Boceprevir
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alberto Plate [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Boceprevir is an antiviral and protease inhibitor that is FDA approved for the treatment of chronic hepatitis C genotype 1 infection, in combination with peginterferon alfa and ribavirin, in adult patients with compensated liver disease, including cirrhosis, who are previously untreated or who have failed previous interferon and ribavirin therapy, including prior null responders, partial responders, and relapsers. Common adverse reactions include fatigue, anemia, nausea, headache and dysgeusia.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Boceprevir must be administered in combination with peginterferon alfa and ribavirin. The dose of Boceprevir is 800 mg (four 200-mg capsules) three times daily (every 7 to 9 hours) with food (a meal or light snack). Refer to the prescribing information for peginterferon alfa and ribavirin for instructions on dosing.
- The following dosing recommendations differ for some subgroups from the dosing studied in the Phase 3 trials. Response-Guided Therapy (RGT) is recommended for most individuals, but longer dosing is recommended in targeted subgroups (e.g., patients with cirrhosis).
Boceprevir/Peginterferon alfa/Ribavirin Combination Therapy: Patients Without Cirrhosis Who Are Previously Untreated or Who Previously Failed Interferon and Ribavirin Therapy
- Initiate therapy with peginterferon alfa and ribavirin for 4 weeks (Treatment Weeks 1вАУ4).
- Add Boceprevir 800 mg (four 200-mg capsules) orally three times daily (every 7 to 9 hours) to peginterferon alfa and ribavirin regimen after 4 weeks of treatment. Based on the patient's HCV-RNA levels at Treatment Week (TW) 8, TW12 and TW24, use the following guidelines to determine duration of treatment (see TABLE 1).

- Consideration should be given to treating previously untreated patients who are poorly interferon responsive (as determined at TW4) with 4 weeks peginterferon alfa and ribavirin followed by 44 weeks of Boceprevir 800 mg orally three times daily (every 7 to 9 hours) in combination with peginterferon alfa and ribavirin in order to maximize rates of SVR.
Boceprevir/Peginterferon alfa/Ribavirin Combination Therapy: Patients with Cirrhosis
- Prior to initiating therapy in patients with compensated cirrhosis, see use in specific populations for additional information.
- Patients with compensated cirrhosis should receive 4 weeks peginterferon alfa and ribavirin followed by 44 weeks Boceprevir 800 mg (four 200-mg capsules) three times daily (every 7 to 9 hours) in combination with peginterferon alfa and ribavirin.
Dose Modification
- Dose reduction of Boceprevir is not recommended.
- If a patient has a serious adverse reaction potentially related to peginterferon alfa and/or ribavirin, the peginterferon alfa and/or ribavirin dose should be reduced or discontinued. Refer to the prescribing information for peginterferon alfa and ribavirin for additional information about how to reduce and/or discontinue the peginterferon alfa and/or ribavirin dose. Boceprevir must not be administered in the absence of peginterferon alfa and ribavirin. If peginterferon alfa or ribavirin is permanently discontinued, Boceprevir must also be discontinued.
Discontinuation of Dosing Based on Treatment Futility
- Discontinuation of therapy is recommended in all patients with 1) HCV-RNA levels of greater than or equal to 1000 IU per mL at TW8; or 2) HCV-RNA levels of greater than or equal to 100 IU per mL at TW12; or 3) confirmed detectable HCV-RNA levels at TW24.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Boceprevir in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Boceprevir in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Boceprevir FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Boceprevir in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Boceprevir in pediatric patients.
Contraindications
- Contraindications to peginterferon alfa and ribavirin also apply to Boceprevir combination treatment. Refer to the respective prescribing information for a list of the contraindications for peginterferon alfa and ribavirin.
- Boceprevir in combination with peginterferon alfa and ribavirin is contraindicated in:
- Pregnant women and men whose female partners are pregnant because of the risks for birth defects and fetal death associated with ribavirin.
- Patients with a history of a hypersensitivity reaction to boceprevir.
- Coadministration with drugs that are highly dependent on CYP3A4/CYP3A5 for clearance, and for which elevated plasma concentrations are associated with serious and/or life-threatening events, including those in TABLE 2, is contraindicated.
- Coadministration with potent CYP3A4/CYP3A5 inducers, where significantly reduced boceprevir plasma concentrations may be associated with reduced efficacy, including those in TABLE 2, is contraindicated.

Warnings
Embryofetal Toxicity (Use with Ribavirin and Peginterferon Alfa)
- Ribavirin may cause birth defects and/or death of the exposed fetus. Extreme care must be taken to avoid pregnancy in female patients and in female partners of male patients. Ribavirin therapy should not be started unless a report of a negative pregnancy test has been obtained immediately prior to initiation of therapy. Refer to the prescribing information for ribavirin for additional information.
- Women of childbearing potential and men must use at least two forms of effective contraception during treatment and for at least 6 months after treatment has concluded. One of these forms of contraception can be a combined oral contraceptive product containing at least 1 mg of norethindrone. Oral contraceptives containing lower doses of norethindrone and other forms of hormonal contraception have not been studied or are contraindicated. Routine monthly pregnancy tests must be performed during this time.
Anemia (Use with Ribavirin and Peginterferon Alfa)
- Anemia has been reported with peginterferon alfa and ribavirin therapy. The addition of Boceprevir to peginterferon alfa and [ribavirin]] is associated with an additional decrease in hemoglobin concentrations. Complete blood counts (with white blood cell differential counts) should be obtained pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate. If hemoglobin is less than 10 g per dL, a decrease in dosage of ribavirin is recommended; and if hemoglobin is less than 8.5 g per dL, discontinuation of ribavirin is recommended. If ribavirin is permanently discontinued for management of anemia, then peginterferon alfa and Boceprevir must also be discontinued. Refer to the prescribing information for ribavirin for additional information regarding dose reduction and/or discontinuation.
- In clinical trials with Boceprevir the proportion of subjects who experienced hemoglobin values less than 10 g per dL and less than 8.5 g per dL was higher in subjects treated with the combination of Boceprevir with Peginterferon alfa 2a®/Ribavirin® than in those treated with Peginterferon alfa 2a/Ribavirin alone (see TABLE 4). With the interventions used for anemia management in the clinical trials, the average additional decrease of hemoglobin was approximately 1 g per dL.
- In clinical trials, the median time to onset of hemoglobin less than 10 g per dL from the initiation of therapy was similar among subjects treated with the combination of Boceprevir and Peginterferon alfa 2a/Ribavirin (71 days with a range of 15-337 days), compared to those who received Peginterferon alfa 2a/Ribavirin (71 days with a range of 8-337 days). Certain adverse reactions consistent with symptoms of anemia, such as dyspnea, exertional dyspnea, dizziness and syncope were reported more frequently in subjects who received the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin than in those treated with Peginterferon alfa 2a/Ribavirin alone.
