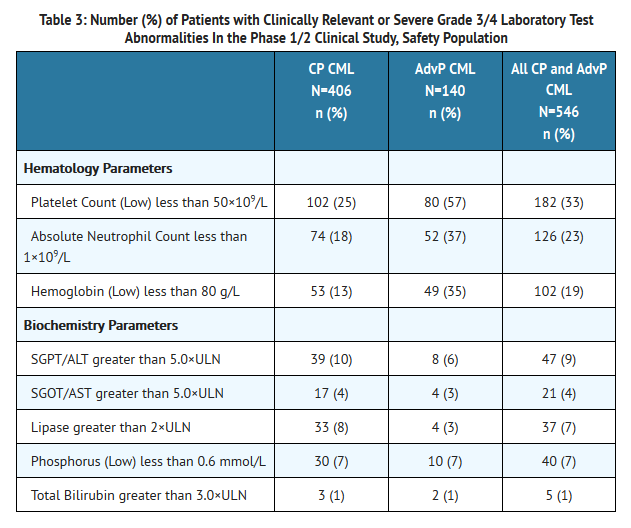
- Table 3 identifies the clinically relevant or severe Grade 3/4 laboratory test abnormalities for the Phase 1/2 CML safety population.
Additional Data from Multiple Clinical Trials
- The following adverse reactions were reported in patients in clinical trials with Bosutinib (less than 10% of Bosutinib-treated patients). They represent an evaluation of the adverse reaction data from 870 patients with Ph+ leukemia who received at least 1 dose of single-agent Bosutinib. These adverse reactions are presented by system organ class and are ranked by frequency. These adverse reactions are included based on clinical relevance and ranked in order of decreasing seriousness within each category.
Blood and Lymphatic System Disorders
- 1% and less than 10% - febrile neutropenia
Cardiac Disorders
- 1% and less than 10% - pericardial effusion; 0.1% and less than 1% - pericarditis
Ear and Labyrinth Disorders
- 1% and less than 10% - tinnitus
Gastrointestinal Disorders
- 1% and less than 10% - gastritis; 0.1% and less than 1% - acute pancreatitis, gastrointestinal hemorrhage
General Disorders and Administrative Site Conditions
- 1% and less than 10% - chest pain, pain
Hepatobiliary Disorders
- 1% and less than 10% - hepatotoxicity, abnormal hepatic function; 0.1% and less than 1% - liver injury
Immune System Disorders
- 1% and less than 10% - drug hypersensitivity; 0.1% and less than 1% - anaphylactic shock
Infections and Infestations
- 1% and less than 10% - pneumonia, influenza, bronchitis
Investigations
- 1% and less than 10% - electrocardiogram QT prolonged, increased blood creatine phosphokinase, increased blood creatinine
Metabolism and Nutrition Disorder
- 1% and less than 10% - hyperkalemia, dehydration
Musculoskeletal and Connective Tissue Disorder
- 1% and less than 10% - myalgia
Nervous System Disorders
- 1% and less than 10% - dysgeusia
Renal and Urinary Disorders
- 1% and less than 10% - acute renal failure, renal failure
Respiratory, Thoracic and Mediastinal Disorders
- 1% and less than 10% - pleural effusion; 0.1% and less than 1% - acute pulmonary edema, respiratory failure, pulmonary hypertension.
Skin and Subcutaneous Disorders
- 1% and less than 10% - urticaria, pruritus, acne; 0.1% and less than 1% - erythema multiforme, exfoliative rash, drug eruption
- Gastrointestinal hemorrhage includes the following preferred terms: gastrointestinal hemorrhage, gastric hemorrhage, upper gastrointestinal hemorrhage
- Chest pain includes the following preferred terms: chest pain, chest discomfort *Hepatotoxicity includes the following preferred terms: hepatotoxicity, toxic hepatitis, cytolytic hepatitis
- Abnormal hepatic function includes the following preferred terms: abnormal hepatic function, liver disorder 5 Pneumonia includes the following preferred terms: pneumonia, bronchopneumonia, lobar pneumonia, primary atypical pneumonia
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Bosutinib in the drug label.
