Hypoglycemia pathophysiology: Difference between revisions
No edit summary |
No edit summary |
||
| Line 12: | Line 12: | ||
[[File:Insulin mechanism 1.gif|500px|center|thumb: Insulin cellular effect, source: Wikipedia]] | [[File:Insulin mechanism 1.gif|500px|center|thumb: Insulin cellular effect, source: Wikipedia]] | ||
<br clear="left" />The actions of [[insulin]] on the human metabolism include:<ref name="pmid24783939">{{cite journal| author=Ahmad K| title=Insulin sources and types: a review of insulin in terms of its mode on diabetes mellitus. | journal=J Tradit Chin Med | year= 2014 | volume= 34 | issue= 2 | pages= 234-7 | pmid=24783939 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24783939 }}</ref> | <br clear="left" />The actions of [[insulin]] on the human metabolism include:<ref name="pmid24783939">{{cite journal| author=Ahmad K| title=Insulin sources and types: a review of insulin in terms of its mode on diabetes mellitus. | journal=J Tradit Chin Med | year= 2014 | volume= 34 | issue= 2 | pages= 234-7 | pmid=24783939 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24783939 }}</ref> | ||
* [[Insulin]] decreases [[Blood sugar|blood glucose]] concentration by inducing | * [[Insulin]] decreases [[Blood sugar|blood glucose]] concentration by inducing uptake of the [[glucose]] by peripheral cells. This function is as a result of increase [[GLUT4]] transporter insertion in the [[cell membrane]] of [[Muscle|muscles]] and [[Adipose tissue|fat tissues]] which allow [[glucose]] to enter the cell. | ||
* | * The increase of [[DNA replication]] and [[protein synthesis]] via control of [[Amino acid|amino acids]] uptake. | ||
* Induction of [[glycogen]] synthesis when [[glucose]] levels are high. | * Induction of [[glycogen]] synthesis when [[glucose]] levels are high. | ||
* | * The increase of cellular [[potassium]] uptake. | ||
* Decreased [[gluconeogenesis]] and [[glycogenolysis]]: Decreased production of [[glucose]] from noncarbohydrate substrates, primarily in the [[liver]] (the vast majority of endogenous [[insulin]] arriving into the [[liver]] never leaves the [[Liver|liver)]]. | * Decreased [[gluconeogenesis]] and [[glycogenolysis]]: Decreased production of [[glucose]] from noncarbohydrate substrates, primarily in the [[liver]] (the vast majority of endogenous [[insulin]] arriving into the [[liver]] never leaves the [[Liver|liver)]]. | ||
* | * The increase of [[lipid]] synthesis: [[insulin]] forces [[fat cells]] to take in [[blood glucose]], which is converted into [[triglycerides]]; a decrease of [[insulin]] causes the reverse. | ||
* | * The decrease of [[lipolysis]]: [[insulin]] forces reduction in conversion of [[Lipid|fat cell lipid]] stores into [[blood]] [[fatty acids]] and [[glycerol]]; a decrease of [[insulin]] causes the reverse. | ||
* | * The decrease of [[proteolysis]]: [[insulin]] decreases the breakdown of [[protein]]. | ||
* | * The decrease of renal [[sodium]] excretion. | ||
=== Pathogenesis of hypoglycemia in diabetics === | === Pathogenesis of hypoglycemia in diabetics === | ||
| Line 29: | Line 29: | ||
Hypoglycemia stimulates secretion of [[glucagon]]. This happens when blood glucose level falls between 65–70 mmHg. Failure to secrete glucagon may be the result of [[Beta cell|beta-cell]] failure and high [[insulin]] level that inhibits [[glucagon]] secretion.<ref name="pmid157348532">{{cite journal| author=Raju B, Cryer PE| title=Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. | journal=Diabetes | year= 2005 | volume= 54 | issue= 3 | pages= 757-64 | pmid=15734853 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15734853 }}</ref> | Hypoglycemia stimulates secretion of [[glucagon]]. This happens when blood glucose level falls between 65–70 mmHg. Failure to secrete glucagon may be the result of [[Beta cell|beta-cell]] failure and high [[insulin]] level that inhibits [[glucagon]] secretion.<ref name="pmid157348532">{{cite journal| author=Raju B, Cryer PE| title=Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. | journal=Diabetes | year= 2005 | volume= 54 | issue= 3 | pages= 757-64 | pmid=15734853 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15734853 }}</ref> | ||
