Haloalkane
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview

The haloalkanes (also known as halogenoalkanes or alkyl halides) are a group of chemical compounds, consisting of alkanes, such as methane or ethane, with one or more halogens linked, such as chlorine or fluorine, making them a type of organic halide. They are known under many chemical and commercial names. As fire extinguishants, propellants and solvents they have or had wide use. Some haloalkanes (those containing chlorine or bromine) are believed to have negative effects on the environment such as ozone depletion. The most widely known family within this group are the chlorofluorocarbons (CFCs).
General
A haloalkane also known as alkyl halogenide, halogenalkane or halogenoalkane, and alkyl halide is a chemical compound derived from an alkane by substituting one or more hydrogen atoms with halogen atoms. Substitution with fluorine, chlorine, bromine and iodine results in fluoroalkanes, chloroalkanes, bromoalkanes and iodoalkanes, respectively. Mixed compounds are also possible, the best-known examples being the chlorofluorocarbons (CFCs) which are mainly responsible for ozone depletion. Haloalkanes are used in semiconductor device fabrication, as refrigerants, foam blowing agents, solvents, aerosol spray propellants, fire extinguishing agents, and chemical reagents.
Freon is a trade name for a group of chlorofluorocarbons used primarily as a refrigerant. The word Freon is a registered trademark belonging to DuPont.
There are 3 types of haloalkane. In primary (1°) haloalkanes the carbon which carries the halogen atom is only attached to one other alkyl group. However CH3Br is also a primary haloalkane, even though there is no alkyl group. In secondary (2°) haloalkanes the carbon that carries the halogen atom is attached to 2 alkyl groups. In tertiary (3°) haloalkanes the carbon that carries the halogen atom is attached to 3 alkyl groups.
Chloro fluoro compounds (CFC, HCFC)
Chlorofluorocarbons (CFC) are compounds containing chlorine, fluorine and carbon only, that is they contain no hydrogen. They were formerly used widely in industry, for example as refrigerants, propellants, and cleaning solvents. Their use has been regularly prohibited by the Montreal Protocol, because of effects on the ozone layer (see ozone depletion). They are also a powerful greenhouse gas, in terms of carbon dioxide equivalence (over a time period of one hundred years) between 5000 and 8100 per kg. [1]
Hydrochlorofluorocarbons (HCFCs) are of a class of haloalkanes where not all hydrogen has been replaced by chlorine or fluorine. They are used primarily as chlorofluorocarbon (CFC) substitutes, as the ozone depleting effects are only about 10% of the CFCs.
Hydro fluoro compounds (HFC)
Hydrofluorocarbons (HFCs) contain no chlorine. They are composed entirely of carbon, hydrogen, and fluorine. They have an even lower global warming potential than HCFCs, and no known effects at all on the ozone layer. Only compounds containing chlorine and bromine are thought to harm the ozone layer. Fluorine itself is not ozone-toxic. [2] However, HFCs and perfluorocarbons do have activity in the entirely different realm of greenhouse gases, which do not destroy ozone, but do cause global warming. Two groups of haloalkanes, hydrofluorocarbons (HFCs) and perfluorocarbons (PFCs), are targets of the Kyoto Protocol. [2]
Polymer haloalkanes
Chlorinated or fluorinated alkenes can be used for polymerization, resulting in polymer haloalkanes with notable chemical resistance properties. Important examples include polychloroethene (polyvinyl chloride, PVC), and polytetrafluoroethylene (PTFE, Teflon), but many more halogenated polymers exist.
History
Original development
Carbon tetrachloride was used in fire extinguishers and glass "anti-fire grenades" from the late nineteenth century until around the end of World War II. Experimentation with chloroalkanes for fire suppression on military aircraft began at least as early as the 1920s.