- In clinical trials with Boceprevir, dose modifications (generally of Peginterferon alfa 2a/Ribavirin) due to anemia occurred twice as often in subjects treated with the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin (26%) compared to Peginterferon alfa 2a/Ribavirin (13%). The proportion of subjects who discontinued study drug due to anemia was 1% in subjects treated with the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin and 1% in subjects who received Peginterferon alfa 2a/Ribavirin. The use of erythropoiesis stimulating agents (ESAs) was permitted for management of anemia, at the investigator's discretion, with or without ribavirin dose reduction in the Phase 2 and 3 clinical trials. The proportion of subjects who received an ESA was 43% in those treated with the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin compared to 24% in those treated with Peginterferon alfa 2a/Ribavirin alone. The proportion of subjects who received a transfusion for the management of anemia was 3% of subjects treated with the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin compared to less than 1% in subjects who received Peginterferon alfa 2a/Ribavirin alone.
- Thromboembolic events have been associated with ESA use in other disease states; and have also been reported with peginterferon alfa use in hepatitis C patients. Thromboembolic events were reported in clinical trials with Boceprevir among subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin, and among those receiving Peginterferon alfa 2a/Ribavirin alone, regardless of ESA use. No definite causality assessment or benefit risk assessment could be made for these events due to the presence of confounding factors and lack of randomization of ESA use.
- A randomized, parallel-arm, open-label clinical trial was conducted in previously untreated CHC subjects with genotype 1 infection to compare use of an ESA versus ribavirin dose reduction for initial management of anemia during therapy with Boceprevir in combination with peginterferon alfa-2b and ribavirin. Similar SVR rates were reported in subjects who were randomized to receive ribavirin dose reduction compared to subjects who were randomized to receive an ESA. In this trial, use of ESAs was associated with an increased risk of thromboembolic events including pulmonary embolism, acute myocardial infarction, cerebrovascular accident, and deep vein thrombosis compared to ribavirin dose reduction alone. The treatment discontinuation rate due to anemia was similar in subjects randomized to receive ribavirin dose reduction compared to subjects randomized to receive ESA (2% in each group). The transfusion rate was 4% in subjects randomized to receive ribavirin dose reduction and 2% in subjects randomized to receive ESA. Ribavirin dose reduction is recommended for the initial management of anemia.
Neutropenia (Use with Ribavirin and Peginterferon Alfa)
- In Phase 2 and 3 clinical trials, seven percent of subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin had neutrophil counts of less than 0.5 × 109 per L compared to 4% of subjects receiving Peginterferon alfa 2a/Ribavirin alone (see TABLE 4). Three subjects experienced severe or life-threatening infections associated with neutropenia, and two subjects experienced life-threatening neutropenia while receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin. Complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate. Decreases in neutrophil counts may require dose reduction or discontinuation of peginterferon alfa and ribavirin. If peginterferon alfa and ribavirin are permanently discontinued, then Boceprevir must also be discontinued. Refer to the prescribing information for peginterferon alfa and ribavirin for additional information regarding dose reduction or discontinuation.
Pancytopenia (Use with Ribavirin and Peginterferon Alfa)
- Serious cases of pancytopenia have been reported postmarketing in patients receiving Boceprevir in combination with peginterferon alfa and ribavirin. Complete blood counts (with white blood cell differential counts) should be obtained at pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate. Refer to the prescribing information for ribavirin and peginterferon alfa for guidelines for discontinuation of therapy based on laboratory parameters.
Hypersensitivity
- Serious acute hypersensitivity reactions (e.g., urticaria, angioedema) have been observed during combination therapy with Boceprevir, peginterferon alfa and ribavirin. If such an acute reaction occurs, combination therapy should be discontinued and appropriate medical therapy immediately instituted.
Drug Interactions
- See TABLE 2 for a listing of drugs that are contraindicated for use with Boceprevir due to potentially life-threatening adverse events, significant drug interactions or loss of virologic activity. Please refer to TABLE 5 for established and other potentially significant drug interactions.
Laboratory Tests
- HCV-RNA levels should be monitored at Treatment Weeks 4, 8, 12, and 24, at the end of treatment, during treatment follow-up, and for other time points as clinically indicated. Use of a sensitive real-time reverse-transcription polymerase chain reaction (RT-PCR) assay for monitoring HCV-RNA levels during treatment is recommended. The assay should have a lower limit of HCV-RNA quantification of equal to or less than 25 IU per mL, and a limit of HCV-RNA detection of approximately 10 to 15 IU per mL. For the purposes of assessing Response-Guided Therapy milestones, a confirmed "detectable but below limit of quantification" HCV-RNA result should not be considered equivalent to an "undetectable" HCV-RNA result (reported as "Target Not Detected" or "HCV-RNA Not Detected"). Complete blood count (with white blood cell differential counts) should be obtained at pretreatment, and at Treatment Weeks 2, 4, 8, and 12, and should be monitored closely at other time points, as clinically appropriate.
- Refer to the prescribing information for peginterferon alfa and ribavirin for pre-treatment, on-treatment and post-treatment laboratory testing recommendations including hematology, biochemistry (including hepatic function tests), and pregnancy testing requirements.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of Boceprevir cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice.
The following serious and otherwise important adverse drug reactions (ADRs) are discussed in detail in another section of the labeling:
The most commonly reported adverse reactions (more than 35% of subjects regardless of investigator's causality assessment) in adult subjects were fatigue, anemia, nausea, headache, and dysgeusia when Boceprevir was used in combination with Peginterferon alfa 2a and Ribavirin.
- The safety of the combination of Boceprevir 800 mg three times daily with Peginterferon alfa 2a/Ribavirin was assessed in 2095 subjects with chronic hepatitis C in one Phase 2, open-label trial and two Phase 3, randomized, double-blind, placebo-controlled clinical trials. SPRINT-1 (subjects who were previously untreated) evaluated the use of Boceprevir in combination with Peginterferon alfa 2a/Ribavirin with or without a four-week lead-in period with Peginterferon alfa 2a/Ribavirin compared to Peginterferon alfa 2a/Ribavirin alone. SPRINT-2 (subjects who were previously untreated) and RESPOND-2 (subjects who had failed previous therapy) evaluated the use of Boceprevir 800 mg three times daily in combination with Peginterferon alfa 2a/Ribavirin with a four-week lead-in period with Peginterferon alfa 2a/Ribavirin compared to Peginterferon alfa 2a/Ribavirin alone. The population studied had a mean age of 49 years (3% of subjects were older than 65 years of age), 39% were female, 82% were white and 15% were black.
- During the four week lead-in period with Peginterferon alfa 2a/Ribavirin in subjects treated with the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin, 28/1263 (2%) subjects experienced adverse reactions leading to discontinuation of treatment. During the entire course of treatment, the proportion of subjects who discontinued treatment due to adverse reactions was 13% for subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin and 12% for subjects receiving Peginterferon alfa 2a/Ribavirin alone. Events resulting in discontinuation were similar to those seen in previous studies with Peginterferon alfa 2a/Ribavirin. Only anemia and fatigue were reported as events that led to discontinuation in more than 1% of subjects in any arm.
- Adverse reactions that led to dose modifications of any drug (primarily Peginterferon alfa 2a and Ribavirin) occurred in 39% of subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin compared to 24% of subjects receiving Peginterferon alfa 2a/Ribavirin alone. The most common reason for dose reduction was anemia, which occurred more frequently in subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin than in subjects receiving Peginterferon alfa 2a/Ribavirin alone.
