Insulinoma pathophysiology
|
Insulinoma Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Insulinoma pathophysiology On the Web |
|
American Roentgen Ray Society Images of Insulinoma pathophysiology |
|
Risk calculators and risk factors for Insulinoma pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Amandeep Singh M.D.[2]
Overview
Insulinoma arises from β islet cells, which are endocrine cells that are normally involved in the production of insulin. It is thought that insulinoma is mediated by mTOR/P70S6K signaling pathway. Thus, inhibitors of mTOR (rapamycin) or dual PI3K/mTOR (NVP-BEZ2235) have become new drugs for treating insulinoma. YY1 gene is mutated by T372R mutation that causes a defect in mitochondrial function for glucose-stimulated insulin action which is thought to be involved in mTOR pathway. The progression to hypoglycemia is actually because of decreased glucose synthesis rather than increased use due to the direct effect of insulin on the liver. Insulinoma is transmitted in an autosomal dominant pattern when it is associated with MEN 1 syndrome. They are usually small (90%), sporadic (90%), solitary (90%) and benign (90%) tumors. On gross pathology insulinomas are encapsulated and have a gray to red-brown appearance. On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with amyloid in the fibrovascular stroma, are characteristic findings of insulinoma. It is also evaluated for the mitotic index (mitosis per 10 high power field) and immunohistochemistry staining by Chromogranin A, synaptophysin, and Ki-67 index. The structure of tumor cells observed under electron microscopy as group A characterized by abundant well-granulated typical β cells with a trabecular arrangement and group B as scarce well-granulated typical β cells and a medullary arrangement.
Pathophysiology
Pathogenesis
- Insulinoma is a rare benign pancreatic neuroendocrine tumor that arises from β islet cells, which are cells that are normally involved in the production of insulin. Few insulinomas can also produce other hormones such as Serotonin, gastrin, ACTH, glucagon, and somatostatin. [1]
- They are usually small (90%), sporadic (90%), solitary (90%) and benign (90%) tumors.
- It usually occurs sporadically (90%) but 10% are found to be associated with MEN 1 syndrome.[2] Those associated with the MEN1 syndrome are usually malignant and higher recurrence rate (21% at 10 and 20 years) than in those without MEN 1 (5% at 10 and 7% at 20 years). [3]
- It is thought that insulinoma is mediated by mTOR/P70S6K signaling pathway. Thus, inhibitors of mTOR (rapamycin) or dual PI3K/mTOR (NVP-BEZ2235) have become new drugs for treating insulinoma.
- Everolimus (an oral mTOR inhibitor) has a better glycemic control in people having an insulinoma.[4][5]
- Mitochondria play a key role in glucose and insulin coupling to assure insulin secretion after glucose stimulation in pancreatic β cells. Coupling is impaired due to abnormal mitochondrial function in β cells causing the death of the cell.[6] This mitochondrial function is regulated by YY1.[7]
- T372R mutation increases the transcription of YY1.[8]
- The progression to hypoglycemia is the result of decreased glucose synthesis rather than increased use due to the direct effect of insulin on the liver.[9]
- The neuroglycopenic symptoms appear eventually due to decreased blood glucose. Hypoglycemia stimulates catecholamine release which produces adrenergic symptoms.[10]
Genetics
- Insulinoma is transmitted in an autosomal dominant pattern when it is associated with MEN 1 syndrome.