American engineer Thomas Midgley developed chlorofluorocarbons (CFC) in 1928 as a replacement for ammonia (NH3), chloromethane (CH3Cl), and sulfur dioxide (SO2), which are toxic but were in common use at the time as refrigerants. The new compound developed had to have a low boiling point and be non-toxic and generally non-reactive. In a demonstration for the American Chemical Society, Midgley flamboyantly demonstrated all these properties by inhaling a breath of the gas and using it to blow out a candle.[citation needed]
Midgley specifically developed CCl2F2. However, one of the attractive features is that there exists a whole family of the compounds, each having a unique boiling point which can suit different applications. In addition to their original application as refrigerants, chlorofluoroalkanes have been used as propellants in aerosol cans, cleaning solvents for circuit boards, and blowing agents for making expanded plastics (such as the expanded polystyrene used in packaging materials and disposable coffee cups).
Development of alternatives
During World War II, various early chloroalkanes were in standard use in military aircraft by some combatants, but these early halons suffered from excessive toxicity. Nevertheless, after the war they slowly became more common in civil aviation as well.
In the 1960s, fluoroalkanes and bromofluoroalkanes became available and were quickly recognized as being among the most effective fire-fighting materials discovered. Much early research with Halon 1301 was conducted under the auspices of the US Armed Forces, while Halon 1211 was, initially, mainly developed in the UK. By the late 1960s they were standard in many applications where water and dry-powder extinguishers posed a threat of damage to the protected property, including computer rooms, telecommunications switches, laboratories, museums and art collections. Beginning with warships, in the 1970s, bromofluoroalkanes also progressively came to be associated with rapid knockdown of severe fires in confined spaces with minimal risk to personnel.
Work on alternatives for chlorofluorocarbons in refrigerants began in the late 1970s after the first warnings of damage to stratospheric ozone were published in the journal Nature in 1974 by Molina and Rowland (who shared the 1995 Nobel Prize for Chemistry for their work). Adding hydrogen and thus creating hydrochlorofluorocarbons (HCFC), chemists made the compounds less stable in the lower atmosphere, enabling them to break down before reaching the ozone layer. Later alternatives dispense with the chlorine, creating hydrofluorocarbons (HFC) with even shorter lifetimes in the lower atmosphere.
By the early 1980s, bromofluoroalkanes were in common use on aircraft, ships and large vehicles as well as in computer facilities and galleries. However, concern was beginning to be felt about the impact of chloroalkanes and bromoalkanes on the ozone layer. The Vienna Convention on Ozone Layer Protection did not cover bromofluoroalkanes as it was thought, at the time, that emergency discharge of extinguishing systems was too small in volume to produce a significant impact, and too important to human safety for restriction.
However, by the time of the Montreal Protocol it was realised that deliberate and accidental discharges during system tests and maintenance accounted for substantially larger volumes than emergency discharges, and consequently halons were brought into the treaty, albeit with many exceptions.
Phase out
Use of certain chloroalkanes as solvents for large scale application, such as dry cleaning, have been phased out, for example, by the IPPC directive on greenhouse gases in 1994 and by the Volatile Organic Compounds (VOC) directive of the EU in 1997. Permitted chlorofluoroalkane uses are medicinal only.
Finally, bromofluoroalkanes have been largely phased out and the possession of such equipment is prohibited in some countries like the Netherlands and Belgium, from 1 January 2004, based on the Montreal Protocol and guidelines of the European Union.
Production of new stocks ceased in most (probably all) countries as of 1994. However many countries still require aircraft to be fitted with halon fire suppression systems because no safe and completely satisfactory alternative has been discovered for this application. There are also a few other, highly specialised, uses. These programs recycle halon through "halon banks" coordinated by the Halon Recycling Corporation[3] to ensure that discharge to the atmosphere occurs only in a genuine emergency and to conserve remaining stocks.
On September 21, 2007, approximately 200 countries agreed to accelerate the elimination of hydrochlorofluorocarbons entirely by 2020 in a United Nations-sponsored Montreal summit. Developing nations were given until 2030. Many nations, such as the United States and China, who had previously resisted such efforts, signed the treaty. [4]
Nomenclature
IUPAC nomenclature
The formal naming of haloalkanes should follow IUPAC nomenclature, which put the halogen as a prefix to the alkane. For example, ethane with bromine becomes bromoethane, methane with four chlorine groups becomes tetrachloromethane. However, many of these compounds have already an established trivial name, which is endorsed by the IUPAC nomenclature, for example chloroform (trichloromethane) and methylene chloride (dichloromethane). For unambiguity, this article follows the systematic naming scheme throughout.