- Serious adverse events were reported in 11% of subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin and in 8% of subjects receiving Peginterferon alfa 2a/Ribavirin.
- Adverse events (regardless of investigator's causality assessment) reported in greater than or equal to 10% of subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin and reported at a rate of greater than or equal to 5% than Peginterferon alfa 2a/Ribavirin alone in SPRINT-1, SPRINT-2, and RESPOND-2 are presented in TABLE 3.
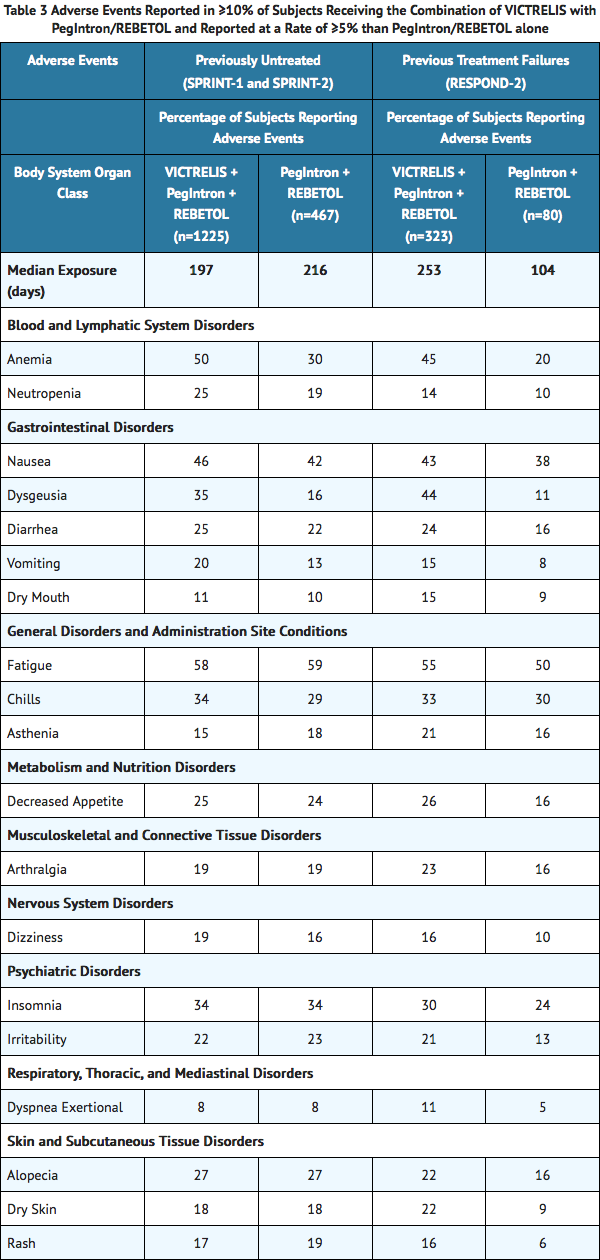
Other Important Adverse Reactions Reported in Clinical Trials
- Among subjects (previously untreated subjects or those who failed previous therapy) who received Boceprevir in combination with peginterferon alfa and ribavirin, the following adverse drug reactions were reported. These events are notable because of their seriousness, severity, or increased frequency in subjects who received Boceprevir in combination with peginterferon alfa and ribavirin compared with subjects who received only peginterferon alfa and ribavirin.
Gastrointestinal Disorders
- Dysgeusia (alteration of taste) was an adverse event reported at an increased frequency in subjects receiving Boceprevir in combination with peginterferon alfa and ribavirin compared with subjects receiving peginterferon alfa and ribavirin alone. Adverse events such as dry mouth, nausea, vomiting and diarrhea were also reported at an increased frequency in subjects receiving Boceprevir in combination with peginterferon alfa and ribavirin.
Laboratory Values
- Changes in selected hematological parameters during treatment of adult subjects with the combination of Boceprevir with Peginterferon alfa 2a and Ribavirin are described in TABLE 4.
Hemoglobin
- Decreases in hemoglobin may require a decrease in dosage or discontinuation of ribavirin. If ribavirin is permanently discontinued, then peginterferon alfa and Boceprevir must also be discontinued.
Neutrophils and Platelets
- The proportion of subjects with decreased neutrophil and platelet counts was higher in subjects treated with Boceprevir in combination with Peginterferon alfa 2a/Ribavirin compared to subjects receiving Peginterferon alfa 2a/Ribavirin alone. Three percent of subjects receiving the combination of Boceprevir with Peginterferon alfa 2a/Ribavirin had platelet counts of less than 50 × 109 per L compared to 1% of subjects receiving Peginterferon alfa 2a/Ribavirin alone. Decreases in neutrophils or platelets may require a decrease in dosage or interruption of peginterferon alfa, or discontinuation of therapy. If peginterferon alfa is permanently discontinued, then ribavirin and Boceprevir must also be discontinued.
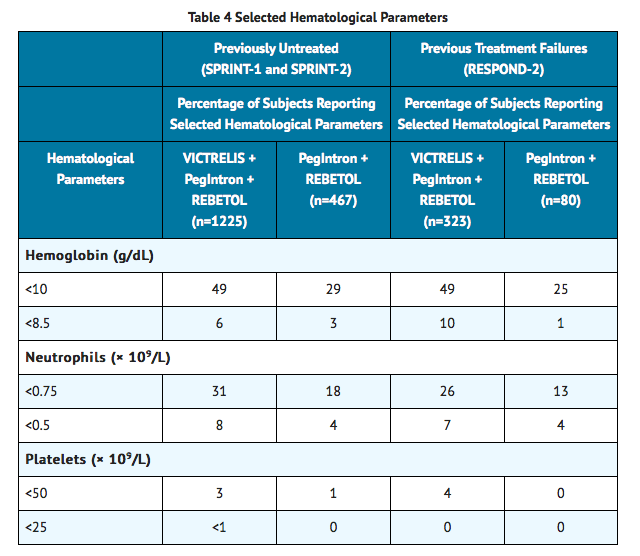
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of Boceprevir in combination with peginterferon alfa and ribavirin. Because these reactions are reported voluntarily from a population of uncertain size, it is not always possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Blood and Lymphatic System Disorders: agranulocytosis, pancytopenia, thrombocytopenia.
- Gastrointestinal Disorders: mouth ulceration, stomatitis.
- Infections and Infestations: pneumonia, sepsis.
- Skin and Subcutaneous Tissue Disorders: angioedema, urticaria; drug rash with eosinophilia and systemic symptoms (DRESS) syndrome, exfoliative rash, exfoliative dermatitis, Stevens-Johnson syndrome, toxic skin eruption, toxicoderma.
Drug Interactions
Potential for Boceprevir to Affect Other Drugs
- Boceprevir is a strong inhibitor of CYP3A4/CYP3A5. Drugs metabolized primarily by CYP3A4/CYP3A5 may have increased exposure when administered with Boceprevir which could increase or prolong their therapeutic and adverse effects. Boceprevir does not inhibit CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6 or CYP2E1 in vitro. In addition, boceprevir does not induce CYP1A2, CYP2B6, CYP2C8, CYP2C9, CYP2C19 or CYP3A4/5 in vitro.