Drug Interactions
Drugs That May Increase Bosutinib Plasma Concentrations
- CYP3A or P-glycoprotein (P-gp) inhibitors: Avoid the concomitant use of strong or moderate CYP3A and/or P-gp inhibitors with Bosutinib as an increase in bosutinib plasma concentration is expected. In a dedicated cross-over drug-interaction trial in healthy volunteers (N=24), concomitant ketoconazole (strong CYP3A inhibitor) increased bosutinib Cmax 5.2-fold and AUC 8.6-fold compared to Bosutinib alone.
Drugs That May Decrease Bosutinib Plasma Concentrations
- CYP3A Inducers: Avoid the concomitant use of strong or moderate CYP3A inducers with Bosutinib as a large reduction in exposure is expected. In a dedicated cross-over drug-interaction trial in healthy volunteers (N=24), concomitant rifampin (strong CYP3A inducer) decreased bosutinib Cmax by 86% and AUC by 94% compared to Bosutinib alone .
- Proton Pump Inhibitors: In a dedicated cross-over drug-interaction trial in healthy volunteers (N=24), concomitant lansoprazole (PPI) decreased bosutinib Cmax by 46% and AUC by 26% compared to Bosutinib alone .
- Consider using short-acting antacids or H2 blockers instead of PPIs to avoid a reduction in bosutinib exposure. Separate antacid or H2 blocker dosing and Bosutinib dosing by more than 2 hours.
Drugs That May Have Their Plasma Concentrations Altered By Bosutinib
- Substrates of P-glycoprotein: An in vitro study suggests that Bosutinib may have the potential to increase the plasma concentrations of drugs that are P-gp substrates, such as digoxin.
Use in Specific Populations
Pregnancy
- Based on its mechanism of action and findings in animals, Bosutinib can cause fetal harm when administered to a pregnant woman. Studies in animals showed reproductive toxicities. If Bosutinib is used during pregnancy, or if the patient becomes pregnant while taking Bosutinib, the patient should be apprised of the potential hazard to the fetus.
- Fetal exposure to bosutinib-derived radioactivity during pregnancy was demonstrated in a placental-transfer study in pregnant rats. Bosutinib was administered orally to pregnant rats during the period of organogenesis at doses of 1, 3 and 10 mg/kg/day. This study did not expose pregnant rats to enough bosutinib to fully evaluate adverse outcomes.
- In a study conducted in rabbits, bosutinib was administered orally to pregnant animals during the period of organogenesis at doses of 3, 10 and 30 mg/kg/day. At the maternally-toxic dose of 30 mg/kg/day of bosutinib, there were fetal anomalies (fused sternebrae, and two fetuses had various visceral observations), and an approximate 6% decrease in fetal body weight. The dose of 30 mg/kg/day resulted in exposures (AUC) approximately 4 times those in humans at the 500 mg/day dose of bosutinib.
Embryofetal Toxicity
- There are no adequate and well controlled studies of Bosutinib in pregnant women. Bosutinib can cause fetal harm when administered to a pregnant woman. Bosutinib caused embryofetal toxicities in rabbits at maternal exposures that were greater than the clinical exposure at the recommended bosutinib dose of 500 mg/day. Females of reproductive potential should be advised to avoid pregnancy while being treated with Bosutinib. If this drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard to the fetus'
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Bosutinib in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Bosutinib during labor and delivery.
Nursing Mothers
- It is not known whether bosutinib is excreted in human milk. Bosutinib and/or its metabolites were excreted in the milk of lactating rats. Radioactivity was present in the plasma of suckling offspring 24 to 48 hours after lactating rats received a single oral dose of radioactive bosutinib. Because many drugs are excreted in human milk and because of the potential for serious adverse reactions in nursing infants from Bosutinib, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
The safety and efficacy of Bosutinib in patients less than 18 years of age have not been established..