Alternative nomenclature for refrigerants
The refrigerant naming system is mainly used for fluorinated and chlorinated short alkanes for refrigerant use. In the US the standard is specified in ANSI/ASHRAE Standard 34-1992, with additional annual supplements.[5] The specified ANSI/ASHRAE prefixes were FC (fluorocarbon) or R (refrigerant), but today most are prefixed by a more specific classification:
- CFC—list of chlorofluorocarbons
- HCFC—list of hydrochlorofluorocarbons
- HFC—list of hydrofluorocarbons
- FC—list of fluorocarbons
- PFC—list of perfluorocarbons (completely fluorinated)
The decoding system for CFC-01234a is:
- 0 = Number of double bonds (omitted if zero)
- 1 = Carbon atoms -1 (omitted if zero)
- 2 = Hydrogen atoms +1
- 3 = Fluorine atoms
- 4 = Replaced by Bromine ("B" prefix added)
- a = Letter added to identify isomers, the "normal" isomer in any number has the smallest mass difference on each carbon, and a, b, or c are added as the masses diverge from normal.
Other coding systems are in use as well.
Overview of named compounds
| + Template:Chembox header colspan="4" | Overview of haloalkanes | |||
|---|---|---|---|
| Template:Chembox header colspan="4" | This table gives an overview of most haloalkanes in general use or commonly known. Listing includes bulk commodity products as well as laboratory chemicals. | |||
| Template:Chembox header colspan=1 | Systematic name | Template:Chembox header colspan=1 | Common/Trivial name(s) |
Template:Chembox header colspan=1 | Code | Template:Chembox header colspan=1 | Chem. formula |
| Halomethanes | |||
| Chloromethane | Methyl chloride | CH3Cl | |
| Dichloromethane | Methylene chloride | CH2Cl2 | |
| Trichloromethane | Chloroform | CHCl3 | |
| Tetrachloromethane | Carbon tetrachloride, Freon 10 | CFC-10 | CCl4 |
| Tetrafluoromethane | Carbon tetrafluoride, Freon 14 | CFC-14 | CF4 |
| Trichlorofluoromethane | Freon-11, R-11 | CFC-11 | CCl3F |
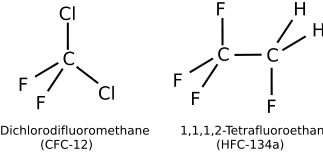
| Dichlorodifluoromethane | Freon-12, R-12 | CFC-12 | CCl2F2 |
| Chlorotrifluoromethane | CFC-13 | CClF3 | |
| Chlorodifluoromethane | R-22 | HCFC-22 | CHClF2 |
| Trifluoromethane | Fluoroform | HFC-23 | CHF3 |
| Chlorofluoromethane | Freon 31 | CH2ClF | |
| Difluoromethane | HFC-32 | CH2F2 | |
| Fluoromethane | Methyl fluoride | HFC-41 | CH3F |
| Dibromomethane | Methylene bromide | CH2Br2 | |
| Tribromomethane | Bromoform | CHBr3 | |
| Bromochloromethane | Halon 1011 | CH2BrCl | |
| Bromochlorodifluoromethane | BCF, Halon 1211 BCF, or Freon 12B1 | Halon 1211 | CBrClF2 |
| Bromotrifluoromethane | BTM, Halon 1301 BTM, or Freon 13BI | Halon 1301 | CBrF3 |
| Trifluoroiodomethane | Trifluoromethyl iodide | Freon 13T1 | CF3I |
| Haloethanes | |||
| 1,1,1-Trichloroethane | Methyl chloroform, tri | Cl3C-CH3 | |
| Hexachloroethane | CFC-110 | C2Cl6 | |
| 1,1,2-Trichloro-1,2,2-trifluoroethane | Trichlorotrifluoroethane | CFC-113 | Cl2FC-CClF2 |
| 1,1,1-trichloro-2,2,2-trifluoroethane | CFC-113a | Cl3C-CF3 | |
