Nalorphine: Difference between revisions
m (Protected "Nalorphine": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Kiran Singh (talk | contribs) No edit summary |
||
| (2 intermediate revisions by 2 users not shown) | |||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| IUPAC_name | | Verifiedfields = changed | ||
| image | | Watchedfields = changed | ||
| verifiedrevid = 402503832 | |||
| | | IUPAC_name = (5α,6α)-17-allyl- 7,8-didehydro- 4,5-epoxymorphinan- 3,6-diol | ||
| | | image =Nalorphine.png | ||
<!--Clinical data--> | |||
| tradename = | |||
| Drugs.com = {{drugs.com|international|nalorphine}} | |||
| | | pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | ||
| | | pregnancy_US = <!-- A / B / C / D / X --> | ||
| pregnancy_category = | |||
| legal_AU = S4 | |||
| legal_CA = <!-- / Schedule I, II, III, IV, V, VI, VII, VIII --> | |||
| pregnancy_AU | | legal_UK = <!-- GSL / P / POM / CD / Class A, B, C --> | ||
| pregnancy_US | | legal_US = <!-- OTC / Rx-only / Schedule I, II, III, IV, V --> | ||
| pregnancy_category= | | legal_status = | ||
| legal_AU | | routes_of_administration = | ||
| legal_CA | |||
| legal_UK | <!--Pharmacokinetic data--> | ||
| legal_US | | bioavailability = | ||
| legal_status | | protein_bound = | ||
| routes_of_administration = | | metabolism = | ||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 62-67-9 | |||
| ATC_prefix = V03 | |||
| ATC_suffix = AB02 | |||
| PubChem = 5284595 | |||
| IUPHAR_ligand = 1629 | |||
| DrugBank_Ref = {{drugbankcite|correct|drugbank}} | |||
| DrugBank = | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 4447643 | |||
| UNII_Ref = {{fdacite|changed|FDA}} | |||
| UNII = U59WB2WRY2 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 415284 | |||
<!--Chemical data--> | |||
| C=19 | H=21 | N=1 | O=3 | |||
| molecular_weight = 311.375 g/mol | |||
| smiles = O[C@H]2\C=C/[C@H]5[C@@H]4N(CC[C@@]51c3c(O[C@H]12)c(O)ccc3C4)C\C=C | |||
| InChI = 1/C19H21NO3/c1-2-8-20-9-7-19-12-4-6-15(22)18(19)23-17-14(21)5-3-11(16(17)19)10-13(12)20/h2-6,12-13,15,18,21-22H,1,7-10H2/t12-,13+,15-,18-,19-/m0/s1 | |||
| InChIKey = UIQMVEYFGZJHCZ-SSTWWWIQBT | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C19H21NO3/c1-2-8-20-9-7-19-12-4-6-15(22)18(19)23-17-14(21)5-3-11(16(17)19)10-13(12)20/h2-6,12-13,15,18,21-22H,1,7-10H2/t12-,13+,15-,18-,19-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = UIQMVEYFGZJHCZ-SSTWWWIQSA-N | |||
| synonyms = <small>(−)−(5''R'',6''S'')-9α-allyl- 4,5-epoxymorphin- 7-en- 3,6-diol</small> | |||
}} | }} | ||
__Notoc__ | |||
{{SI}} | {{SI}} | ||
{{CMG}} | |||
==Overview== | |||
'''Nalorphine''' ([[International Nonproprietary Name|INN]]; '''Lethidrone''', '''Nalline'''), also known as '''''N''-allyl-normorphine''', is a mixed [[opioid]] [[agonist–antagonist]]. It acts at two opioid receptors, at the [[mu receptor]] it has antagonistic effects and at the [[kappa receptor]]s it exerts high-efficacy agonistic characteristics. It is used to reverse opioid overdose and (starting in the 1950s) in a challenge test to determine opioid dependence.<ref>[http://www.time.com/time/magazine/article/0,9171,808859,00.html "Medicine: Drug Detector"], ''[[Time (magazine)|Time]]'', Dec. 24, 1956</ref> | |||
==Synthesis== | |||
