Somatostatin
| VALUE_ERROR (nil) | |||||||
|---|---|---|---|---|---|---|---|
| Identifiers | |||||||
| Aliases | |||||||
| External IDs | GeneCards: [1] | ||||||
| Orthologs | |||||||
| Species | Human | Mouse | |||||
| Entrez |
|
| |||||
| Ensembl |
|
| |||||
| UniProt |
|
| |||||
| RefSeq (mRNA) |
|
| |||||
| RefSeq (protein) |
|
| |||||
| Location (UCSC) | n/a | n/a | |||||
| PubMed search | n/a | n/a | |||||
| Wikidata | |||||||
| |||||||
Somatostatin, also known as growth hormone-inhibiting hormone (GHIH) or by several other names, is a peptide hormone that regulates the endocrine system and affects neurotransmission and cell proliferation via interaction with G protein-coupled somatostatin receptors and inhibition of the release of numerous secondary hormones. Somatostatin inhibits insulin and glucagon secretion.[1]
Somatostatin has two active forms produced by the alternative cleavage of a single preproprotein: one consisting of 14 amino acids (shown in infobox to right), the other consisting of 28 amino acids.[2][3]
Among the vertebrates, there exist six different somatostatin genes that have been named SS1, SS2, SS3, SS4, SS5 and SS6.[4] Zebrafish have all six.[4] The six different genes, along with the five different somatostatin receptors, allow somatostatin to possess a large range of functions.[5] Humans have only one somatostatin gene, SST.[6][7][8]
Nomenclature
Synonyms of "somatostatin" include:
- growth hormone–inhibiting hormone (GHIH)
- growth hormone release–inhibiting hormone (GHRIH)
- somatotropin release–inhibiting factor (SRIF)
- somatotropin release–inhibiting hormone (SRIH)
Production
Digestive system
Somatostatin is secreted by delta cells at several locations in the digestive system, namely the pyloric antrum, the duodenum and the pancreatic islets.[9]
Somatostatin released in the pyloric antrum travels via the portal venous system to the heart, then enters the systemic circulation to reach the locations where it will exert its inhibitory effects. In addition, somatostatin release from delta cells can act in a paracrine manner.[9]
In the stomach, somatostatin acts directly on the acid-producing parietal cells via a G-protein coupled receptor (which inhibits adenylate cyclase, thus effectively antagonising the stimulatory effect of histamine) to reduce acid secretion.[9] Somatostatin can also indirectly decrease stomach acid production by preventing the release of other hormones, including gastrin, secretin and histamine which effectively slows down the digestive process.
Brain
Somatostatin is produced by neuroendocrine neurons of the ventromedial nucleus of the hypothalamus. These neurons project to the median eminence, where somatostatin is released from neurosecretory nerve endings into the hypothalamohypophysial system through neuron axons. Somatostatin is then carried to the anterior pituitary gland, where it inhibits the secretion of growth hormone from somatotrope cells. The somatostatin neurons in the periventricular nucleus mediate negative feedback effects of growth hormone on its own release; the somatostatin neurons respond to high circulating concentrations of growth hormone and somatomedins by increasing the release of somatostatin, so reducing the rate of secretion of growth hormone.