| 1,2-Dichloro-1,1,2,2-tetrafluoroethane | Dichlorotetrafluoroethane | CFC-114 | ClF2C-CClF2 |
| 1-Chloro-1,1,2,2,2-pentafluoroethane | Chloropentafluoroethane | CFC-115 | ClF2C-CF3 |
| 2-Chloro-1,1,1,2-tetrafluoroethane | HFC-124 | CHF2CF3 | |
| 1,1,2,2,2-pentafluoroethane | Pentafluoroethane | HFC-125 | CHF2CF3 |
| 1,1,2,2-Tetrafluoroethane | HFC-134 | F2HC-CHF2 | |
| 1,1,1,2-Tetrafluoroethane | R-134a | HFC-134a, Suva-134a | F3C-CH2F |
| 1,1-Dichloro-1-fluoroethane | HCFC-141b | Cl2FC-CH3 | |
| 1-Chloro-1,1-difluoroethane | HCFC-142b | ClF2C-CH3 | |
| 1,2-Dichloroethane | Ethylene dichloride | Freon 150 | ClH2C-CH2Cl |
| 1,1-Dichloroethane | Ethylidene dichloride | Freon 150a | Cl2HC-CH3 |
| 1,1-Difluoroethane | HFC-152a | F2HC-CH3 | |
| Longer haloalkanes, polymers | |||
| 1,1,1,2,3,3,3-Heptafluoropropane | HFC-227ea, FE-227, FM-200 | F3C-CHF-CF3 | |
| Decafluorobutane | perfluorobutane | R610, PFB, CEA-410 | F3C-CF2-CF2-CF3 |
| Polychloroethene | polyvinyl chloride, PVC | -[CHCl-CH2]x- | |
| Polytetrafluoroethene | Polytetrafluoroethylene, PTFE, Teflon |
-[CF2-CF2]x- | |
Synthesis
Alkyl halides can be synthesized from alkanes, alkenes, or alcohols.
From alkanes
Alkanes react with halogens by free radical halogenation. In this reaction a hydrogen atom is removed from the alkane, then replaced by a halogen atom by reaction with a diatomic halogen molecule. Thus:
- Step 1: X2 → 2 X· (Initiation step)
- Step 2: X· + R-H → R· + HX (1st propagation step)
- Step 3: R· + X2 → R-X + X· (2nd propagation step)
Steps 2 and 3 keep repeating, each providing the reactive intermediate needed for the other step. This is called a radical chain reaction. This reaction continues until the radicals are used up by one of three termination steps.
- Step 4: R· + X· → R-X (1st termination step)
- Step 5: 2 X· → X2 (2nd termination step)
- Step 6: 2 R· → R-R (3rd termination step)
Note that Step 4 results in the same product as Step 3, the desired haloalkane, but through the destruction of two radicals. Step 5 is just the reverse of Step 1 and Step 6 accounts for the small contamination of this reaction by larger alkanes and their subsequent haloalkanes.
From alkenes
Preparation of haloalkane:
- An alkene reacts with a dry hydrogen halide (HX) like hydrogen chloride (HCl) or hydrogen bromide (HBr) to form a haloalkane. The double bond of the alkene is replaced by two new bonds, one with the halogen and one with the hydrogen atom of the hydrohalic acid. Markovnikov's rule states that in this reaction, the halogen is more likely to become attached to the more substituted carbon. This is a electrophilic addition reaction. It gives a Markovnikov addition product. For example:
- H3C-CH=CH2 + HBr → H3C-CHBr-CH3 (primary product) + H3C-CH2-CH2Br (secondary product).
Water must be absent otherwise there will be a side product(water). The reaction is necessarily to be carried out in a dry inert solvent such as CCl4 or directly in the gaseous phase.