- Boceprevir is a potential inhibitor of p-glycoprotein (P-gp) based on in vitro studies. In a drug interaction trial conducted with digoxin, Boceprevir had limited p-glycoprotein inhibitory potential at clinically relevant concentrations.
Potential for Other Drugs to Affect Boceprevir
- Boceprevir is primarily metabolized by aldo-ketoreductase (AKR). In drug interaction trials conducted with AKR inhibitors diflunisal and ibuprofen, boceprevir exposure did not increase to a clinically significant extent. Boceprevir may be coadministered with AKR inhibitors.
- Boceprevir is partly metabolized by CYP3A4/CYP3A5. It is also a substrate for p-glycoprotein. Coadministration of Boceprevir with drugs that induce or inhibit CYP3A4/CYP3A5 could decrease or increase exposure to boceprevir.
Established and Other Potential Significant Drug Interactions
- TABLE 5 provides recommendations based on established or potentially clinically significant drug interactions. Boceprevir is contraindicated with drugs that are potent inducers of CYP3A4/CYP3A5 and drugs that are highly dependent on CYP3A4/CYP3A5 for clearance, and for which elevated plasma concentrations are associated with serious and/or life-threatening events.

Use in Specific Populations
Pregnancy
- Boceprevir must be administered in combination with peginterferon alfa and ribavirin
- Significant teratogenic and/or embryocidal effects have been demonstrated in all animal species exposed to ribavirin; and therefore ribavirin is contraindicated in women who are pregnant and in the male partners of women who are pregnant. Interferons have abortifacient effects in animals and should be assumed to have abortifacient potential in humans.
- Extreme caution must be taken to avoid pregnancy in female patients and female partners of male patients while taking this combination. Women of childbearing potential and their male partners should not receive ribavirin unless they are using effective contraception (two reliable forms) during treatment with ribavirin and for 6 months after treatment. One of these reliable forms of contraception can be a combined oral contraceptive product containing at least 1 mg of norethindrone. Oral contraceptives containing lower doses of norethindrone and other forms of hormonal contraception have not been studied or are contraindicated.
- In case of exposure during pregnancy, a Ribavirin Pregnancy Registry has been established to monitor maternal-fetal outcomes of pregnancies in female patients and female partners of male patients exposed to ribavirin during treatment and for 6 months following cessation of treatment. Physicians and patients are encouraged to report such cases by calling 1-800-593-2214.
- Boceprevir must not be used as a monotherapy. There are no adequate and well-controlled studies with Boceprevir in pregnant women. No effects on fetal development have been observed in rats and rabbits at boceprevir AUC exposures approximately 11.8- and 2.0-fold higher, respectively, than those in humans at the recommended dose of 800 mg three times daily.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Boceprevir in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Boceprevir during labor and delivery.
Nursing Mothers
- It is not known whether Boceprevir is excreted into human breast milk. Levels of boceprevir and/or metabolites in the milk of lactating rats were slightly higher than levels observed in maternal blood. Peak blood concentrations of boceprevir and/or metabolites in nursing pups were less than 1% of those of maternal blood concentrations. Because of the potential for adverse reactions from the drug in nursing infants, a decision must be made whether to discontinue nursing or discontinue treatment with Boceprevir, taking into account the importance of the therapy to the mother.
Pediatric Use
- The safety, efficacy, and pharmacokinetic profile of Boceprevir in pediatric patients have not been studied.
Geriatic Use
- Clinical studies of Boceprevir did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. In general, caution should be exercised in the administration and monitoring of Boceprevir in geriatric patients due to the greater frequency of decreased hepatic function, concomitant diseases and other drug therapy.
Gender
There is no FDA guidance on the use of Boceprevir with respect to specific gender populations.
Race
There is no FDA guidance on the use of Boceprevir with respect to specific racial populations.
Renal Impairment
- No dosage adjustment of Boceprevir is required for patients with any degree of renal impairment.
Hepatic Impairment
- No dose adjustment of Boceprevir is required for patients with mild, moderate or severe hepatic impairment. Safety and efficacy of Boceprevir have not been studied in patients with decompensated cirrhosis.
- In published observational studies of patients with compensated cirrhosis treated with first generation HCV protease inhibitors, including boceprevir, in combination with peginterferon alfa and ribavirin, platelet count < 100,000/mm3 and serum albumin < 3.5 g/dL were baseline characteristics that were identified as predictors of death or serious complications (severe infection or hepatic decompensation) during therapy.
- The potential risks and benefits of Boceprevir in combination with peginterferon alfa and ribavirin should be carefully considered before initiating therapy in patients with compensated cirrhosis who have platelet count < 100,000/mm3 and serum albumin < 3.5 g/dL at baseline. If therapy is initiated, close monitoring for signs of infections and worsening liver function is warranted.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Boceprevir in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Boceprevir in patients who are immunocompromised.
Organ Transplantation
- The safety and efficacy of Boceprevir alone or in combination with peginterferon alfa and ribavirin for the treatment of chronic hepatitis C genotype 1 infection in liver or other organ transplant recipients have not been studied.
Administration and Monitoring
Administration
There is limited information regarding Boceprevir Administration in the drug label.
Monitoring
There is limited information regarding Boceprevir Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Boceprevir and IV administrations.
Overdosage
- Daily doses of 3600 mg have been taken by healthy volunteers for 5 days without untoward symptomatic effects.
- There is no specific antidote for overdose with Boceprevir. Treatment of overdosage with Boceprevir should consist of general supportive measures, including monitoring of vital signs, and observation of the patient's clinical status.
Pharmacology
Mechanism of Action
- Boceprevir is a direct acting antiviral drug against the hepatitis C virus
Structure
- Boceprevir has the following chemical name: (1R,5S)-N-3-Amino-1-(cyclobutylmethyl)-2,3-dioxopropyl-3-[2(S)-(1,1-dimethylethyl)amino]carbonylamino-3,3-dimethyl-1-oxobutyl-6,6-dimethyl-3-azabicyclo3.1.0hexan-2(S)-carboxamide. The molecular formula is C27H45N5O5 and its molecular weight is 519.7. Boceprevir has the following structural formula:
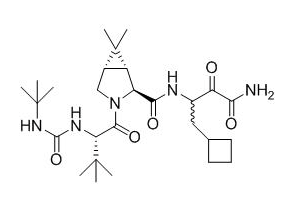
Pharmacodynamics
- The effect of boceprevir 800 mg and 1200 mg on QTc interval was evaluated in a randomized, multiple-dose, placebo-, and active-controlled (moxifloxacin 400 mg) 4-way crossover thorough QT study in 36 healthy subjects. In the study with demonstrated ability to detect small effects, the upper bound of the one-sided 95% confidence interval for the largest placebo-adjusted, baseline-corrected QTc based on individual correction method (QTcI) was below 10 ms, the threshold for regulatory concern. The dose of 1200 mg yields a boceprevir maximum exposure increase of approximately 15% which may not cover exposures due to coadministration with strong CYP3A4 inhibitors or use in patients with severe hepatic impairment. However, at the doses studied in the thorough QT study, no apparent concentration-QT relationship was identified. Thus, there is no expectation of a QTc effect under a higher exposure scenario.