* '''[[Epinephrine]]''' | * '''[[Epinephrine]]''' | ||
[[Epinephrine]] response to hypoglycemia becomes suppressed in many patients.<ref name="pmid8450063">{{cite journal| author=Dagogo-Jack SE, Craft S, Cryer PE| title=Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. | journal=J Clin Invest | year= 1993 | volume= 91 | issue= 3 | pages= 819-28 | pmid=8450063 | doi=10.1172/JCI116302 | pmc=288033 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8450063 }}</ref> This happens when [[blood glucose]] level falls between 65–70mmHg. A suppressed [[epinephrine]] response causes defective glucose counter-regulation and hypoglycemia unawareness occurs.<ref name="pmid18387080">{{cite journal| author=Geddes J, Schopman JE, Zammitt NN, Frier BM| title=Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. | journal=Diabet Med | year= 2008 | volume= 25 | issue= 4 | pages= 501-4 | pmid=18387080 | doi=10.1111/j.1464-5491.2008.02413.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18387080 }}</ref> This may be due to shifting the [[glycemic]] threshold for the sympathoadrenal response to a lower [[plasma glucose]] concentration. [[Brain|The brain]] is the first organ to be affected by decreased blood glucose level. Impairment of judgment and [[Seizure]]s may occur resulting in [[coma]]. | [[Epinephrine]] response to hypoglycemia becomes suppressed in many patients.<ref name="pmid8450063">{{cite journal| author=Dagogo-Jack SE, Craft S, Cryer PE| title=Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. | journal=J Clin Invest | year= 1993 | volume= 91 | issue= 3 | pages= 819-28 | pmid=8450063 | doi=10.1172/JCI116302 | pmc=288033 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8450063 }}</ref> This happens when [[blood glucose]] level falls between 65–70mmHg. A suppressed [[epinephrine]] response causes defective [[glucose]] counter-regulation and hypoglycemia unawareness occurs.<ref name="pmid18387080">{{cite journal| author=Geddes J, Schopman JE, Zammitt NN, Frier BM| title=Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. | journal=Diabet Med | year= 2008 | volume= 25 | issue= 4 | pages= 501-4 | pmid=18387080 | doi=10.1111/j.1464-5491.2008.02413.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18387080 }}</ref> This may be due to shifting the [[glycemic]] threshold for the [[Adrenal|sympathoadrenal]] response to a lower [[plasma glucose]] concentration. [[Brain|The brain]] is the first organ to be affected by decreased [[blood glucose]] level. Impairment of judgment and [[Seizure]]s may occur resulting in [[coma]]. | ||
=== Pathogenesis of hypoglycemia in [[insulinoma]]: === | === Pathogenesis of hypoglycemia in [[insulinoma]]: === | ||
* [[Insulinoma]] is a rare benign [[pancreatic neuroendocrine tumor]] that arises from [[Islet cell|β islet cells]].<sup>[[Insulinoma pathophysiology#cite note-AlJadir2015-1|[1]]]</sup> | * [[Insulinoma]] is a rare benign [[pancreatic neuroendocrine tumor]] that arises from [[Islet cell|β islet cells]].<sup>[[Insulinoma pathophysiology#cite note-AlJadir2015-1|[1]]]</sup> | ||
* It usually occurs sporadically but 10% are found to be associated with [[MEN 1]] syndrome.<sup>[[Insulinoma pathophysiology#cite note-pmid18672144-2|[2]]]</sup> | * It usually occurs sporadically but 10% are found to be associated with [[MEN 1]] syndrome.<sup>[[Insulinoma pathophysiology#cite note-pmid18672144-2|[2]]]</sup> | ||
* It is thought that [[insulinoma]] is mediated by mutation in mTOR/P70S6K signaling pathway. An oral mTOR inhibitor ([[Everolimus]]) may make better [[Glycemic control|glycemic control]]<nowiki/> in people having [[insulinoma]].<sup>[[Insulinoma pathophysiology#cite note-pmid19129539-4|[4]]]</sup> | * It is thought that [[insulinoma]] is mediated by a mutation in mTOR/P70S6K signaling pathway. An oral mTOR inhibitor ([[Everolimus]]) may make better [[Glycemic control|glycemic control]]<nowiki/> in people having an [[insulinoma]].<sup>[[Insulinoma pathophysiology#cite note-pmid19129539-4|[4]]]</sup> | ||