- Alkenes also react with halogens (X2) to form haloalkanes with two neighboring halogen atoms(Dihaloalkane). This is sometimes known as "decolorizing" the halogen, since the reagent X2 is colored and the product is usually colorless. For example:
- 2H3C-CH=CH2 + Br2 → 2H3C-CHBr-CH2Br
From alcohols
Tertiary alkanol reacts with hydrochloric acid directly to produce tertiary chloroalkane, but if primary or secondary alkanol is used, an activator such as zinc chloride is needed. Alternatively the conversion may be performed directly using thionyl chloride which is called the Darzen's process.The Darzen's process is one of the most convenient methods known because the bi-products are gaseous and thus escape, leaving behind pure alkyl chloride. Alkanol may likewise be converted to bromoalkane using hydrobromic acid or phosphorus tribromide or iodoalkane using red phosphorus and iodine (equivalent to phosphorus triiodide). Two examples:
- (H3C)3C-OH + HCl → (H3C)3C-Cl + 2 H2O
- CH3-(CH2)6-OH + SOCl2 → CH3-(CH2)6-Cl + SO2 + HCl
By substitution of alcohol in the absence of water
Halogenating agents are:
- Phosphorus pentachloride
- Thionyl chloride
- hydrogen chloride
- Phosphorus with Bromine
- Phosphorus with Iodine
- Hydrogen chloride with zinc chloride
Reactions of haloalkanes
Haloalkanes are reactive towards nucleophiles. They are polar molecules: the carbon to which the halogen is attached is slightly electropositive where the halogen is slightly electronegative. This results in an electron deficient (electrophilic) carbon which, inevitably, attracts nucleophiles.
Substitution reactions
Substitution reactions involve the replacement of the halogen with another molecule - thus leaving saturated hydrocarbons, as well as the halogen product.
Hydrolysis - a reaction in which water breaks a bond--is a good example of the nucleophilic nature of halogenoalkanes. The polar bond attracts a hydroxide ion, OH-. (NaOH(aq) being a common source of this ion). This OH- is a nucleophile with a clearly negative charge, as it has excess electrons it donates them to the carbon, which results in a covalent bond between the two. Thus C-X is broken by heterolytic fission resulting in a halide ion, X-. As can be seen, the OH is now attached to the alkyl group, creating an alcohol. (Hydrolysis of bromoethane, for example, yields ethanol).
One should note that within the halogen series, the C-X bond weakens as one goes to heavier halogens, and this affects the rate of reaction. Thus, the C-I of an iodoalkane generally reacts faster than the C-F of a fluoroalkane.
Apart from hydrolysis, there are a few other isolated examples of nucleophilic substitution:
- Ammonia (NH3) and bromoethane yields a mixture of ethylamine, diethylamine, and triethylamine (as their bromide salts), and tetraethylammonium bromide.
- Cyanide (CN-) added to bromoethane will form propionitrile (CH3CH2CN), a nitrile, and Br-. Nitriles can be further hydrolyzed into carboxylic acids.
Elimination reactions
Rather than creating a molecule with the halogen substituted with something else, one can completely eliminate both the halogen and a nearby hydrogen, thus forming an alkene. For example, with bromoethane and NaOH in ethanol, the hydroxide ion OH- attracts a hydrogen atom - thus removing a hydrogen and bromine from bromoethane. This results in C2H4 (ethylene), H2O and Br-.
Applications
Propellant
One major use of CFCs has been as propellants in aerosol inhalers for drugs used to treat asthma. The conversion of these devices and treatments from CFC to halocarbons that do not have the same effect on the ozone layer is well under way. The hydrofluoroalkane propellant's ability to solubilize medications and excipients is markedly different from CFCs and as a result requires a considerable amount of effort to reformulate (a significant amount of development effort has also been required to develop non-CFC alternatives to CFC-based refrigerants, particularly for applications where the refrigeration mechanism cannot be modified or replaced). They have now been outlawed in all 50 U.S. states universally.