Somatostatin is also produced by several other populations that project centrally, i.e., to other areas of the brain, and somatostatin receptors are expressed at many different sites in the brain. In particular, populations of somatostatin neurons occur in the arcuate nucleus,[citation needed] the hippocampus,[citation needed] and the brainstem nucleus of the solitary tract.[citation needed]
Actions
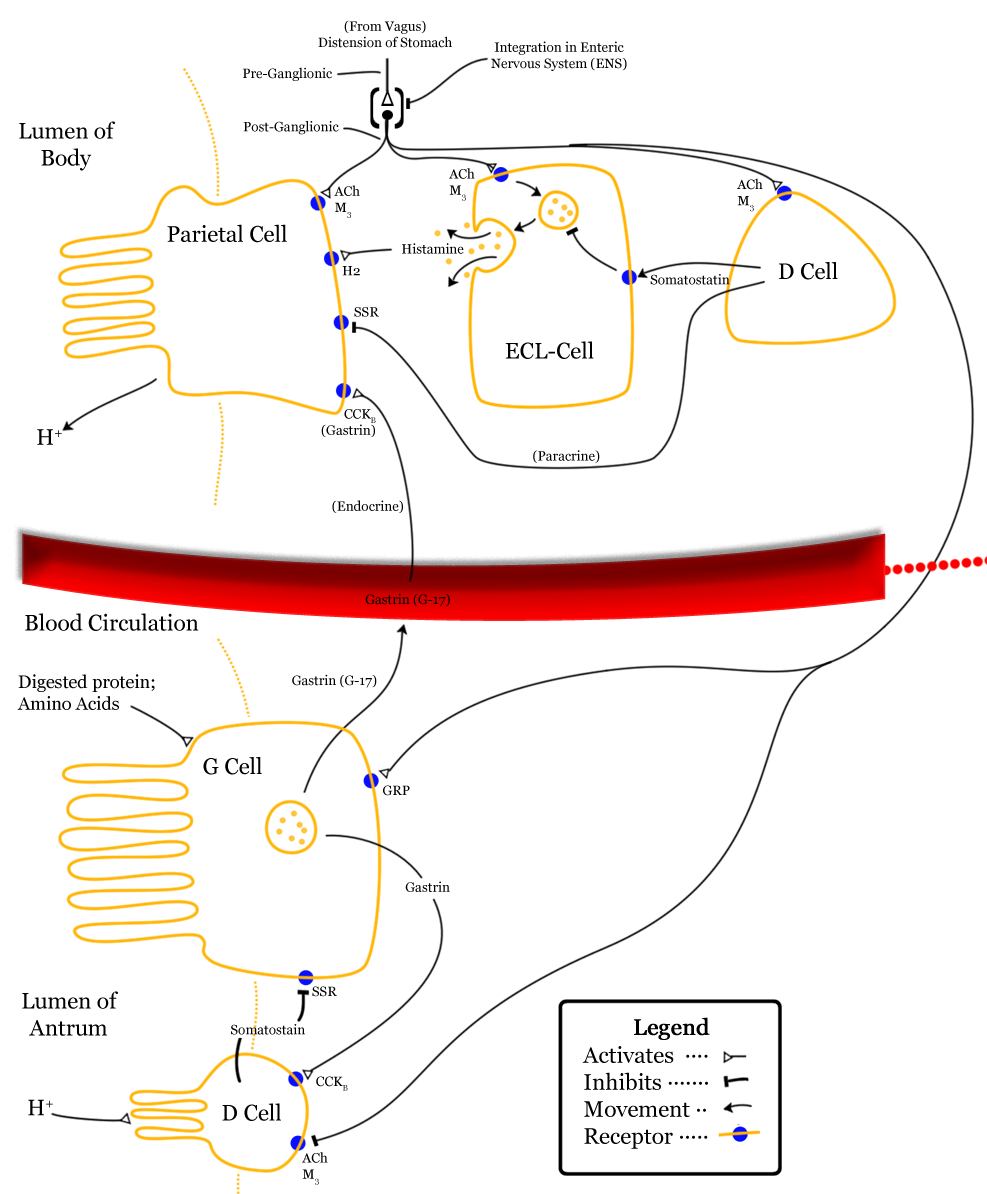
Somatostatin is classified as an inhibitory hormone,[2] and is induced by low pH.[citation needed]. Its actions are spread to different parts of the body. Somatostatin release is inhibited by the Vagus nerve.[10]
Anterior pituitary
In the anterior pituitary gland, the effects of somatostatin are:
- Inhibit the release of growth hormone (GH)[11] (thus opposing the effects of growth hormone–releasing hormone (GHRH))
- Inhibit the release of thyroid-stimulating hormone (TSH)[12]
- Inhibit adenylyl cyclase in parietal cells
- Inhibits the release of prolactin (PRL)
Gastrointestinal system
- Somatostatin is homologous with cortistatin (see somatostatin family) and suppresses the release of gastrointestinal hormones
- Decreases the rate of gastric emptying, and reduces smooth muscle contractions and blood flow within the intestine[11]
- Suppresses the release of pancreatic hormones
- Suppresses the exocrine secretory action of the pancreas
Synthetic substitutes
This section needs additional citations for verification. (March 2009) (Learn how and when to remove this template message) |
Octreotide (brand name Sandostatin, Novartis Pharmaceuticals) is an octapeptide that mimics natural somatostatin pharmacologically, though is a more potent inhibitor of growth hormone, glucagon, and insulin than the natural hormone, and has a much longer half-life (about 90 minutes, compared to 2–3 minutes for somatostatin). Since it is absorbed poorly from the gut, it is administered parenterally (subcutaneously, intramuscularly, or intravenously). It is indicated for symptomatic treatment of carcinoid syndrome and acromegaly. It is also finding increased use in polycystic diseases of the liver and kidney.