* [[Mitochondria]] play a key role in [[glucose]] and [[insulin]] coupling to assure insulin secretion after [[glucose]] stimulation in [[pancreatic]] β cells. Coupling is impaired due to abnormal [[mitochondrial]] function in β cells causes the death of the cell.<sup>[[Insulinoma pathophysiology#cite note-pmid22766318-6|[6]]]</sup> | * [[Mitochondria]] play a key role in [[glucose]] and [[insulin]] coupling to assure insulin secretion after [[glucose]] stimulation in [[pancreatic]] [[Beta cells|β cells]]. Coupling is impaired due to abnormal [[mitochondrial]] function in [[Beta cells|β cells]] causes the death of the cell.<sup>[[Insulinoma pathophysiology#cite note-pmid22766318-6|[6]]]</sup> | ||
* [[YY1]] regulates this [[mitochondria]]<nowiki/>l function.<sup>[[Insulinoma pathophysiology#cite note-pmid18046414-7|[7]]]</sup> T372R mutation | * [[YY1]] regulates this [[mitochondria]]<nowiki/>l function.<sup>[[Insulinoma pathophysiology#cite note-pmid18046414-7|[7]]]</sup> T372R mutation increases the [[transcription]] of YY1.<sup>[[Insulinoma pathophysiology#cite note-CaoGao2013-8|[8]]]</sup> The understanding of role and functions of [[YY1]] in [[Beta cells|β cells]] in near future might prove to be therapeutic potentials. | ||
* The progression to [[hypoglycemia]] is actually because of decreased [[glucose]] synthesis rather than increased use due to the direct effect of [[insulin]] on the [[liver]].<sup>[[Insulinoma pathophysiology#cite note-RizzaHaymond1981-9|[9]]]</sup> | * The progression to [[hypoglycemia]] is actually because of decreased [[glucose]] synthesis rather than increased use due to the direct effect of [[insulin]] on the [[liver]].<sup>[[Insulinoma pathophysiology#cite note-RizzaHaymond1981-9|[9]]]</sup> | ||
* The neuroglycopenic symptoms appear eventually due to decreased [[blood glucose]]. [[Hypoglycemia]] stimulates [[catecholamine]] release which produces [[adrenergic]] symptoms.<sup>[[Insulinoma pathophysiology#cite note-pmid1305178-10|[10]]]</sup> | * The neuroglycopenic symptoms appear eventually due to decreased [[blood glucose]]. [[Hypoglycemia]] stimulates [[catecholamine]] release which produces [[adrenergic]] symptoms.<sup>[[Insulinoma pathophysiology#cite note-pmid1305178-10|[10]]]</sup> | ||
=== Pathogenesis of hypoglycemia in non-islet-cell tumors hypoglycemia(NICTH): === | === Pathogenesis of hypoglycemia in non-islet-cell tumors hypoglycemia(NICTH): === | ||
* Non-islet-cell tumors are large tumors of [[mesenchymal]] or [[Epithelial cells|epithelial cell]] types originate from the [[Pancreas|pancreas.]] | * Non-islet-cell [[tumors]] are large tumors of [[mesenchymal]] or [[Epithelial cells|epithelial cell]] types originate from the [[Pancreas|pancreas.]] | ||
* NICTH appears to be increased [[glucose]] utilization and inhibition of glucose release from the [[liver]]. | * NICTH appears to be increased [[glucose]] utilization and inhibition of glucose release from the [[liver]]. | ||
* This happens as a result of tumor production of incompletely processed [[IGF2|IGF-2]].<ref name="pmid19088155">{{cite journal| author=Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER et al.| title=Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 3 | pages= 709-28 | pmid=19088155 | doi=10.1210/jc.2008-1410 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19088155 }}</ref> | * This happens as a result of tumor production of incompletely processed [[IGF2|IGF-2]].<ref name="pmid19088155">{{cite journal| author=Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER et al.| title=Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 3 | pages= 709-28 | pmid=19088155 | doi=10.1210/jc.2008-1410 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19088155 }}</ref> | ||
| Line 64: | Line 64: | ||
== Gross pathology == | == Gross pathology == | ||
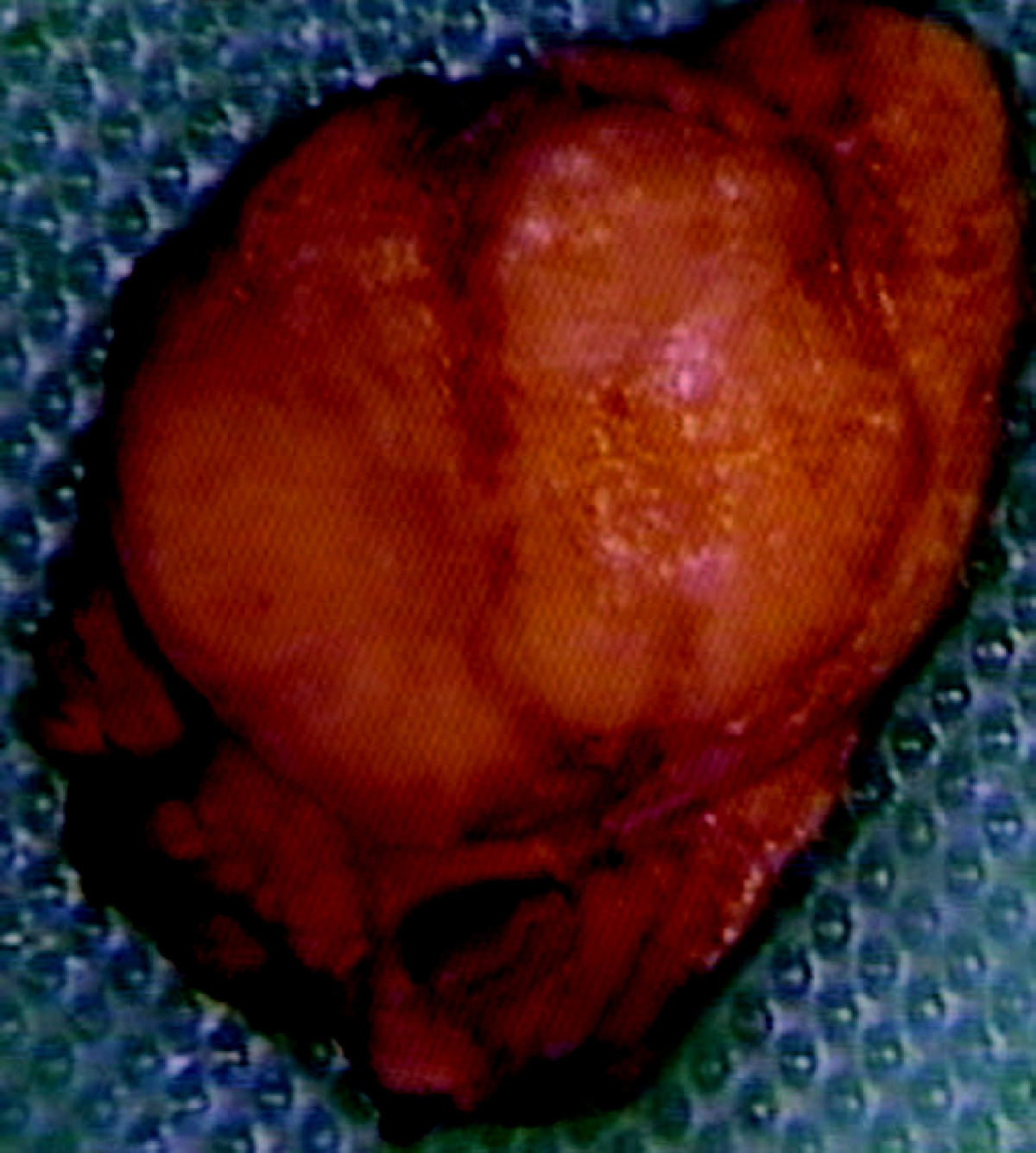