Fire extinguishing
At high temperatures, halons decompose to release halogen atoms that combine readily with active hydrogen atoms, quenching the flame propagation reaction even when adequate fuel, oxygen and heat remains. The chemical reaction in a flame proceeds as a free radical chain reaction; by sequestering the radicals which propagate the reaction, halons are able to "poison" the fire at much lower concentrations than are required by fire suppressants using the more traditional methods of cooling, oxygen deprivation, or fuel dilution.
For example, Halon 1301 total flooding systems are typically used at concentrations no higher than 7% v/v in air, and can suppress many fires at 2.9% v/v. By contrast, carbon dioxide fire suppression flood systems are operated from 34% concentration by volume (surface-only combustion of liquid fuels) up to 75% (dust traps). Carbon dioxide can cause severe distress at concentrations of 3 to 6%, and has caused death by respiratory paralysis in a few minutes at 10% concentration. Halon 1301 causes only slight giddiness at its effective concentration of 5%, and even at 15% persons remain conscious but impaired and suffer no long term effects. (Experimental animals have also been exposed to 2% concentrations of Halon 1301 for 30 hours per week for 4 months, with no discernible health effects at all.) Halon 1211 also has low toxicity, although it is more toxic than Halon 1301, and thus considered unsuitable for flooding systems.
However, Halon 1301 fire suppression is not completely non-toxic; very high temperature flame, or contact with red-hot metal, can cause decomposition of Halon 1301 to toxic byproducts. The presence of such byproducts is readily detected because they include hydrobromic acid and hydrofluoric acid, which are intensely irritating. Halons are very effective on Class A (organic solids), B (flammable liquids and gases) and C (electrical) fires, but they are totally unsuitable for Class D (metal) fires, as they will not only produce toxic gas and fail to halt the fire, but in some cases pose a risk of explosion. Halons can be used on Class K (kitchen oils and greases) fires, but offer no advantages over specialised foams.
Halon 1211 is typically used in hand-held extinguishers, in which a stream of liquid halon is directed at a smaller fire by a user. The stream evaporates under reduced pressure, producing strong local cooling, as well as a high concentration of halon in the immediate vicinity of the fire. In this mode, extinguishment is achieved by cooling and oxygen deprivation at the core of the fire, as well as radical quenching over a larger area. After fire suppression, the halon moves away with the surrounding air, leaving no residue.
Halon 1301 is more usually employed in total flooding systems. In these systems, banks of halon cylinders are kept pressurised to about 4 MPa (600 PSI) with compressed nitrogen, and a fixed piping network leads to the protected enclosure. On triggering, the entire measured contents of one or more cylinders are discharged into the enclosure in a few seconds, through nozzles designed to ensure uniform mixing throughout the room. The quantity dumped is pre-calculated to achieve the desired concentration, typically 3-7% v/v. This level is maintained for some time, typically with a minimum of ten minutes and sometimes up to a twenty minute 'soak' time, to ensure all items have cooled so reignition is unlikely to occur, then the air in the enclosure is purged, generally via a fixed purge system that is activated by the proper authorities. During this time the enclosure may be entered by persons wearing SCBA. (There exists a common myth that this is because halon is highly toxic; in fact it is because it can cause giddiness and mildly impaired perception, and also due to the risk of combustion byproducts.)
Flooding systems may be manually operated or automatically triggered by a VESDA or other automatic detection system. In the latter case, a warning siren and strobe lamp will first be activated for a few seconds to warn personnel to evacuate the area. The rapid discharge of halon and consequent rapid cooling fills the air with fog, and is accompanied by a loud, disorienting noise.