[[File:Nalorphine synthesis.png|thumb|center|700px|Nalorphine synthesis:<ref>{{Cite doi|10.1021/ja01256a036}}</ref>]] | |||
More recently, it has become much more commonplace to use [[ethyl chloroformate]] instead of [[cyanogen bromide]] for the [[Von Braun reaction|Von Braun degradation]] demethylation step. See for example the [[list of phenyltropanes]] or the synthesis of [[paroxetine]] for further examples of this. | |||
==See also== | |||
==References== | |||
{{Reflist|2}} | |||
{{Antidotes}} | {{Antidotes}} | ||
{{Hallucinogens}} | |||
[[Category:Drug]] | |||
[[Category:Alcohols]] | |||
[[Category:Alkenes]] | |||
[[Category:Morphinans]] | |||
[[Category:Opioid antagonists]] | [[Category:Opioid antagonists]] | ||
[[Category: | [[Category:Phenols]] | ||
[[Category:Semisynthetic opioids]] | |||
Latest revision as of 17:11, 9 April 2015
 | |
| Clinical data | |
|---|---|
| Synonyms | (−)−(5R,6S)-9α-allyl- 4,5-epoxymorphin- 7-en- 3,6-diol |
| AHFS/Drugs.com | International Drug Names |
| ATC code | |
| Legal status | |
| Legal status |
|
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| IUPHAR/BPS | |
| ChemSpider | |
| UNII | |
| ChEMBL | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C19H21NO3 |
| Molar mass | 311.375 g/mol |
| 3D model (JSmol) | |
| |
| |
| | |
|
WikiDoc Resources for Nalorphine |
|
Articles |
|---|
|
Most recent articles on Nalorphine |
|
Media |
|
Evidence Based Medicine |
|
Clinical Trials |
|
Ongoing Trials on Nalorphine at Clinical Trials.gov Clinical Trials on Nalorphine at Google
|
|
Guidelines / Policies / Govt |
|
US National Guidelines Clearinghouse on Nalorphine
|
|
Books |
|
News |
|
Commentary |
|
Definitions |
|
Patient Resources / Community |
|
Patient resources on Nalorphine Discussion groups on Nalorphine Patient Handouts on Nalorphine Directions to Hospitals Treating Nalorphine Risk calculators and risk factors for Nalorphine
|
|
Healthcare Provider Resources |
|
Causes & Risk Factors for Nalorphine |
|
Continuing Medical Education (CME) |
|
International |
|
|
|
Business |
|
Experimental / Informatics |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Nalorphine (INN; Lethidrone, Nalline), also known as N-allyl-normorphine, is a mixed opioid agonist–antagonist. It acts at two opioid receptors, at the mu receptor it has antagonistic effects and at the kappa receptors it exerts high-efficacy agonistic characteristics. It is used to reverse opioid overdose and (starting in the 1950s) in a challenge test to determine opioid dependence.[1]
Synthesis
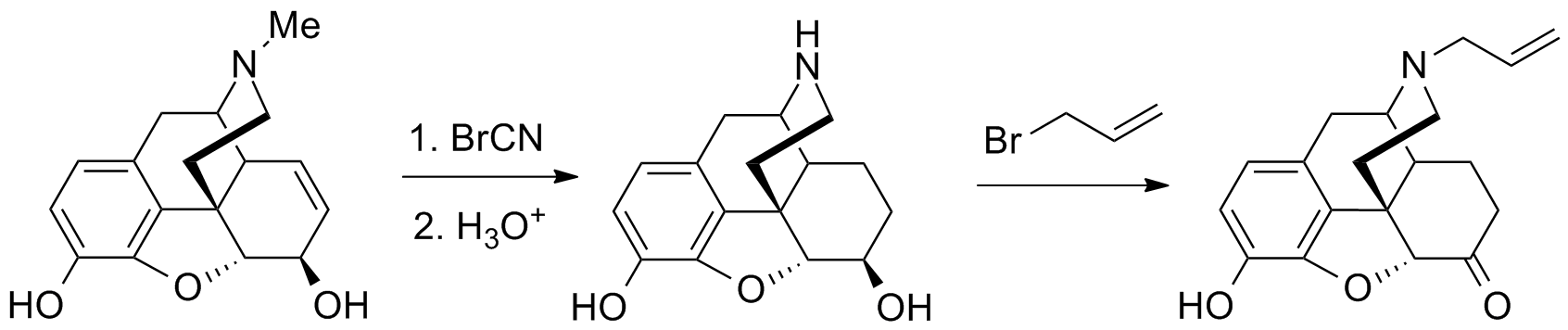
More recently, it has become much more commonplace to use ethyl chloroformate instead of cyanogen bromide for the Von Braun degradation demethylation step. See for example the list of phenyltropanes or the synthesis of paroxetine for further examples of this.
See also
References
- ↑ "Medicine: Drug Detector", Time, Dec. 24, 1956
- ↑ Template:Cite doi
- Pages with script errors
- Template:drugs.com link with non-standard subpage
- Articles with changed EBI identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugboxes which contain changes to verified fields
- Drugboxes which contain changes to watched fields
- Drug
- Alcohols
- Alkenes
- Morphinans
- Opioid antagonists
- Phenols
- Semisynthetic opioids