On gross pathology insulinomas have a gray to red brown appearance, encapsulated and are usually small and solitary tumors. Although there is a case report of a large(9cm), pedunculated and weighing more than 100g.<ref name="pmid15522939">{{cite journal| author=Mittendorf EA, Liu YC, McHenry CR| title=Giant insulinoma: case report and review of the literature. | journal=J Clin Endocrinol Metab | year= 2005 | volume= 90 | issue= 1 | pages= 575-80 | pmid=15522939 | doi=10.1210/jc.2004-0825 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15522939 }} </ref> | On gross pathology insulinomas have a gray to red-brown appearance, encapsulated and are usually small and solitary tumors. Although there is a case report of a large(9cm), pedunculated and weighing more than 100g.<ref name="pmid15522939">{{cite journal| author=Mittendorf EA, Liu YC, McHenry CR| title=Giant insulinoma: case report and review of the literature. | journal=J Clin Endocrinol Metab | year= 2005 | volume= 90 | issue= 1 | pages= 575-80 | pmid=15522939 | doi=10.1210/jc.2004-0825 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15522939 }} </ref> | ||
* Almost all [[Insulinoma|insulinomas]] are present throughout the [[pancreas]] and extrapancreatic ones causing hypoglycemia are rare(<2%).<ref name="pmid23430217">{{cite journal| author=Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y et al.| title=Diagnosis and management of insulinoma. | journal=World J Gastroenterol | year= 2013 | volume= 19 | issue= 6 | pages= 829-37 | pmid=23430217 | doi=10.3748/wjg.v19.i6.829 | pmc=3574879 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23430217 }}</ref> | * Almost all [[Insulinoma|insulinomas]] are present throughout the [[pancreas]] and extrapancreatic ones causing hypoglycemia are rare(<2%).<ref name="pmid23430217">{{cite journal| author=Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y et al.| title=Diagnosis and management of insulinoma. | journal=World J Gastroenterol | year= 2013 | volume= 19 | issue= 6 | pages= 829-37 | pmid=23430217 | doi=10.3748/wjg.v19.i6.829 | pmc=3574879 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23430217 }}</ref> | ||
* Various other findings are noted on gross pathology such as:<ref name="pmid17312378">{{cite journal| author=de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M et al.| title=Well-differentiated pancreatic tumor/carcinoma: insulinoma. | journal=Neuroendocrinology | year= 2006 | volume= 84 | issue= 3 | pages= 183-8 | pmid=17312378 | doi=10.1159/000098010 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17312378 }}</ref> | * Various other findings are noted on [[gross pathology]] such as:<ref name="pmid17312378">{{cite journal| author=de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M et al.| title=Well-differentiated pancreatic tumor/carcinoma: insulinoma. | journal=Neuroendocrinology | year= 2006 | volume= 84 | issue= 3 | pages= 183-8 | pmid=17312378 | doi=10.1159/000098010 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17312378 }}</ref> | ||
* Size of the tumor | * Size of the [[tumor]] | ||
* [[Metastasis]] to lymph nodes | * [[Metastasis]] to lymph nodes | ||
* | * Extra[[pancreatic]] involvement | ||
* Distant [[metastasis]] | * Distant [[metastasis]] | ||
[[File:Insulinoma.jpg|center|300px|thumb|Gross pathology of insulinoma, source: By Edward Alabraba et al. - Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World Journal of Surgical Oncology 2009, 7:18doi:10.1186/1477-7819-7-18, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=6686376]] | [[File:Insulinoma.jpg|center|300px|thumb|Gross pathology of insulinoma, source: By Edward Alabraba et al. - Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World Journal of Surgical Oncology 2009, 7:18doi:10.1186/1477-7819-7-18, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=6686376]] | ||
| Line 76: | Line 76: | ||
== Microscopic pathology == | == Microscopic pathology == | ||
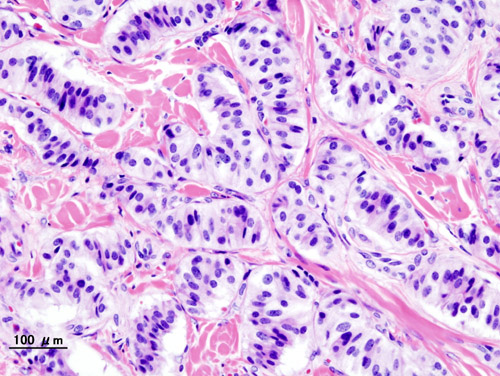