Due to environmental concerns, alternatives are being deployed.[6]
Halon 1301 is also used in the F-16 fighters to prevent the fuel vapors in the fuel tanks from becoming explosive; when the aircraft enters area with the possibility of unfriendly fire, Halon 1301 is injected into the fuel tanks for one-time use. Due to environmental concerns, trifluoroiodomethane (CF3I) is being considered as an alternative.[7]
Environmental issues
Template:Pollution Since the late 1970s the use of CFCs has been heavily regulated because of their destructive effects on the ozone layer. After the development of his electron capture detector, James Lovelock was the first to detect the widespread presence of CFCs in the air, finding a concentration of 60 parts per trillion of CFC-11 over Ireland. In a self-funded research expedition ending in 1973, Lovelock went on to measure the concentration of CFC-11 in both the Arctic and Antarctic, finding the presence of the gas in each of 50 air samples collected, but incorrectly concluding that CFC's are not hazardous to the environment. The experiment did however provide the first useful data on the presence of CFC's in the atmosphere. The damage caused by CFC's discovered by Sherry Rowland and Mario Molina who, after hearing a lecture on the subject of Lovelocks work, embarked on research resulting in the first published paper suggesting the connection in 1974. It turns out that one of CFCs' most attractive features—their unreactivity—has been instrumental in making them one of the most significant pollutants. CFCs' lack of reactivity gives them a lifespan which can exceed 100 years in some cases. This gives them time to diffuse into the upper stratosphere. Here, the sun's ultraviolet radiation is strong enough to break off the chlorine atom, which on its own is a highly reactive free radical. This catalyzes the break up of ozone into oxygen by means of a variety of mechanisms, of which the simplest is:
- Cl· + O3 → ClO· + O2
- ClO· + O3 → Cl· + 2 O2
Since the chlorine is regenerated at the end of these reactions, a single Cl atom can destroy many thousands of ozone molecules. Reaction schemes similar to this one (but more complicated) are believed to be the cause of the ozone hole observed over the poles and upper latitudes of the Earth. Decreases in stratospheric ozone may lead to increases in skin cancer.
In 1975, the US state of Oregon enacted the world's first ban of CFCs (legislation introduced by Walter F. Brown). The United States and several European countries banned the use of CFCs in aerosol spray cans in 1978, but continued to use them in refrigeration, foam blowing, and as solvents for cleaning electronic equipment. By 1985, scientists observed a dramatic seasonal depletion of the ozone layer over Antarctica. International attention to CFCs resulted in a meeting of world diplomats in Montreal in 1987. They forged a treaty, the Montreal Protocol, which called for drastic reductions in the production of CFCs. On March 2, 1989, 12 European Community nations agreed to ban the production of all CFCs by the end of the century. In 1990, diplomats met in London and voted to significantly strengthen the Montreal Protocol by calling for a complete elimination of CFCs by the year 2000. By the year 2010 CFCs should be completely eliminated from developing countries as well.
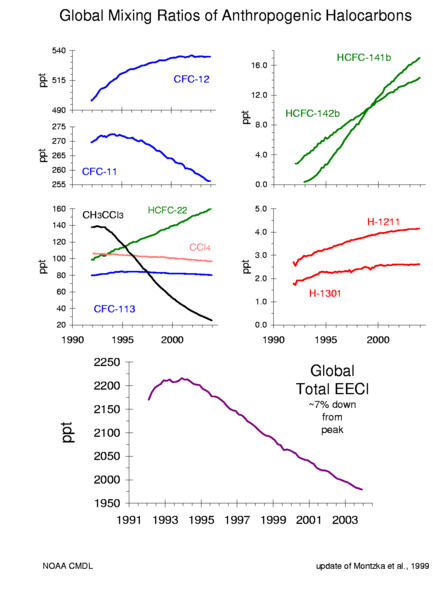
Because the only available CFC gases in countries adhering to the treaty is from recycling, their prices have gone up considerably. A worldwide end to production should also terminate the smuggling of this material, such as from Mexico to the United States.
A number of substitutes for CFCs have been introduced. Hydrochlorofluorocarbons (HCFCs) are much more reactive than CFCs, so a large fraction of the HCFCs emitted break down in the troposphere, and hence are removed before they have a chance to affect the ozone layer. Nevertheless, a significant fraction of the HCFCs do break down in the stratosphere and they have contributed to more chlorine buildup there than originally predicted. Development of non-chlorine based chemical compounds as a substitute for CFCs and HCFCs continues. One such class are the hydrofluorocarbons (HFCs), which contain only hydrogen and fluorine. One of these compounds, HFC-134a, is now used in place of CFC-12 in automobile air conditioners; which itself may contribute to global warming (see HFC-134a).