Pharmacokinetics
- Boceprevir capsules contain a 1:1 mixture of two diastereomers, SCH534128 and SCH534129. In plasma the diastereomer ratio changes to 2:1, favoring the active diastereomer, SCH534128. Plasma concentrations of boceprevir described below consist of both diastereomers SCH534128 and SCH534129, unless otherwise specified.
- In healthy subjects who received 800 mg three times daily alone, boceprevir drug exposure was characterized by AUC(т) of 5408 ng × hr per mL (n=71), Cmax of 1723 ng per mL (n=71), and Cmin of 88 ng per mL (n=71). Pharmacokinetic results were similar between healthy subjects and HCV-infected subjects.
Absorption
- Boceprevir was absorbed following oral administration with a median Tmax of 2 hours. Steady state AUC, Cmax, and Cmin increased in a less-than-dose-proportional manner and individual exposures overlapped substantially at 800 mg and 1200 mg, suggesting diminished absorption at higher doses. Accumulation is minimal (0.8- to 1.5-fold) and pharmacokinetic steady state is achieved after approximately 1 day of three times daily dosing. The absolute bioavailability of boceprevir has not been studied.
- Effects of Food on Oral Absorption: Boceprevir should be administered with food. Food enhanced the exposure of boceprevir by up to 65% at the 800 mg three times daily dose, relative to the fasting state. The bioavailability of boceprevir was similar regardless of meal type (e.g., high-fat vs. low-fat) or whether taken 5 minutes prior to eating, during a meal, or immediately following completion of the meal. Therefore, Boceprevir may be taken without regard to either meal type or timing of the meal.
Distribution
- Boceprevir has a mean apparent volume of distribution (Vd/F) of approximately 772 L at steady state in healthy subjects. Human plasma protein binding is approximately 75% following a single dose of boceprevir 800 mg. Boceprevir is administered as an approximately equal mixture of two diastereomers, SCH534128 and SCH534129, which rapidly interconvert in plasma. The predominant diastereomer, SCH534128, is pharmacologically active and the other diastereomer is inactive.
Metabolism
- Studies in vitro indicate that boceprevir primarily undergoes metabolism through the aldo-keto reductase (AKR)-mediated pathway to ketone-reduced metabolites that are inactive against HCV. After a single 800-mg oral dose of 14C-boceprevir, the most abundant circulating metabolites were a diastereomeric mixture of ketone-reduced metabolites with a mean exposure approximately 4-fold greater than that of boceprevir. Boceprevir also undergoes, to a lesser extent, oxidative metabolism mediated by CYP3A4/5.
Drug Interactions
- Drug interaction studies were performed with boceprevir and drugs likely to be coadministered or drugs commonly used as probes for pharmacokinetic interactions. The effects of coadministration of boceprevir on AUC, Cmax and Cmin are summarized in TABLE 6 (effects of coadministered drugs on boceprevir) and TABLE 7 (effects of boceprevir on coadministered drugs).
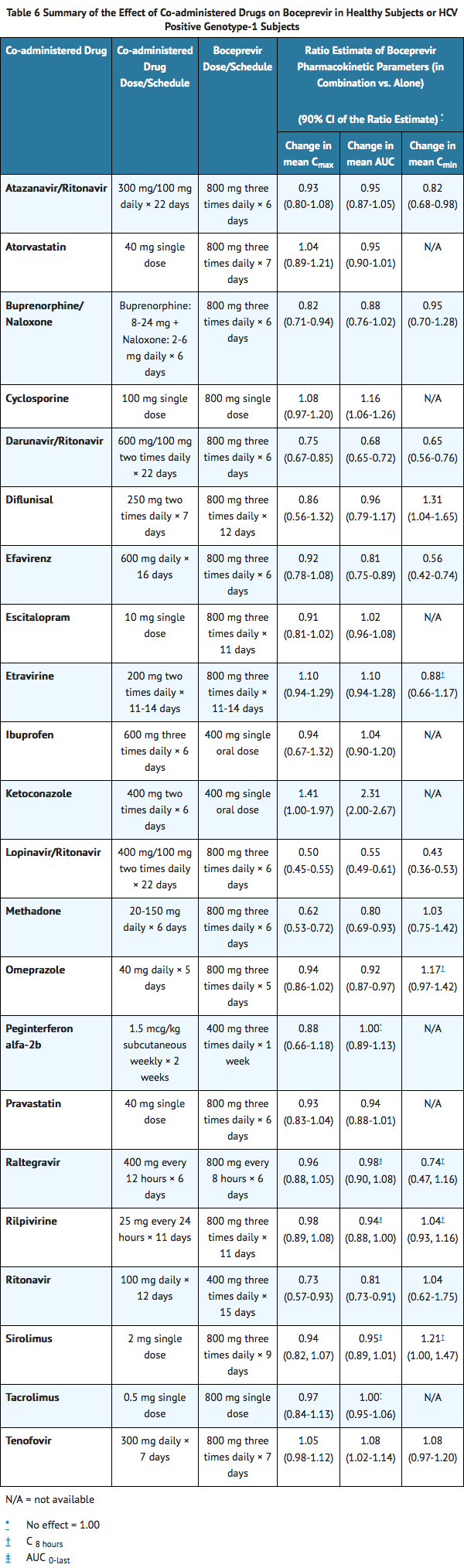
Elimination
- Boceprevir is eliminated with a mean plasma half-life (t½) of approximately 3.4 hours. Boceprevir has a mean total body clearance (CL/F) of approximately 161 L per hr. Following a single 800 mg oral dose of 14C-boceprevir, approximately 79% and 9% of the dose was excreted in feces and urine, respectively, with approximately 8% and 3% of the dosed radiocarbon eliminated as boceprevir in feces and urine. The data indicate that boceprevir is eliminated primarily by the liver.
Nonclinical Toxicology
Carcinogenesis and Mutagenesis
- Use with Ribavirin and Peginterferon alfa: Ribavirin is genotoxic in in vitro and in vivo assays. Ribavirin was not oncogenic in mouse and rat carcinogenicity studies at doses less than the maximum recommended daily human dose. Please refer to the prescribing information for ribavirin for additional information.
- Two-year carcinogenicity studies in mice and rats were conducted with boceprevir. Mice were administered doses of up to 500 mg per kg in males and 650 mg per kg in females, and rats were administered doses of up to 125 mg per kg in males and 100 mg per kg in females. In mice, no significant increases in the incidence of drug-related neoplasms were observed at the highest doses tested resulting in boceprevir AUC exposures approximately 2.3- and 6.0-fold higher in males and females, respectively, than those in humans at the recommended dose of 800 mg three times daily. In rats, no increases in the incidence of drug-related neoplasms were observed at the highest doses tested resulting in boceprevir AUC exposures similar to those in humans at the recommended dose of 800 mg three times daily.