Geriatic Use
- In the Phase 1/2 clinical trial of Bosutinib in patients with Ph+ CML, 20% were age 65 and over, 4% were 75 and over. No overall differences in safety or effectiveness were observed between these patients and younger patients, and other reported clinical experience has not identified differences in responses between the elderly and younger patients, but greater sensitivity of some older individuals cannot be ruled out.
Gender
There is no FDA guidance on the use of Bosutinib with respect to specific gender populations.
Race
There is no FDA guidance on the use of Bosutinib with respect to specific racial populations.
Renal Impairment
- Reduce the Bosutinib dose in patients with CLcr less than 30 mL/min at baseline. For patients with CLcr 30 to 50 mL who cannot tolerate a 500 mg dose, follow dose adjustment recommendations for toxicity. In a dedicated renal impairment trial, compared to volunteers with normal renal function, the exposure (AUC) of bosutinib increased by 60% and 35% in subjects with CLcr less than 30 mL/min and CLcr 30 to 50 mL/min, respectively.
- Bosutinib has not been studied in patients undergoing hemodialysis.
Hepatic Impairment
- Treat with a dose of 200 mg daily in patients with any baseline hepatic impairment. In a dedicated hepatic impairment trial, the exposure to bosutinib increased (Cmax increased 1.5- to 2.3-fold and the AUC increased 1.9- to 2.4-fold) in patients with hepatic impairment (Child-Pugh classes A, B, and C; N=18) compared to matched healthy volunteers (N=9)
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Bosutinib in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Bosutinib in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
- Perform monthly hepatic enzyme tests for the first three months of treatment with Bosutinib and as clinically indicated. In patients with transaminase elevations, monitor liver enzymes more frequently. Withhold, dose reduce, or discontinue Bosutinib as necessary
IV Compatibility
There is limited information regarding IV Compatibility of Bosutinib in the drug label.
Overdosage
- Experience with Bosutinib overdose in clinical studies was limited to isolated cases. There were no reports of any serious adverse events associated with the overdoses. Patients who take an overdose of Bosutinib should be observed and given appropriate supportive treatment.
Pharmacology
There is limited information regarding Bosutinib Pharmacology in the drug label.
Mechanism of Action
- Bosutinib is a tyrosine kinase inhibitor. Bosutinib inhibits the Bcr-Abl kinase that promotes CML; it is also an inhibitor of Src-family kinases including Src, Lyn, and Hck. Bosutinib inhibited 16 of 18 imatinib-resistant forms of Bcr-Abl expressed in murine myeloid cell lines. Bosutinib did not inhibit the T315I and V299L mutant cells. In mice, treatment with bosutinib reduced the size of CML tumors relative to controls and inhibited growth of murine myeloid tumors expressing several imatinib-resistant forms of Bcr-Abl.
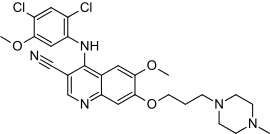
Structure
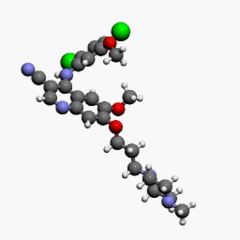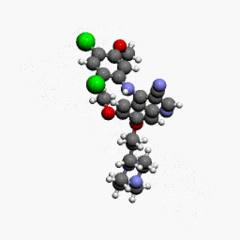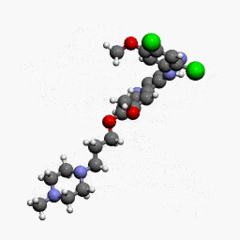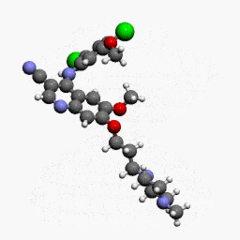
- Bosutinib is a kinase inhibitor. The chemical name for bosutinib monohydrate is 3-Quinolinecarbonitrile, 4-[(2,4-dichloro-5-methoxyphenyl)amino]-6-methoxy-7-[3-(4-methyl-1-piperazinyl) propoxy]-, hydrate (1:1). Its chemical formula is C26H29Cl2N5O3•H2O (monohydrate); its molecular weight is 548.46 (monohydrate), equivalent to 530.46 (anhydrous). Bosutinib monohydrate has the following chemical structure:

- Bosutinib monohydrate is a white to yellowish-tan powder. Bosutinib monohydrate has a pH dependent solubility across the physiological pH range. At or below pH 5, bosutinib monohydrate behaves as a highly soluble compound. Above pH 5, the solubility of bosutinib monohydrate reduces rapidly.