There is concern that halons are being broken down in the atmosphere to bromine, which reacts with ozone, leading to depletion of the ozone layer (this is similar to the case of chlorofluorocarbons such as freon). These issues are complicated: the kinds of fires that require halon extinguishers to be put out will typically cause more damage to the ozone layer than the halon itself, not to mention human and property damage. However, fire extinguisher systems must be tested regularly, and these tests may lead to damage. As a result, some regulatory measures have been taken, and halons are being phased out in most of the world.
In the United States, purchase and use of freon gases is regulated by the Environmental Protection Agency, and substantial fines have been levied for their careless venting. Also, licenses, good for life, are required to buy or use these chemicals. The EPA website discusses these rules in great detail, and also lists numerous private companies that are approved to give examinations for these certificates.
There are two kinds of licenses. Obtaining a "Section 609" license to use CFCs to recharge old (pre-1993 model year) car air conditioners is fairly easy and requires only an online multiple choice test offered by several companies. Companies that use unlicensed technicians for CFC recharge operations are subject to a US$15,000 fine per technician by the EPA.
The "Section 608" license, needed to recharge CFC-using stationary and non-automobile mobile units, is also multiple choice but more difficult. A general knowledge test is required, plus separate exams for small size (such as home refrigerator) units, and for high and low pressure systems. These are respectively called Parts I, II, and III. A person who takes and passes all tests receives a "Universal" license; otherwise, one that is endorsed only for the respectively passed Parts. While the general knowledge and Part I exams can be taken online, taking them before a proctor (which has to be done for Parts II and III) lets the applicant pass these tests with lower scores.
Safety
Haloalkanes in copper tubing open to the environment can turn into phosgene gas after coming in contact with extreme heat, such as while brazing or in a fire situation. Other ways that phosgene can be created is by passing the haloalkane through an internal combustion engine, or by inhaling it through a lit cigarette, cigar or pipe. Phosgene is a substance that was used as a chemical weapon in World War I. Low exposure can cause irritation, but high levels cause fluid to collect in the lungs, possibly resulting in death.
See also
References
- ↑ http://www.epa.gov/fedrgstr/EPA-AIR/1995/October/Day-11/pr-1117.html
- ↑ Lerner & K. Lee Lerner, Brenda Wilmoth (2006). "Environmental issues : essential primary sources."". Thomson Gale. Retrieved 2006-09-11.
- ↑ http://www.halon.org/
- ↑ http://ap.google.com/article/ALeqM5jSMKuV-tCweZi386Aiz07LHT0yFQ
- ↑ http://resourcecenter.ashrae.org/store/ashrae/newstore.cgi?itemid=23260&view=item&categoryid=311&categoryparent=311&page=1&loginid=2022241
- ↑ http://p2library.nfesc.navy.mil/P2_Opportunity_Handbook/3_III_2.html
- ↑ http://www.afrlhorizons.com/Briefs/0012/ML0008.html
- Information on Fluorocarbons and their applications
- B. S. Furnell et al., Vogel's Textbook of Practical Organic Chemistry, 5th edition, Longman/Wiley, New York, 1989.
- Nomenclature FAQ
- Numbering Scheme for Ozone-Depleting Substances and their Substitutes
- Class I Ozone-Depleting Substances
- CFC Smuggling
- CFC Illegal Trade
- Numbering Scheme for Ozone-Depleting Substances and their Substitutes
- Class I Ozone-Depleting Substances
- Class II Ozone-Depleting Substances (HCFC's)
- History of Halon use by the US Navy
- Ozone Loss: The Chemical Culprits
bg:Фреон da:CFC-gas de:Halogenalkan eo:Fluorklorkarbonhidrogenaĵoj ko:할로알케인 it:Alogenuri alchilici lv:Halogēnogļūdeņraži lt:Alkilhalidai mk:Алкилхалогенид nl:Halogeenalkaan no:Freon sl:CFC sv:CFC Template:Jb1 Template:WH Template:WS