- Genes involved in the pathogenesis of insulinoma include MEN1 gene. Loss of heterozygosity of MEN1 gene takes place on chromosome 11q13. [11]
Associated Conditions
The following conditions are associated with insulinomas:
- Pancreatic neuroendocrine tumors such as:[3]
- MEN 1
- Von Hippel-Lindau
- Neurofibromatosis type 1
Gross Pathology
- On gross pathology insulinoma is encapsulated and have a gray to red brown appearance.[12]
- They are usually small and solitary tumors, although there is a case report of a large (9 cm), pedunculated insulinoma and weighing more than 100 grams.[13]
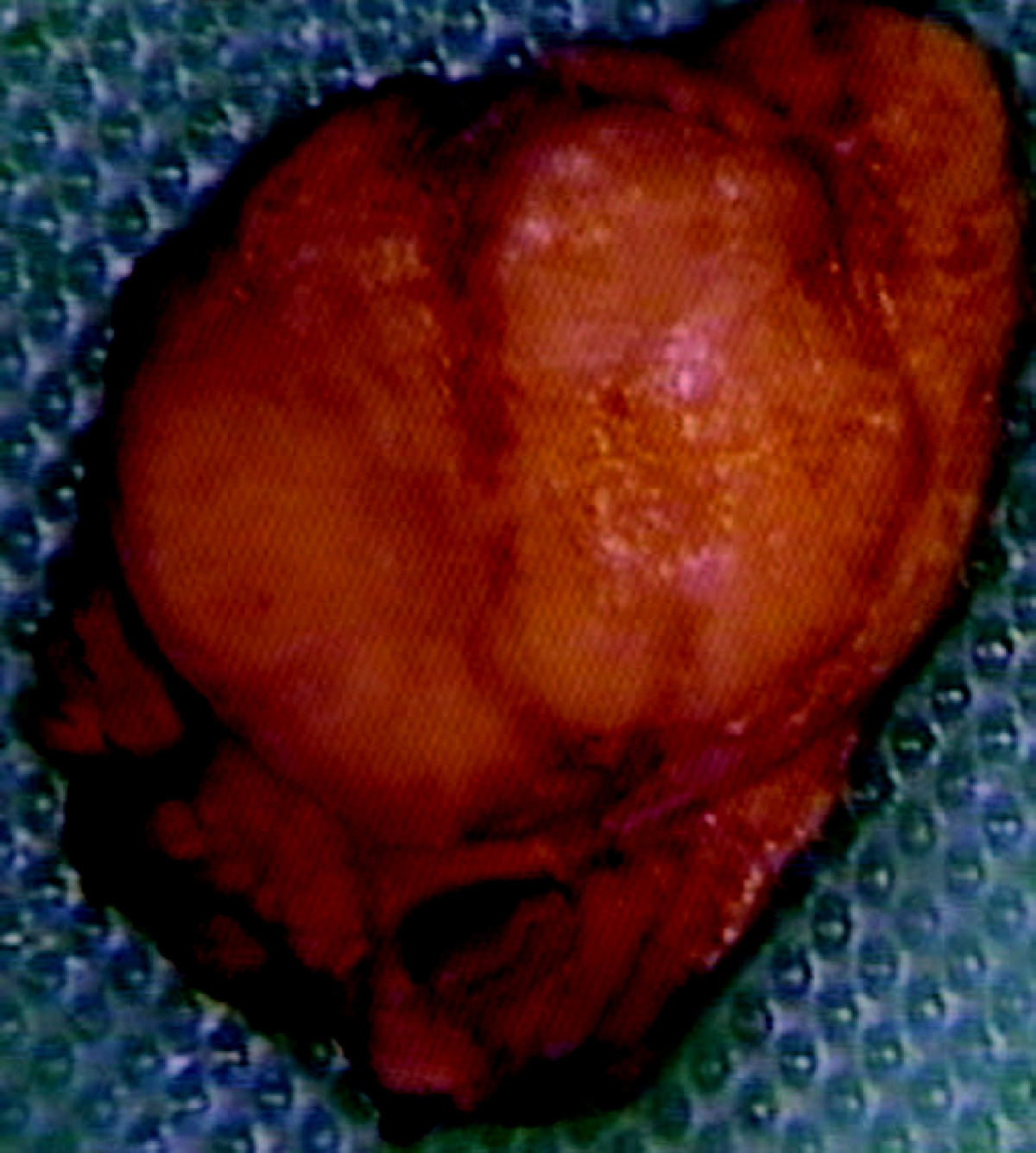
- Almost all insulinomas are present throughout the pancreas and extrapancreatic ones causing hypoglycemia are rare (<2%)[15]
- Various other findings are noted on gross pathology are: [16]
- Size of the tumor
- Metastasis to lymph nodes
- Extrapancreatic involvement
- Distant metastasis
Microscopic Pathology
- On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures particularly with amyloid in fibrovascular stroma are characteristic findings of insulinoma.[17]
- It is also evaluated for the mitotic index (mitosis per 10 high power field) and immunohistochemistry staining by chromogranin A, synaptophysin, and Ki-67 index.[16]
- The structure of tumor cells observed under electron microscopy as:
- Group A characterized by abundant well-granulated typical β cells with trabecular arrangement.
- Group B as scarce well-granulated typical β cells and a medullary arrangement. [18]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[19]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[19]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[19]
References
- ↑ AlJadir, Saadi (2015). "Insulinoma: Literature's Review (Part 1)". Endocrinology&Metabolism International Journal. 2 (3). doi:10.15406/emij.2015.02.00025. ISSN 2473-0815.
- ↑ Callender GG, Rich TA, Perrier ND (2008). "Multiple endocrine neoplasia syndromes". Surg Clin North Am. 88 (4): 863–95, viii. doi:10.1016/j.suc.2008.05.001. PMID 18672144.
- ↑ 3.0 3.1 Service FJ, McMahon MM, O'Brien PC, Ballard DJ (1991). "Functioning insulinoma--incidence, recurrence, and long-term survival of patients: a 60-year study". Mayo Clin Proc. 66 (7): 711–9. PMID 1677058.