- Bosutinib® (bosutinib) tablets are supplied for oral administration in two strengths: a 100 mg yellow, oval, biconvex, film-coated tablet debossed with "Pfizer" on one side and "100" on the other; and a 500 mg red, oval, biconvex, film-coated tablet debossed with "Pfizer" on one side and "500" on the other.
- Each 100 mg Bosutinib tablet contains 103.40 mg of bosutinib monohydrate, equivalent to 100 mg of bosutinib; each 500 mg Bosutinib tablet contains 516.98 mg of bosutinib monohydrate, equivalent to 500 mg of bosutinib. The following inactive ingredients are included in the tablets: microcrystalline cellulose, croscarmellose sodium, poloxamer, povidone, magnesium stearate, polyvinyl alcohol, titanium dioxide, polyethylene glycol, talc, and iron oxide yellow (for 100 mg tablet) and iron oxide red (for 500 mg tablet).
Pharmacodynamics
- The effect of a single dose of bosutinib 500 mg alone and with ketoconazole on the QTc interval was evaluated in a randomized, placebo- and active-controlled (moxifloxacin 400 mg) two or three-period crossover thorough QT study in 60 healthy subjects. No significant changes in placebo adjusted, baseline-corrected QTc were observed.
Pharmacokinetics
Absorption
- Following administration of a single dose of Bosutinib 500 mg with food in patients with cancer, the median time-to-peak concentration (tmax) was 4–6 hours. Bosutinib exhibits dose proportional increases in AUC and Cmax, over the dose range of 200 to 800 mg. After 15 daily doses of Bosutinib (500 mg) with food in patients with CML, the mean (SD) Cmax value was 200 (12) ng/mL, and the mean (SD) AUC was 3650 (425) ng∙h/mL. When given with a high fat meal, the Cmax and AUC of bosutinib increased 1.8- and 1.7-fold, respectively.
Distribution
- After administration of a single dose of Bosutinib 500 mg with food in patients with CML, bosutinib had a mean apparent volume of distribution ± standard deviation of 6080 ± 1230 L.
- Bosutinib was highly bound to human plasma proteins in vitro (94%) and ex vivo in healthy subjects (96%), and binding was not concentration-dependent. Bosutinib is a P-gp substrate and inhibitor in vitro. No studies have been conducted with other transporters.
Metabolism
- Bosutinib is primarily metabolized by CYP3A4. The major circulating metabolites identified in plasma are oxydechlorinated (M2) bosutinib (19% of parent exposure) and N-desmethylated (M5) bosutinib (25% of parent exposure), with bosutinib N-oxide (M6) as a minor circulating metabolite. All the metabolites were deemed inactive.
Elimination
- In patients with CML given single oral doses of Bosutinib 500 mg with food, the mean terminal phase elimination half-life (t1/2) was 22.5 (1.7) hours, and the mean (SD) clearance (Cl/F) was 189 (48) L/h. In six healthy male subjects given a single oral dose of [14C] radiolabeled bosutinib, 91.3% of the dose was recovered in feces and 3% of the dose recovered in urine.
Hepatic Impairment
- In a dedicated hepatic impairment trial, a single dose of Bosutinib 200 mg was administered with food to 18 volunteers with hepatic impairment (Child-Pugh classes A, B, and C) and 9 matched healthy volunteers. Cmax of bosutinib increased 2.4-fold, 2-fold, and 1.5-fold, respectively, in Child-Pugh classes A, B, and C, and bosutinib AUC increased 2.3-fold, 2-fold, and 1.9-fold, respectively.