* On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with [[amyloid]] in a fibrovascular [[stroma]], are characteristic findings of [[Insulinoma|insulinoma.]]<ref>{{cite book | last = Lloyd | first = Ricardo | title = Endocrine pathology : differential diagnosis and molecular advances | publisher = Springer | location = New York London | year = 2010 | isbn = 978-1441910684 }}</ref> | * On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with [[amyloid]] in a fibrovascular [[stroma]], are characteristic findings of [[Insulinoma|insulinoma.]]<ref>{{cite book | last = Lloyd | first = Ricardo | title = Endocrine pathology : differential diagnosis and molecular advances | publisher = Springer | location = New York London | year = 2010 | isbn = 978-1441910684 }}</ref> | ||
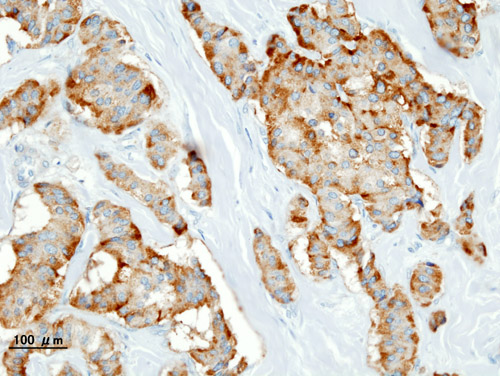
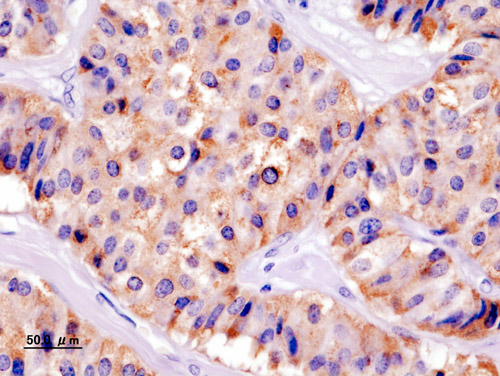
* It is also evaluated for the [[mitotic index]](mitosis per 10 high power field) and immunohistochemistry staining by [[Chromogranin A]], [[synaptophysin]], and [[Ki-67]] index.<ref name="de HerderNiederle2007">{{cite journal|last1=de Herder|first1=Wouter W.|last2=Niederle|first2=Bruno|last3=Scoazec|first3=Jean-Yves|last4=Pauwels|first4=Stanislas|last5=Klöppel|first5=Günter|last6=Falconi|first6=Massimo|last7=Kwekkeboom|first7=Dik J.|last8=Öberg|first8=Kjel|last9=Eriksson|first9=Barbro|last10=Wiedenmann|first10=Bertram|last11=Rindi|first11=Guido|last12=O’Toole|first12=Dermot|last13=Ferone|first13=Diego|title=Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma|journal=Neuroendocrinology|volume=84|issue=3|year=2007|pages=183–188|issn=0028-3835|doi=10.1159/000098010}}</ref> | * It is also evaluated for the [[mitotic index]](mitosis per 10 high-power field) and [[immunohistochemistry]] staining by [[Chromogranin A]], [[synaptophysin]], and [[Ki-67]] index.<ref name="de HerderNiederle2007">{{cite journal|last1=de Herder|first1=Wouter W.|last2=Niederle|first2=Bruno|last3=Scoazec|first3=Jean-Yves|last4=Pauwels|first4=Stanislas|last5=Klöppel|first5=Günter|last6=Falconi|first6=Massimo|last7=Kwekkeboom|first7=Dik J.|last8=Öberg|first8=Kjel|last9=Eriksson|first9=Barbro|last10=Wiedenmann|first10=Bertram|last11=Rindi|first11=Guido|last12=O’Toole|first12=Dermot|last13=Ferone|first13=Diego|title=Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma|journal=Neuroendocrinology|volume=84|issue=3|year=2007|pages=183–188|issn=0028-3835|doi=10.1159/000098010}}</ref> | ||
* The structure of tumor cells observed under [[Electron microscope|electron microscopy]] as Group A characterized by abundant well-granulated typical B cells with the [[Trabeculae|trabecula]]<nowiki/>r arrangement and Group B as scarce well-granulated typical B cells and a medullary arrangement. | * The structure of [[Tumor cell|tumor cells]] observed under [[Electron microscope|electron microscopy]] as Group A characterized by abundant well-granulated typical B cells with the [[Trabeculae|trabecula]]<nowiki/>r arrangement and Group B as scarce well-granulated typical B cells and a medullary arrangement. | ||
[[File:Pancreatic insulinoma histology 2.JPG|300px|thumb|left|Pancreatic insulinoma histopathology, source: CCBY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=507384]] | [[File:Pancreatic insulinoma histology 2.JPG|300px|thumb|left|Pancreatic insulinoma histopathology, source: CCBY-SA 3.0, https://commons.wikimedia.org/w/index.php?curid=507384]] | ||
Revision as of 21:31, 19 September 2017
|
Hypoglycemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Hypoglycemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hypoglycemia pathophysiology |
|
Risk calculators and risk factors for Hypoglycemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Mohammed Abdelwahed M.D[2]
Overview
The pathophysiology of hypoglycemia depends on the failure of physiological defense mechanisms and hormones such as insulin, glucagon and epinephrine to correct hypoglycemia. Most of these defense mechanisms are hormones that control glycogenolysis and gluconeogenesis.
Pathogenesis
Physiological effect of insulin
- Insulin binds to its receptor which starts many protein activation cascades.
- The insulin signal transduction pathway begins when insulin binds to the insulin receptor proteins.
- Once the transduction pathway is completed, the GLUT-4 storage vesicles fuse with the cellular membrane.