Lanreotide (Somatuline, Ipsen Pharmaceuticals)is a medication used in the management of acromegaly and symptoms caused by neuroendocrine tumors, most notably carcinoid syndrome. It is a long-acting analog of somatostatin, like octreotide. It is available in several countries, including the United Kingdom, Australia, and Canada, and was approved for sale in the United States by the Food and Drug Administration on August 30, 2007.
Evolutionary history
Six somatostatin genes have been discovered in vertebrates. The current proposed history as to how these six genes arose is based on the three whole-genome duplication events that took place in vertebrate evolution along with local duplications in teleost fish. An ancestral somatostatin gene was duplicated during the first whole-genome duplication event (1R) to create SS1 and SS2. These two genes were duplicated during the second whole-genome duplication event (2R) to create four new somatostatin genes:SS1, SS2, SS3, and one gene that was lost during the evolution of vertebrates. Tetrapods retained SS1 (also known as SS-14 and SS-28) and SS2 (also known as cortistatin) after the split in the Sarcopterygii and Actinopterygii lineage split. In teleost fish, SS1, SS2, and SS3 were duplicated during the third whole-genome duplication event (3R) to create SS1, SS2, SS4, SS5, and two genes that were lost during the evolution of teleost fish. SS1 and SS2 went through local duplications to give rise to SS6 and SS3.[4]
See also
References
- ↑ "somatostatin". Encyclopædia Britannica. Encyclopædia Britannica Online. Encyclopædia Britannica Inc., 2016. Web. 04 mag. 2016 <http://www.britannica.com/science/somatostatin>.
- ↑ 2.0 2.1 Costoff A. "Sect. 5, Ch. 4: Structure, Synthesis, and Secretion of Somatostatin". Endocrinology: The Endocrine Pancreas. Medical College of Georgia. p. 16. Archived from the original on April 5, 2008. Retrieved 2008-02-19.
- ↑ "somatostatin preproprotein [Homo sapiens]". NCBI Reference Sequence. National Center for Biotechnology Information Support Center (NCBI).
- ↑ 4.0 4.1 4.2 Liu Y, Lu D, Zhang Y, Li S, Liu X, Lin H (September 2010). "The evolution of somatostatin in vertebrates". Gene. 463 (1–2): 21–8. doi:10.1016/j.gene.2010.04.016. PMID 20472043.
- ↑ Gahete MD, Cordoba-Chacón J, Duran-Prado M, Malagón MM, Martinez-Fuentes AJ, Gracia-Navarro F, Luque RM, Castaño JP (July 2010). "Somatostatin and its receptors from fish to mammals". Annals of the New York Academy of Sciences. 1200: 43–52. doi:10.1111/j.1749-6632.2010.05511.x. PMID 20633132.
- ↑ "Entrez Gene: Somatostatin".
- ↑ Shen LP, Pictet RL, Rutter WJ (August 1982). "Human somatostatin I: sequence of the cDNA". Proceedings of the National Academy of Sciences of the United States of America. 79 (15): 4575–9. doi:10.1073/pnas.79.15.4575. PMC 346717. PMID 6126875.
- ↑ Shen LP, Rutter WJ (April 1984). "Sequence of the human somatostatin I gene". Science. 224 (4645): 168–71. doi:10.1126/science.6142531. PMID 6142531.
- ↑ 9.0 9.1 9.2 Boron, Walter F.; Boulpaep, Emile L. (2012). Medical Physiology, 2e Updated Edition, 2nd Edition (2nd ed.). Philadelphia, PA: Elsevier. ISBN 9781437717532.