Renal Impairment
- In a dedicated renal impairment trial, a single dose of Bosutinib 200 mg was administered with food to 26 volunteers with mild (CLcr: 51 to 80 mL/min), moderate (CLcr: 30 to 50 mL/min) or severe renal impairment (CLcr less than 30 mL/min) and to 8 healthy volunteers with normal renal function. Creatinine Clearance for category classification was calculated by the Cockcroft-Gault formula. Subjects with moderate and severe renal impairment had a 35% and 60% increase in AUC compared to healthy volunteers with normal renal function, respectively. Bosutinib exposure was not changed in patients with mild renal impairment. The Bosutinib dose should be reduced in patients with CLcr less than 30 mL/min and patients with CLcr between 30 to 50 mL/min should have their dose reduced if they are unable to tolerate a 500 mg dose.
Drug Interactions
- CYP3A Inhibitors
- In a cross-over trial of 24 healthy volunteers, a single dose of 100 mg of Bosutinib was either administered alone or in combination with five daily doses of 400 mg of ketoconazole under fasting conditions. Ketoconazole increased bosutinib Cmax and AUC 5.2-fold and 8.6-fold, respectively'
- CYP3A Inducers
- P-gp Substrates
- pH Altering Medications
- Bosutinib displays pH-dependent aqueous solubility, in vitro. In a cross-over trial in 24 healthy volunteers, a single oral dose of 400 mg of Bosutinib was either administered alone or in combination with multiple-oral doses of 60 mg of lansoprazole under fasting conditions. Lansoprazole decreased bosutinib Cmax and AUC by 46% and 26%, respectively.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- A 2-year carcinogenicity study was conducted orally in rats at bosutinib doses up to 25 mg/kg/day in males and 15 mg/kg/day in females. The exposures achieved at the high dose were approximately 1.5- to 3-fold the human exposure (based on AUC) at the bosutinib dose of 500 mg/day. The study was negative for carcinogenic findings.
- Bosutinib was not mutagenic or clastogenic in a battery of tests, including the bacteria reverse mutation assay (Ames Test), the in vitro assay using human peripheral blood lymphocytes and the micronucleus test in orally treated male mice.
- In a rat fertility study, drug-treated males were mated with untreated females, or untreated males were mated with drug-treated females. Females were administered the drug from pre-mating through early embryonic development. The dose of 70 mg/kg/day of bosutinib resulted in reduced fertility in males as demonstrated by 16% reduction in the number of pregnancies. There were no lesions in the male reproductive organs at this dose. This dose of 70 mg/kg/day resulted in exposure (AUC) in male rats approximately equal to that in humans at the 500 mg/day dose of bosutinib. Fertility (number of pregnancies) was not affected when female rats were treated with bosutinib. However, there were increased embryonic resorptions at greater than or equal to 10 mg/kg/day of bosutinib (40% of the human exposure), and decreased implantations and reduced number of viable embryos at 30 mg/kg/day of bosutinib (1.4 times the human exposure).
Clinical Studies
- Imatinib-Resistant or -Intolerant Ph+ Chronic Phase (CP), Accelerated Phase (AP) and Blast Phase (BP) CML
- A single-arm, Phase 1/2 open-label, multicenter trial was conducted to evaluate the efficacy and safety of Bosutinib 500 mg once daily in patients with imatinib-resistant or -intolerant CML with separate cohorts for chronic, accelerated, and blast phase disease previously treated with one prior TKI (imatinib) or more than one TKI (imatinib followed by dasatinib and/or nilotinib). The definition of imatinib resistance included (1) failure to achieve or maintain any hematologic improvement within four weeks; (2) failure to achieve a complete hematologic response (CHR) by 3 months, cytogenetic response by 6 months or major cytogenetic response (MCyR) by 12 months; (3) progression of disease after a previous cytogenetic or hematologic response; or (4) presence of a genetic mutation in the BCR-Abl gene associated with imatinib resistance. Imatinib intolerance was defined as inability to tolerate imatinib due to toxicity, or progression on imatinib and inability to receive a higher dose due to toxicity. The definitions of resistance and intolerance to both dasatinib and nilotinib were similar to those for imatinib. The protocol was amended to exclude patients with a known history of the T315I mutation after 396 patients were enrolled in the trial.