- Boceprevir was not genotoxic in a battery of in vitro or in vivo assays, including bacterial mutagenicity, chromosomal aberration in human peripheral blood lymphocytes and mouse micronucleus assays.
Impairment of Fertility
- Use with Ribavirin and Peginterferon alfa: In fertility studies in male animals, ribavirin induced reversible testicular toxicity; while peginterferon alfa may impair fertility in females. Please refer to the prescribing information for ribavirin and peginterferon alfa for additional information.
- Boceprevir-induced reversible effects on fertility and early embryonic development in female rats, with no effects observed at a 75 mg per kg dose level. At this dose, boceprevir AUC exposures are approximately 1.3-fold higher than those in humans at the recommended dose of 800 mg three times daily. Decreased fertility was also observed in male rats, most likely as a consequence of testicular degeneration. No testicular degeneration was observed at a 15 mg per kg dose level resulting in boceprevir AUC exposures of less than those in humans at the recommended dose of 800 mg three times daily. Testicular degeneration was not observed in mice or monkeys administered boceprevir for 3 months at doses of up to 900 or 1000 mg per kg, respectively. At these doses, boceprevir AUC exposures are approximately 6.8- and 4.4-fold higher in mice and monkeys, respectively, than those in humans at the recommended dose of 800 mg three times daily. Additionally, limited clinical monitoring has revealed no evidence of testicular toxicity in human subjects.
Clinical Studies
The efficacy of Boceprevir as a treatment for chronic hepatitis C (genotype 1) infection was assessed in approximately 1500 adult subjects who were previously untreated (SPRINT-2) or who had failed previous peginterferon alfa and ribavirin therapy (RESPOND-2) in Phase 3 clinical studies.
Previously Untreated Subjects
- SPRINT-2 was a randomized, double-blind, placebo-controlled study comparing two therapeutic regimens of Boceprevir 800 mg orally three times daily in combination with PR Peginterferon alfa 2a 1.5 micrograms per kg per week subcutaneously and weight-based dosing with Ribavirin (600вАУ1400 mg per day orally divided twice daily)] to PR alone in adult subjects who had chronic hepatitis C (HCV genotype 1) infection with detectable levels of HCV-RNA and were not previously treated with interferon alfa therapy. Subjects were randomized in a 1:1:1 ratio within two separate cohorts (Cohort 1/non-Black and Cohort 2/Black) and were stratified by HCV genotype (1a or 1b) and by HCV-RNA viral load (less than or equal to 400,000 IU per mL vs. more than 400,000 IU per mL) to one of the following three treatment arms:
- Peginterferon alfa 2a + Ribavirin for 48 weeks (PR48).
- Peginterferon alfa 2a + Ribavirin for four weeks followed by Boceprevir 800 mg three times daily + Peginterferon alfa 2a + Ribavirin for 24 weeks. The subjects were then continued on different regimens based on Treatment Week (TW) 8 through TW24 response-guided therapy (boceprevir-RGT). All subjects in this treatment arm were limited to 24 weeks of therapy with Boceprevir.
- Subjects with undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) and remained undetectable through TW24 discontinued therapy and entered follow-up at the TW28 visit.
- Subjects with detectable HCV-RNA at TW8 or any subsequent treatment week but subsequently achieving undetectable HCV-RNA (Target Not Detected) at TW24 (late responders) were changed in a blinded fashion to placebo at the TW28 visit and continued therapy with Peginterferon alfa 2a + Ribavirin for an additional 20 weeks, for a total treatment duration of 48 weeks.
- Peginterferon alfa 2a + Ribavirin for four weeks followed by Boceprevir 800 mg three times daily + Peginterferon alfa 2a + Ribavirin for 44 weeks (boceprevir-PR48).
All subjects with detectable HCV-RNA in plasma at TW24 were discontinued from treatment. Sustained Virologic Response (SVR) was defined as plasma HCV-RNA less than 25 IU/mL at Follow-up Week 24. Plasma HCV-RNA results at Follow-up Week 12 were used if plasma HCV-RNA results at Follow-up Week 24 were missing.
Mean age of subjects randomized was 49 years. The racial distribution of subjects was as follows: 82% White, 14% Black, and 4% others. The distribution of subjects by gender was 60% men and 40% women.
The addition of Boceprevir to Peginterferon alfa 2a and Ribavirin significantly increased the SVR rates compared to Peginterferon alfa 2a and Ribavirin alone in the combined cohort (63% to 66% in arms containing Boceprevir vs. 38% PR48 control) for randomized subjects who received at least one dose of any study medication (Full-Analysis-Set population). SVR rates for Blacks who received the combination of Boceprevir with Peginterferon alfa 2a and Ribavirin were 42% to 53% in a predefined analysis (see TABLE 10).
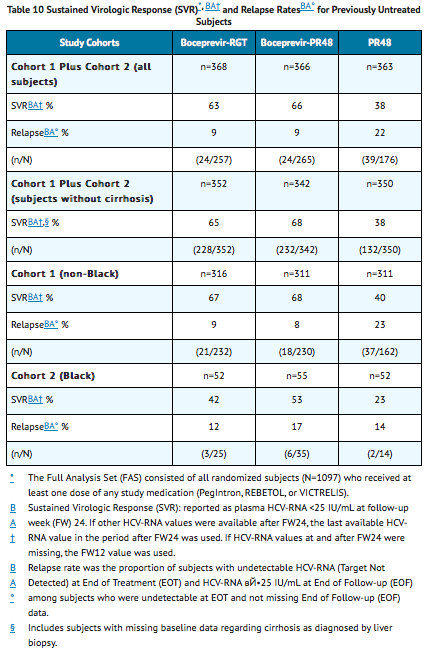
- In subjects with cirrhosis at baseline, sustained virologic response was higher in those who received treatment with the combination of Boceprevir with Peginterferon alfa 2a and Ribavirin for 44 weeks after lead-in therapy with Peginterferon alfa 2a and Ribavirin (10/24, 42%) compared to those who received RGT (5/16 , 31%).
Sustained Virologic Response (SVR) Based on TW8 HCV-RNA Results
- TABLE 11 presents sustained virologic response based on TW8 HCV-RNA results in previously untreated subjects. Fifty-seven percent (208/368) of subjects in the boceprevir-RGT arm and 56% (204/366) of subjects in the boceprevir-PR48 arm had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 17% (60/363) of subjects in the PR48 arm.
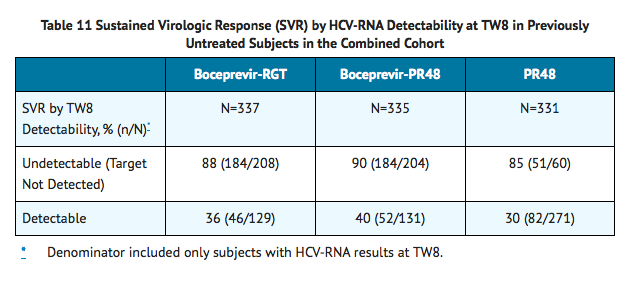
- Among subjects with detectable HCV-RNA at TW8 who had attained undetectable HCV-RNA (Target Not Detected) at TW24 and completed at least 28 weeks of treatment, the SVR rates were 66% (45/68) in boceprevir-RGT arm (4 weeks of Peginterferon alfa 2a and Ribavirin then 24 weeks of Boceprevir with Peginterferon alfa 2a and Ribavirin followed by 20 weeks of Peginterferon alfa 2a and Ribavirin alone) and 75% (55/73) in boceprevir-PR48 arms (4 weeks of Peginterferon alfa 2a and Ribavirin then 44 weeks of Boceprevir with Peginterferon alfa 2a and Ribavirin).