- ↑ Holst JJ, Skak-Nielsen T, Orskov C, Seier-Poulsen S (August 1992). "Vagal control of the release of somatostatin, vasoactive intestinal polypeptide, gastrin-releasing peptide, and HCl from porcine non-antral stomach". Scandinavian Journal of Gastroenterology. 27 (8): 677–85. PMID 1359631.
- ↑ 11.0 11.1 Bowen R (2002-12-14). "Somatostatin". Biomedical Hypertextbooks. Colorado State University. Retrieved 2008-02-19.
- ↑ First Aid for the USMLE Step 1, 2010. Page 286.
- ↑ 13.0 13.1 Costoff A. "Sect. 5, Ch. 4: Structure, Synthesis, and Secretion of Somatostatin". Endocrinology: The Endocrine Pancreas. Medical College of Georgia. p. 17. Archived from the original on March 31, 2008. Retrieved 2008-02-19.
- ↑ van der Meulen T, Donaldson CJ, Cáceres E, Hunter AE, Cowing-Zitron C, Pound LD, Adams MW, Zembrzycki A, Grove KL, Huising MO (July 2015). "Urocortin3 mediates somatostatin-dependent negative feedback control of insulin secretion". Nature Medicine. 21 (7): 769–76. doi:10.1038/nm.3872. PMC 4496282. PMID 26076035.
Further reading
- Florio T, Schettini G (September 2001). "[Somatostatin and its receptors. Role in the control of cell proliferation]". Minerva Endocrinologica. 26 (3): 91–102. PMID 11753230.
- Yamada Y, Reisine T, Law SF, Ihara Y, Kubota A, Kagimoto S, Seino M, Seino Y, Bell GI, Seino S (December 1992). "Somatostatin receptors, an expanding gene family: cloning and functional characterization of human SSTR3, a protein coupled to adenylyl cyclase". Molecular Endocrinology. 6 (12): 2136–42. doi:10.1210/me.6.12.2136. PMID 1337145.
- Yamada Y, Post SR, Wang K, Tager HS, Bell GI, Seino S (January 1992). "Cloning and functional characterization of a family of human and mouse somatostatin receptors expressed in brain, gastrointestinal tract, and kidney". Proceedings of the National Academy of Sciences of the United States of America. 89 (1): 251–5. doi:10.1073/pnas.89.1.251. PMC 48214. PMID 1346068.
- Brazeau P, Vale W, Burgus R, Ling N, Butcher M, Rivier J, Guillemin R (January 1973). "Hypothalamic polypeptide that inhibits the secretion of immunoreactive pituitary growth hormone". Science. 179 (4068): 77–9. doi:10.1126/science.179.4068.77. PMID 4682131.
- Shen LP, Pictet RL, Rutter WJ (August 1982). "Human somatostatin I: sequence of the cDNA". Proceedings of the National Academy of Sciences of the United States of America. 79 (15): 4575–9. doi:10.1073/pnas.79.15.4575. PMC 346717. PMID 6126875.
- Shen LP, Rutter WJ (April 1984). "Sequence of the human somatostatin I gene". Science. 224 (4645): 168–71. doi:10.1126/science.6142531. PMID 6142531.
- Montminy MR, Goodman RH, Horovitch SJ, Habener JF (June 1984). "Primary structure of the gene encoding rat preprosomatostatin". Proceedings of the National Academy of Sciences of the United States of America. 81 (11): 3337–40. doi:10.1073/pnas.81.11.3337. PMC 345502. PMID 6145156.
- Zabel BU, Naylor SL, Sakaguchi AY, Bell GI, Shows TB (November 1983). "High-resolution chromosomal localization of human genes for amylase, proopiomelanocortin, somatostatin, and a DNA fragment (D3S1) by in situ hybridization". Proceedings of the National Academy of Sciences of the United States of America. 80 (22): 6932–6. doi:10.1073/pnas.80.22.6932. PMC 390100. PMID 6196780.
- Panetta R, Greenwood MT, Warszynska A, Demchyshyn LL, Day R, Niznik HB, Srikant CB, Patel YC (March 1994). "Molecular cloning, functional characterization, and chromosomal localization of a human somatostatin receptor (somatostatin receptor type 5) with preferential affinity for somatostatin-28". Molecular Pharmacology. 45 (3): 417–27. PMID 7908405.