- The efficacy endpoints for patients with CP CML previously treated with one prior TKI (imatinib) were the rate of attaining MCyR at week 24 and the duration of MCyR. The efficacy endpoints for patients with CP CML previously treated with both imatinib and at least 1 additional TKI were the cumulative rate of attaining MCyR by week 24 and the duration of MCyR. The efficacy endpoints for patients with previously treated AP and BP CML were confirmed complete hematologic response (CHR) and overall hematologic response (OHR).
- The trial enrolled 546 patients with CP, AP or BP CML. Of the total patient population 73% were imatinib resistant and 27% were imatinib intolerant. In this trial, 53% of patients were males, 65% were Caucasian, and 20% were 65 years old or older. Of the 546 treated patients, 503 were considered evaluable for efficacy. Patients were evaluable for efficacy if they had received at least one dose of Bosutinib and had a valid baseline efficacy assessment. Among evaluable patients, there were 266 patients with CP CML previously treated with one prior TKI (imatinib), 108 patients with CP CML previously treated with both imatinib and at least 1 additional TKI, and 129 patients with advanced phase CML previously treated with at least one TKI.
- Median duration of Bosutinib treatment was 22 months in patients with CP CML previously treated with one TKI (imatinib), 8 months in patients with CP CML previously treated with imatinib and at least 1 additional TKI, 10 months in patients with AP CML previously treated with at least imatinib, and 3 months in patients with BP CML previously treated with at least imatinib.
- The 24 week efficacy results are present in Table 6.
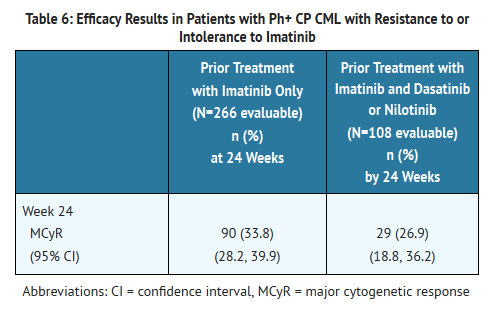
- The minimum follow-up was 23 months for patients with CP CML treated with one prior TKI (imatinib) and 13 months for patients with CP CML treated with imatinib and at least one additional TKI. For the 53.4% of patients with CP CML treated with one prior TKI (imatinib) who achieved a MCyR at any time, the median duration of MCyR was not reached. Among these patients, 52.8% had a MCyR lasting at least 18 months. For the 32.4% of patients with CP CML treated with imatinib and at least one additional TKI who achieved a MCyR at any time, the median duration of MCyR was not reached. Among these patients, 51.4% had a MCyR lasting at least 9 months. Of the 374 evaluable patients with CP CML, 16 patients had confirmed disease transformation to AP or BP while on treatment with Bosutinib.
- The 48 week efficacy results in patients with accelerated and blast phases CML previously treated with at least imatinib are summarized in Table 7.

- The CHR and OHR rates were based on a minimum follow-up of 12 months for patients with AP CML and 18 months for patients with BP CML. Of the 69 evaluable patients with AP CML, 4 patients had confirmed disease transformation to BP while on Bosutinib treatment.
How Supplied
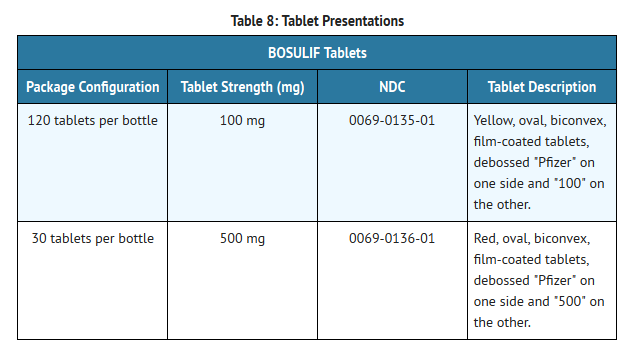
- Bosutinib tablets are supplied for oral administration in two strengths: a 100 mg yellow, oval, biconvex, film-coated tablet debossed with "Pfizer" on one side and "100" on the other; and a 500 mg red, oval, biconvex, film-coated tablet debossed with "Pfizer" on one side and "500" on the other. Bosutinib tablets are available in the following packaging configurations (Table 8)
Storage
- Store at 20°C to 25°C (68°F to 77°F); excursions permitted to 15°C to 30°C (59°F to 86°F) .