- As a result, the GLUT-4 protein channels become embedded into the membrane, allowing glucose to be transported into the cell.[1]
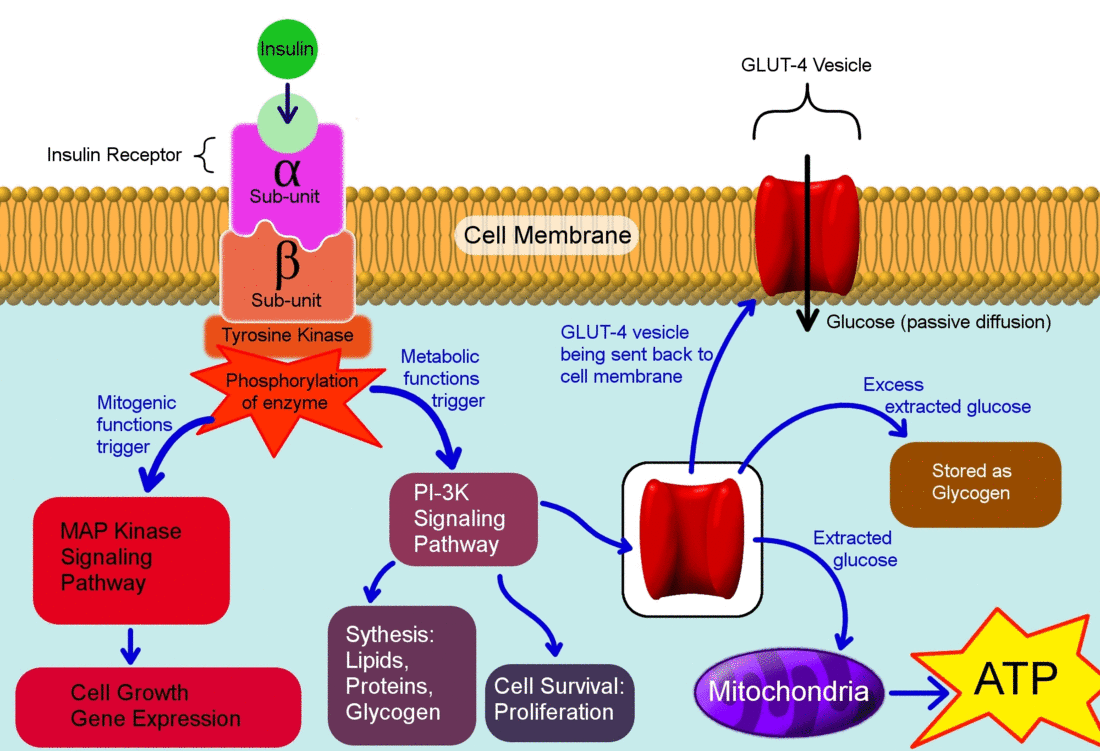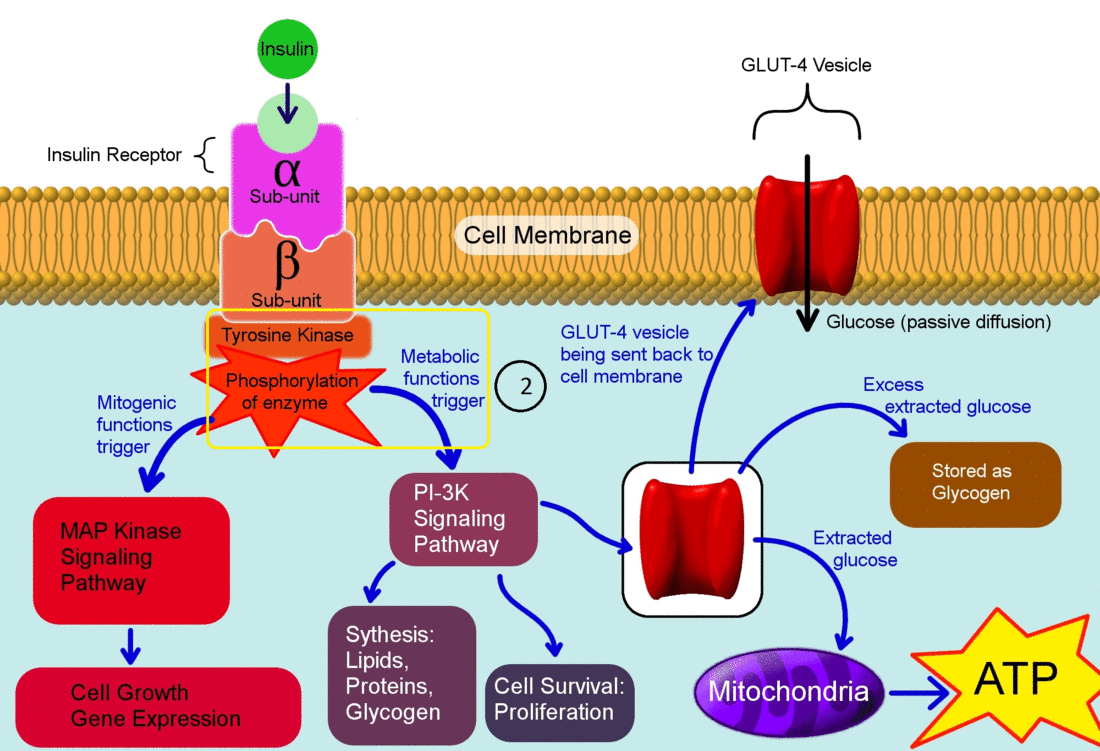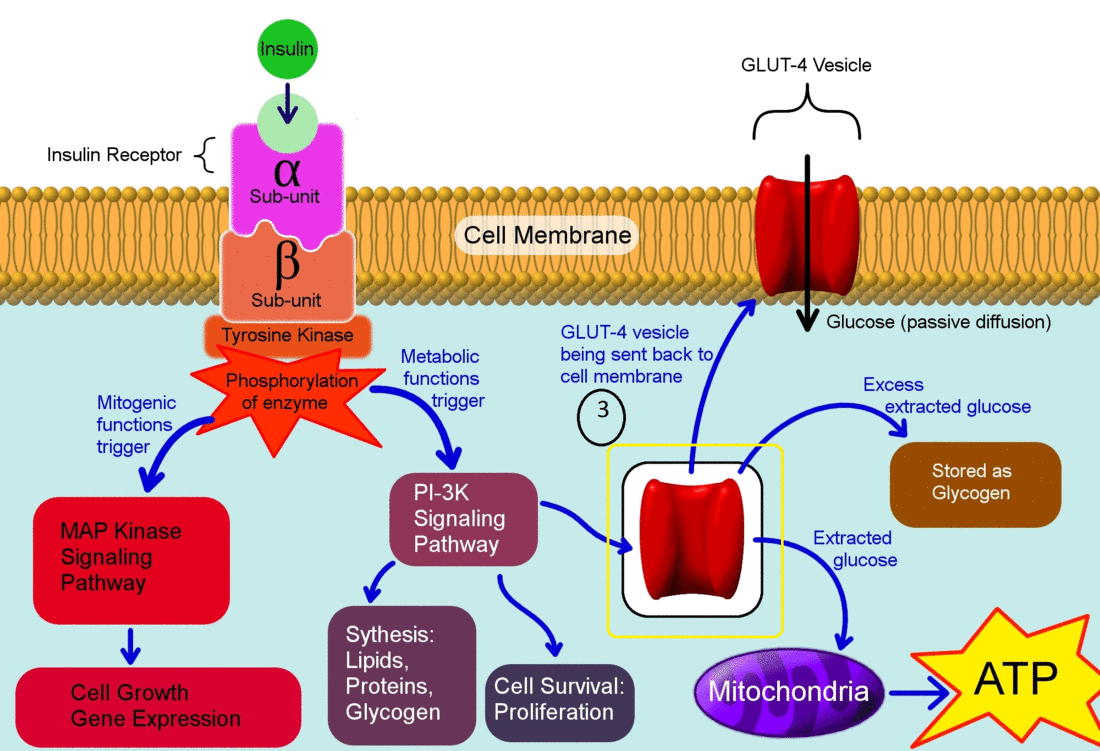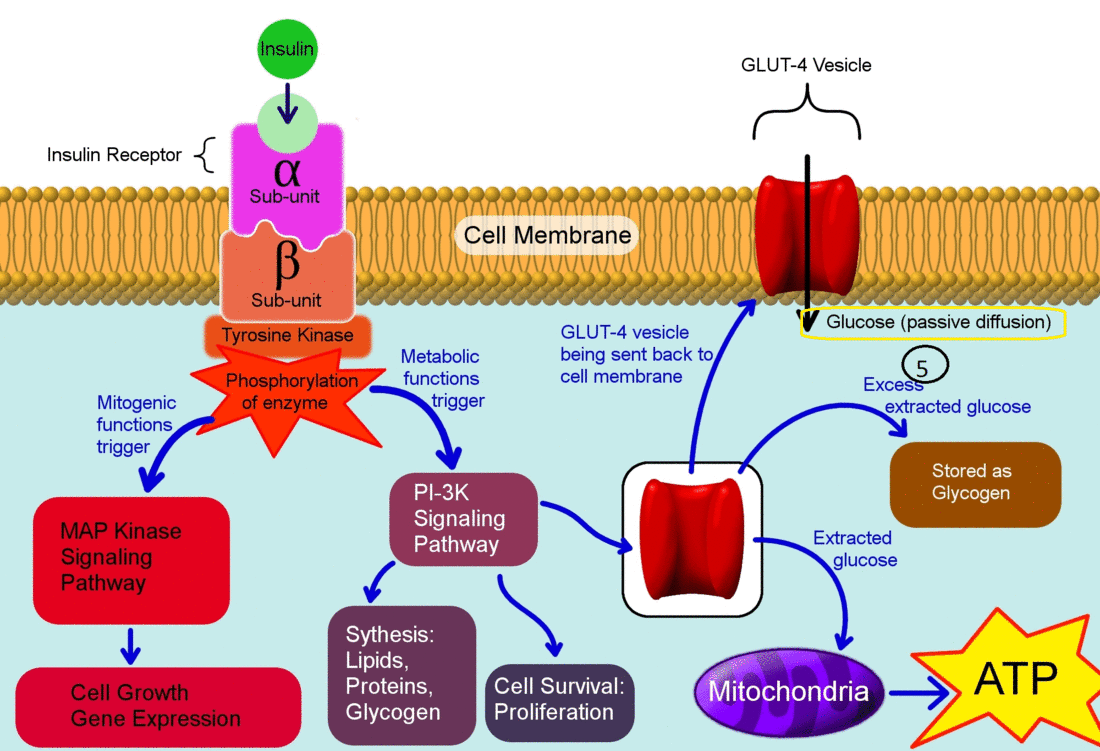
The actions of insulin on the human metabolism include:[2]
- Insulin decreases blood glucose concentration by inducing uptake of the glucose by peripheral cells. This function is as a result of increase GLUT4 transporter insertion in the cell membrane of muscles and fat tissues which allow glucose to enter the cell.
- The increase of DNA replication and protein synthesis via control of amino acids uptake.
- Induction of glycogen synthesis when glucose levels are high.
- The increase of cellular potassium uptake.
- Decreased gluconeogenesis and glycogenolysis: Decreased production of glucose from noncarbohydrate substrates, primarily in the liver (the vast majority of endogenous insulin arriving into the liver never leaves the liver).
- The increase of lipid synthesis: insulin forces fat cells to take in blood glucose, which is converted into triglycerides; a decrease of insulin causes the reverse.