- Demchyshyn LL, Srikant CB, Sunahara RK, Kent G, Seeman P, Van Tol HH, Panetta R, Patel YC, Niznik HB (June 1993). "Cloning and expression of a human somatostatin-14-selective receptor variant (somatostatin receptor 4) located on chromosome 20". Molecular Pharmacology. 43 (6): 894–901. PMID 8100352.
- Kaupmann K, Bruns C, Hoyer D, Seuwen K, Lübbert H (September 1993). "Distribution and second messenger coupling of four somatostatin receptor subtypes expressed in brain". FEBS Letters. 331 (1–2): 53–9. doi:10.1016/0014-5793(93)80296-7. PMID 8405411.
- Aguila MC, Rodriguez AM, Aguila-Mansilla HN, Lee WT (May 1996). "Somatostatin antisense oligodeoxynucleotide-mediated stimulation of lymphocyte proliferation in culture". Endocrinology. 137 (5): 1585–90. doi:10.1210/en.137.5.1585. PMID 8612489.
- Sharma K, Patel YC, Srikant CB (December 1996). "Subtype-selective induction of wild-type p53 and apoptosis, but not cell cycle arrest, by human somatostatin receptor 3". Molecular Endocrinology. 10 (12): 1688–96. doi:10.1210/me.10.12.1688. PMID 8961277.
- Dournaud P, Boudin H, Schonbrunn A, Tannenbaum GS, Beaudet A (February 1998). "Interrelationships between somatostatin sst2A receptors and somatostatin-containing axons in rat brain: evidence for regulation of cell surface receptors by endogenous somatostatin". The Journal of Neuroscience. 18 (3): 1056–71. PMID 9437026.
- Barnea A, Roberts J, Ho RH (January 1999). "Evidence for a synergistic effect of the HIV-1 envelope protein gp120 and brain-derived neurotrophic factor (BDNF) leading to enhanced expression of somatostatin neurons in aggregate cultures derived from the human fetal cortex". Brain Research. 815 (2): 349–57. doi:10.1016/S0006-8993(98)01098-1. PMID 9878821.
- Ferone D, van Hagen PM, van Koetsveld PM, Zuijderwijk J, Mooy DM, Lichtenauer-Kaligis EG, Colao A, Bogers AJ, Lombardi G, Lamberts SW, Hofland LJ (January 1999). "In vitro characterization of somatostatin receptors in the human thymus and effects of somatostatin and octreotide on cultured thymic epithelial cells". Endocrinology. 140 (1): 373–80. doi:10.1210/en.140.1.373. PMID 9886848.
- Brakch N, Lazar N, Panchal M, Allemandou F, Boileau G, Cohen P, Rholam M (February 2002). "The somatostatin-28(1-12)-NPAMAP sequence: an essential helical-promoting motif governing prosomatostatin processing at mono- and dibasic sites". Biochemistry. 41 (5): 1630–9. doi:10.1021/bi011928m. PMID 11814357.
- Oomen SP, van Hennik PB, Antonissen C, Lichtenauer-Kaligis EG, Hofland LJ, Lamberts SW, Löwenberg B, Touw IP (February 2002). "Somatostatin is a selective chemoattractant for primitive (CD34(+)) hematopoietic progenitor cells". Experimental Hematology. 30 (2): 116–25. doi:10.1016/S0301-472X(01)00772-X. PMID 11823046.
- Simonetti M, Di BC (February 2002). "Structural motifs in the maturation process of peptide hormones. The somatostatin precursor. I. A CD conformational study". Journal of Peptide Science. 8 (2): 66–79. doi:10.1002/psc.370. PMID 11860030.
- Genes on human chromosome
- Pages with broken file links
- All articles with unsourced statements
- Articles with unsourced statements from August 2011
- Articles with invalid date parameter in template
- Articles with unsourced statements from May 2014
- Articles needing additional references from March 2009
- All articles needing additional references
- Antidiarrhoeals
- Endocrine system
- Hormones of the somatotropic axis
- Neuropeptides
- Neuroendocrinology
- Pancreatic hormones
- Somatostatin receptor agonists