- ↑ Kulke MH, Bergsland EK, Yao JC (2009). "Glycemic control in patients with insulinoma treated with everolimus". N Engl J Med. 360 (2): 195–7. doi:10.1056/NEJMc0806740. PMID 19129539.
- ↑ Zhan HX, Cong L, Zhao YP, Zhang TP, Chen G, Zhou L; et al. (2012). "Activated mTOR/P70S6K signaling pathway is involved in insulinoma tumorigenesis". J Surg Oncol. 106 (8): 972–80. doi:10.1002/jso.23176. PMID 22711648.
- ↑ Supale S, Li N, Brun T, Maechler P (2012). "Mitochondrial dysfunction in pancreatic β cells". Trends Endocrinol Metab. 23 (9): 477–87. doi:10.1016/j.tem.2012.06.002. PMID 22766318.
- ↑ Cunningham JT, Rodgers JT, Arlow DH, Vazquez F, Mootha VK, Puigserver P (2007). "mTOR controls mitochondrial oxidative function through a YY1-PGC-1alpha transcriptional complex". Nature. 450 (7170): 736–40. doi:10.1038/nature06322. PMID 18046414.
- ↑ Cao, Yanan; Gao, Zhibo; Li, Lin; Jiang, Xiuli; Shan, Aijing; Cai, Jie; Peng, Ying; Li, Yanli; Jiang, Xiaohua; Huang, Xuanlin; Wang, Jiaqian; Wei, Qing; Qin, Guijun; Zhao, Jiajun; Jin, Xiaolong; Liu, Li; Li, Yingrui; Wang, Weiqing; Wang, Jun; Ning, Guang (2013). "Whole exome sequencing of insulinoma reveals recurrent T372R mutations in YY1". Nature Communications. 4. doi:10.1038/ncomms3810. ISSN 2041-1723.
- ↑ Rizza, R. A.; Haymond, M. W.; Verdonk, C. A.; Mandarino, L. J.; Miles, J. M.; Service, F. J.; Gerich, J. E. (1981). "Pathogenesis of Hypoglycemia in Insulinoma Patients: Suppression of Hepatic Glucose Production by Insulin". Diabetes. 30 (5): 377–381. doi:10.2337/diab.30.5.377. ISSN 0012-1797.
- ↑ Abe T (1992). "[Letter from Alabama--Medicaid and Medicare]". Kango. 44 (2): 135–40. PMID 1305178.
- ↑ Shin JJ, Gorden P, Libutti SK (2010). "Insulinoma: pathophysiology, localization and management". Future Oncol. 6 (2): 229–37. doi:10.2217/fon.09.165. PMC 3498768. PMID 20146582.
- ↑ Lloyd, Ricardo (2010). Endocrine pathology : differential diagnosis and molecular advances. New York London: Springer. ISBN 978-1441910684.
- ↑ Mittendorf EA, Liu YC, McHenry CR (2005). "Giant insulinoma: case report and review of the literature". J Clin Endocrinol Metab. 90 (1): 575–80. doi:10.1210/jc.2004-0825. PMID 15522939.
- ↑ Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World Journal of Surgical Oncology 2009, 7:18doi:10.1186/1477-7819-7-18, CC BY 2.0, Source: https://commons.wikimedia.org/w/index.php?curid=6686376
- ↑ Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y, Kobayashi M, Hanazaki K (2013). "Diagnosis and management of insulinoma". World J. Gastroenterol. 19 (6): 829–37. doi:10.3748/wjg.v19.i6.829. PMC 3574879. PMID 23430217.
- ↑ 16.0 16.1 de Herder, Wouter W.; Niederle, Bruno; Scoazec, Jean-Yves; Pauwels, Stanislas; Klöppel, Günter; Falconi, Massimo; Kwekkeboom, Dik J.; Öberg, Kjel; Eriksson, Barbro; Wiedenmann, Bertram; Rindi, Guido; O’Toole, Dermot; Ferone, Diego (2007). "Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma". Neuroendocrinology. 84 (3): 183–188. doi:10.1159/000098010. ISSN 0028-3835.
- ↑ Lloyd, Ricardo (2010). Endocrine pathology : differential diagnosis and molecular advances. New York London: Springer. ISBN 978-1441910684.
- ↑ Berger M, Bordi C, Cüppers HJ, Berchtold P, Gries FA, Münterfering H; et al. (1983). "Functional and morphologic characterization of human insulinomas". Diabetes. 32 (10): 921–31. PMID 6311653.
- ↑ 19.0 19.1 19.2 Neuroendocrine tumor of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas
![Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[19]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[19]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[19]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)