Previous Partial Responders and Relapsers to Interferon and Ribavirin Therapy
- RESPOND-2 was a randomized, parallel-group, double-blind study comparing two therapeutic regimens of Boceprevir 800 mg orally three times daily in combination with PR [[[Peginterferon alfa 2a]] 1.5 micrograms per kg per week subcutaneously and weight-based ribavirin (600вАУ1400 mg per day orally divided twice daily)] compared to PR alone in adult subjects with chronic hepatitis C (HCV genotype 1) infection with demonstrated interferon responsiveness (as defined historically by a decrease in HCV-RNA viral load greater than or equal to 2-log10 by Week 12, but never achieved SVR partial responders or undetectable HCV-RNA at end of prior treatment with a subsequent detectable HCV-RNA in plasma [relapsers]). Subjects with less than 2-log10 decrease in HCV-RNA by week 12 of previous treatment (prior null responders) were not eligible for enrollment in this trial. Subjects were randomized in a 1:2:2 ratio and stratified based on response to their previous qualifying regimen (relapsers vs. partial responders) and by HCV subtype (1a vs. 1b) to one of the following treatment arms:
- Peginterferon alfa 2a + Ribavirin for 48 weeks (PR48)
- Peginterferon alfa 2a + Ribavirin for 4 weeks followed by Boceprevir 800 mg three times daily + Peginterferon alfa 2a + Ribavirin for 32 weeks. The subjects were then continued on different treatment regimens based on TW8 and TW12 response-guided therapy (boceprevir-RGT). All subjects in this treatment arm were limited to 32 weeks of Boceprevir.
- Subjects with undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) and TW12 completed therapy at TW36 visit.
- Subjects with a detectable HCV-RNA at TW8 but subsequently undetectable (Target Not Detected) at TW12 (late responders) were changed in a blinded fashion to placebo at the TW36 visit and continued treatment with Peginterferon alfa 2a + Ribavirin for an additional 12 weeks, for a total treatment duration of 48 weeks.
- Peginterferon alfa 2a + Ribavirin for 4 weeks followed by Boceprevir 800 mg three times daily + Peginterferon alfa 2a + Ribavirin for 44 weeks (boceprevir-PR48).
- All subjects with detectable HCV-RNA in plasma at TW12 were discontinued from treatment. Sustained Virologic Response (SVR) was defined as plasma HCV-RNA less than 25 IU/mL at Follow-up Week 24. Plasma HCV-RNA results at Follow-up Week 12 were used if plasma HCV-RNA results at Follow-up Week 24 were missing.
- Mean age of subjects randomized was 53 years. The racial distribution of subjects was as follows: 85% White, 12% Black, and 3% others. The distribution of subjects by gender was 67% men and 33% women.
- The addition of Boceprevir to the Peginterferon alfa 2a and Ribavirin therapy significantly increased the SVR rates compared to Peginterferon alfa 2a/Ribavirin alone (59% to 66% in arms containing Boceprevir vs. 23% PR48 control) for randomized subjects who received at least one dose of any study medication (Full-Analysis-Set population) (see TABLE 12).
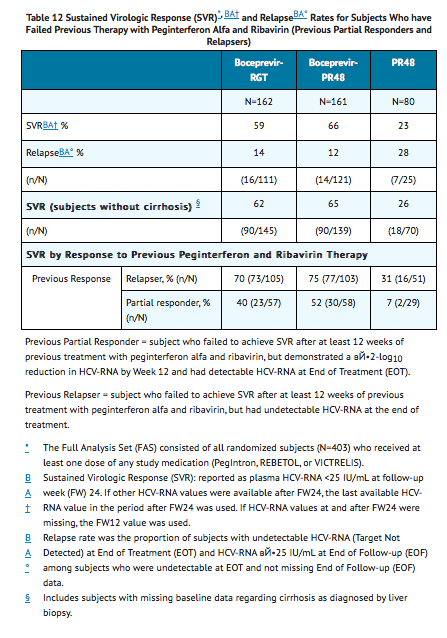
- In subjects with cirrhosis at baseline, sustained virologic response was higher in those who received treatment with the combination of Boceprevir with Peginterferon alfa 2a and Ribavirin for 44 weeks after 4 weeks of lead-in therapy with Peginterferon alfa 2a and Ribavirin (17/22, 77%) compared to those who received RGT (6/17, 35%).
Sustained Virologic Response (SVR) Based on TW8 HCV-RNA Results
- TABLE 13 presents sustained virologic response based on TW8 HCV-RNA results in subjects who were relapsers or partial responders to previous interferon and ribavirin therapy. Forty-six percent (74/162) of subjects in the boceprevir-RGT arm and 52% (84/161) in the boceprevir-PR48 had undetectable HCV-RNA (Target Not Detected) at TW8 (early responders) compared with 9% (7/80) in the PR48 arm.
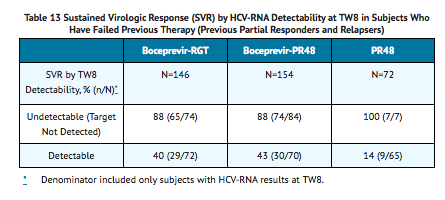
- Among subjects with detectable HCV-RNA at TW8 who attained an undetectable HCV-RNA (Target Not Detected) at TW12 and completed at least 36 weeks of treatment, the SVR rates were 79% (27/34) in boceprevir-RGT arm (4 weeks of Peginterferon alfa 2a and Ribavirin then 32 weeks of Boceprevir with Peginterferon alfa 2a and Ribavirin followed by 12 weeks of Peginterferon alfa 2a and Ribavirin alone) and 72% (29/40) in boceprevir-PR48 arm (4 weeks of Peginterferon alfa 2a and Ribavirin then 44 weeks of Boceprevir with Peginterferon alfa 2a and Ribavirin).
Interferon Responsiveness during Lead-In Therapy with Peginterferon alfa and Ribavirin
Previously Untreated Subjects
- In previously untreated subjects evaluated in SPRINT-2, interferon-responsiveness (defined as greater than or equal to 1-log10 decline in viral load at TW4) was predictive of SVR. Subjects treated with Boceprevir who demonstrated interferon responsiveness at TW4 achieved SVR rates of 81% (203/252) in boceprevir-RGT arm and 79% (200/254) in boceprevir-PR48 arm, compared to 52% (134/260) in subjects treated with Peginterferon alfa 2a/Ribavirin.