Handling and Disposal
- Procedures for proper disposal of anticancer drugs should be considered. Any unused product or waste material should be disposed of in accordance with local requirements, or drug take back programs.
Images
Drug Images
{{#ask: Page Name::Bosutinib |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Bosutinib |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Dosing and Administration
- Instruct patients to take Bosutinib exactly as prescribed, not to change their dose or to stop taking Bosutinib unless they are told to do so by their doctor. If patients miss a dose beyond 12 hours, they should be advised to take the next scheduled dose at its regular time. A double dose should not be taken to make up for any missed dose. Advise patients to take Bosutinib with food. Patients should be advised: "Do not crush or cut tablet. Do not touch or handle crushed or broken tablets."
Gastrointestinal Problems
- Advise patients that they may experience diarrhea, nausea, vomiting, abdominal pain, or blood in their stools with Bosutinib and to seek medical attention promptly for these symptoms.
Low Blood Cell Counts
- Advise patients of the possibility of developing low blood cell counts and to immediately report fever, any suggestion of infection, or signs or symptoms suggestive of bleeding or easy bruising.
Liver Problems
- Advise patients of the possibility of developing liver function abnormalities and to immediately report jaundice.
Fluid Retention
- Advise patients of the possibility of developing fluid retention (swelling, weight gain, or shortness of breath) and to seek medical attention promptly if these symptoms arise.
Other Adverse Reactions
- Advise patients that they may experience other adverse reactions such as respiratory tract infections, rash, fatigue, loss of appetite, headache, dizziness, back pain, arthralgia, or pruritus with Bosutinib and to seek medical attention if symptoms are significant. There is a possibility of anaphylactic shock.
Pregnancy and Breast-feeding
- Advise patients that Bosutinib can cause fetal harm when administered to a pregnant woman. Advise women of the potential hazard to the fetus and to avoid becoming pregnant. If Bosutinib is used during pregnancy, or if the patient becomes pregnant while taking Bosutinib, the patient should be apprised of the potential hazard to the fetus. Because a potential risk to the nursing infant cannot be excluded, women that are taking Bosutinib should not breast-feed or provide breast milk to infants.
- Counsel females of reproductive potential to use effective contraceptive measures to prevent pregnancy during and for at least 30 days after completing treatment with Bosutinib. Instruct patients to contact their physicians immediately if they become pregnant during treatment. Advise patients not to take Bosutinib treatment while pregnant or breastfeeding. If a patient wishes to restart breastfeeding after treatment, advise her to discuss the appropriate timing with her physician.
Drug Interactions
- Advise patients that Bosutinib and certain other medicines, including over the counter medications or herbal supplements (such as St. John's wort) can interact with each other and may alter the effects of Bosutinib
Precautions with Alcohol
- Alcohol-Bosutinib interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- ®
Look-Alike Drug Names
- A® — B®
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Bosutinib
|Pill Name=No image.jpg
|Drug Name=
|Pill Ingred=|+sep=;
|Pill Imprint=
|Pill Dosage={{{dosageValue}}} {{{dosageUnit}}}
|Pill Color=|+sep=;
|Pill Shape=
|Pill Size (mm)=
|Pill Scoring=
|Pill Image=
|Drug Author=
|NDC=
}}
{{#subobject:
|Label Page=Bosutinib |Label Name=Bosutinib08.png
}}
{{#subobject:
|Label Page=Bosutinib |Label Name=Bosutinib09.png
}}