- Subjects treated with Boceprevir who demonstrated poor interferon responsiveness (defined as less than 1-log10 decline in viral load at TW4), achieved SVR rates of 28% (27/97) in boceprevir-RGT arm and 38% (36/95) in boceprevir-PR48 arm, compared to 4% (3/83) in subjects treated with Peginterferon alfa 2a/Ribavirin. Subjects with less than a 0.5-log10 decline in viral load at TW4 achieved SVR rates of 28% (13/47) in boceprevir-RGT arm and 30% (11/37) in boceprevir-PR48 arm, compared to 0% (0/25) in subjects treated with Peginterferon alfa 2a/Ribavirin. Subjects with less than a 0.5-log10 decline in viral load at TW4 with peginterferon alfa plus ribavirin therapy alone are predicted to have a null response (less than 2-log10 viral load decline at TW12) to peginterferon alfa and ribavirin.
Previous Partial Responders and Relapsers to Interferon and Ribavirin Therapy
- In subjects who were previous relapsers and partial responders evaluated in RESPOND-2, interferon-responsiveness (defined as greater than or equal to 1-log10 decline in viral load at TW4) was predictive of SVR. Subjects treated with Boceprevir who demonstrated interferon responsiveness at TW4 achieved SVR rates of 74% (81/110) in boceprevir-RGT arm and 79% (90/114) in boceprevir-PR48 arm, compared to 27% (18/67) in subjects treated with Peginterferon alfa 2a/Ribavirin. Subjects treated with Boceprevir who demonstrated poor interferon responsiveness (defined as less than 1-log10 decline in viral load at TW4) achieved SVR rates of 33% (15/46) in boceprevir-RGT arm and 34% (15/44) in boceprevir-PR48 arm, compared to 0% (0/12) in subjects treated with Peginterferon alfa 2a/Ribavirin.
Prior Null Responders to Interferon and Ribavirin Therapy
- PROVIDE was an open-label, single-arm trial of Boceprevir 800 mg orally three times daily in combination with peginterferon alfa-2b 1.5 micrograms per kg per week subcutaneously and weight-based ribavirin (600 вАУ 1,400 mg per day orally divided twice daily) in adult subjects with chronic hepatitis C (HCV) genotype 1 infection who did not achieve SVR while in the peginterferon alfa/ribavirin control arms of previous Phase 2 and 3 trials of combination therapy with Boceprevir Subjects who enrolled in PROVIDE within 2 weeks after the last dose of peginterferon alfa/ribavirin in the prior trial received Boceprevir 800 mg three times daily + peginterferon alfa-2b + ribavirin for 44 weeks. Subjects who were not able to enroll in this trial within 2 weeks received Peginterferon alfa 2a/Ribavirin lead-in for 4 weeks followed by Boceprevir 800 mg three times daily + peginterferon alfa-2b + ribavirin for 44 weeks.
Among subjects who were null responders in the peginterferon alfa/ribavirin control arm of the prior trial, SVR (reported as plasma HCV-RNA <25 IU/mL at follow-up week 24) was 38% (20/52) and the relapse rate was 13% (3/23).
Use of Ribavirin Dose Reduction versus Erythropoiesis Stimulating Agent (ESA) in the Management of Anemia in Previously Untreated Subjects
- A randomized, parallel-arm, open-label study was conducted to compare two strategies for the management of anemia (use of ESA versus ribavirin dose reduction) in 687 subjects with previously untreated CHC genotype 1 infection who became anemic during therapy with Boceprevir 800 mg orally three times daily plus peginterferon alfa-2b 1.5 micrograms per kg per week subcutaneously and weight-based ribavirin (600 вАУ 1,400 mg orally per day divided twice daily). The study enrolled subjects with serum hemoglobin concentrations of less than 15 g per dL. Subjects were treated for 4 weeks with peginterferon alfa-2b and ribavirin followed by up to 44 weeks of Boceprevir plus peginterferon alfa-2b and ribavirin. If a subject became anemic (serum hemoglobin of approximately less than or equal to 10 g per dL within the treatment period), the subject was randomized in a 1:1 ratio to either ribavirin dose reduction (N=249) or use of erythropoietin 40,000 units subcutaneously once weekly for the management of the anemia (N=251). If serum hemoglobin concentrations continued to decrease to less than or equal to 8.5 g per dL, subjects could be treated with additional anemia interventions, including the addition of erythropoietin (18% of those in the ribavirin dose reduction arm) or ribavirin dose reduction (37% of those in the ESA arm).
- Mean age of subjects randomized was 49 years. The racial distribution of subjects was as follows: 77% White, 19% Black, and 4% other. The distribution of subjects by gender was 37% men and 63% women.
- The overall intent-to-treat SVR rate for all enrolled subjects (including those subjects who were not randomized to RBV dose reduction or ESA for the management of anemia) was 63% (431/687). The SVR rate in subjects randomized who received ribavirin dose reduction was 71% (178/249), similar to the SVR rate of 71% (178/251) in subjects randomized to receive an ESA. The relapse rates in subjects randomized to receive ribavirin dose reduction or an ESA were 10% (19/196) and 10% (19/197), respectively.
How Supplied
- Boceprevir 200 mg capsules are comprised of a red-colored cap with the Merck logo printed in yellow ink, and a yellow-colored body with "314" printed in red ink. The capsules are packaged into a carton with 28 bottles containing 12 capsules (NDC 0085-0314-02).
Storage
- Boceprevir Capsules should be refrigerated at 2вАУ8°C (36вАУ46°F) until dispensed. Avoid exposure to excessive heat. For patient use, refrigerated capsules of Boceprevir can remain stable until the expiration date printed on the label. Boceprevir can also be stored at room temperature up to 25°C (77°F) for 3 months. Keep container tightly closed.
Images
Drug Images
{{#ask: Page Name::Boceprevir |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
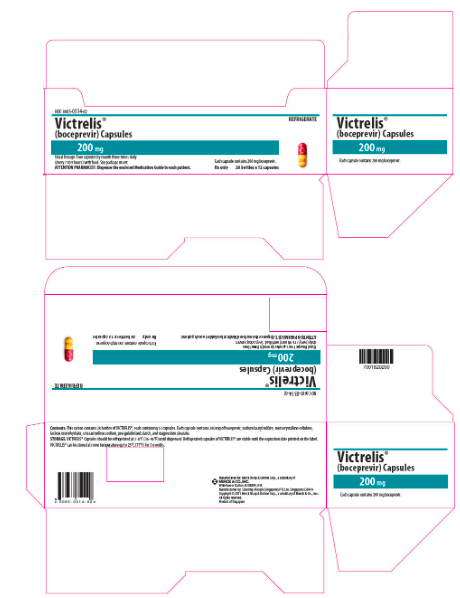
{{#ask: Label Page::Boceprevir |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Boceprevir Patient Counseling Information in the drug label.
Precautions with Alcohol
- Alcohol-Boceprevir interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
Look-Alike Drug Names
There is limited information regarding Boceprevir Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ 1.0 1.1 Kiser JJ, Burton JR, Anderson PL, Everson GT (May 2012). "Review and Management of Drug Interactions with Boceprevir and Telaprevir". Hepatology. 55 (5): 1620–8. doi:10.1002/hep.25653. PMC 3345276.