- The decrease of lipolysis: insulin forces reduction in conversion of fat cell lipid stores into blood fatty acids and glycerol; a decrease of insulin causes the reverse.
- The decrease of proteolysis: insulin decreases the breakdown of protein.
- The decrease of renal sodium excretion.
Pathogenesis of hypoglycemia in diabetics
The pathophysiology of hypoglycemia mainly relies on the failure of physiological defense mechanisms and hormones such as insulin, glucagon and epinephrine to correct hypoglycemia. Most of these hormones control glycogenolysis and gluconeogenesis, including:
The most important and the first mechanism to counter-regulate hypoglycemia is the ability to suppress insulin release. This happens early when blood glucose level is between 80–85 mmHg. This cannot occur in patients with absolute beta-cell failure, type 1 diabetes mellitus, and long-standing type 2 diabetes.[3] High insulin levels inhibit hepatic glycogenolysis causing more hypoglycemia.
Hypoglycemia stimulates secretion of glucagon. This happens when blood glucose level falls between 65–70 mmHg. Failure to secrete glucagon may be the result of beta-cell failure and high insulin level that inhibits glucagon secretion.[4]
Epinephrine response to hypoglycemia becomes suppressed in many patients.[5] This happens when blood glucose level falls between 65–70mmHg. A suppressed epinephrine response causes defective glucose counter-regulation and hypoglycemia unawareness occurs.[6] This may be due to shifting the glycemic threshold for the sympathoadrenal response to a lower plasma glucose concentration. The brain is the first organ to be affected by decreased blood glucose level. Impairment of judgment and Seizures may occur resulting in coma.
Pathogenesis of hypoglycemia in insulinoma:
- Insulinoma is a rare benign pancreatic neuroendocrine tumor that arises from β islet cells.[1]
- It usually occurs sporadically but 10% are found to be associated with MEN 1 syndrome.[2]
- It is thought that insulinoma is mediated by a mutation in mTOR/P70S6K signaling pathway. An oral mTOR inhibitor (Everolimus) may make better glycemic control in people having an insulinoma.[4]
- Mitochondria play a key role in glucose and insulin coupling to assure insulin secretion after glucose stimulation in pancreatic β cells. Coupling is impaired due to abnormal mitochondrial function in β cells causes the death of the cell.[6]
- YY1 regulates this mitochondrial function.[7] T372R mutation increases the transcription of YY1.[8] The understanding of role and functions of YY1 in β cells in near future might prove to be therapeutic potentials.
- The progression to hypoglycemia is actually because of decreased glucose synthesis rather than increased use due to the direct effect of insulin on the liver.[9]
- The neuroglycopenic symptoms appear eventually due to decreased blood glucose. Hypoglycemia stimulates catecholamine release which produces adrenergic symptoms.[10]
Pathogenesis of hypoglycemia in non-islet-cell tumors hypoglycemia(NICTH):
- Non-islet-cell tumors are large tumors of mesenchymal or epithelial cell types originate from the pancreas.
- NICTH appears to be increased glucose utilization and inhibition of glucose release from the liver.
- This happens as a result of tumor production of incompletely processed IGF-2.[7]
- Incompletely processed IGF-2 also suppresses glucagon and growth hormone release.[8]
- The net result is continued glucose utilization by skeletal muscle and inhibition of glucose release, glycogenolysis, and gluconeogenesis in the liver.[8]
Genetics
Genes associated with diabetes include the following:[9]
- Currently, 58 genomic regions are found to be associated with Type 1 DM.
- The major susceptibility gene for type1 DM is located on HLA region of chromosome 6. It accounts for 40-50% of the genetic risk for type1 DM. This region encodes for class II major histocompatibility complex (MHC) molecules. Class II major histocompatibility complex (MHC) molecules play an important role in presenting antigen to helper T cells and initiating an immune response.
- Other major susceptibility genes which were associated with Type1 DM include polymorphisms in the promoter region of the insulin gene, the CTLA-4 gene, interleukin 2 receptor, CTLA4, and PTPN22.
- Presence of certain genes confers protection against the development of the disease. Haplotype DQA1*0102, DQB1*0602 is extremely rare in individuals with type1 DM (<1%) and appears to provide protection from type1 DM.
Genetics associated with syndrome:[10]
- Deregulation of imprinted gene expression in the chromosome 11p15.5 region can result in the BWS phenotype.
- The critical BWS genes in that region include insulin-like growth factor 2 (IGF2), H19, cyclin-dependent kinase inhibitor 1C (CDKN1C), potassium channel voltage-gated KQT-like subfamily member 1 (KCNQ1), and KCNQ1-overlapping transcript 1 (KCNQ1OT1, or long QT intronic transcript 1).
Genes associated with autoimmune hypoglycemia include the following:[11]
- Serological HLA typing demonstrated the patient had HLA-DR4.
- DNA typing showed she had HLA-DRB1*0401 and HLA-DRB1*0406 is strikingly associated with patients with insulin autoimmune syndrome who have polyclonal insulin autoantibodies.
Gross pathology
On gross pathology insulinomas have a gray to red-brown appearance, encapsulated and are usually small and solitary tumors. Although there is a case report of a large(9cm), pedunculated and weighing more than 100g.[12]
- Almost all insulinomas are present throughout the pancreas and extrapancreatic ones causing hypoglycemia are rare(<2%).[13]
- Various other findings are noted on gross pathology such as:[14]
- Size of the tumor
- Metastasis to lymph nodes
- Extrapancreatic involvement
- Distant metastasis
Microscopic pathology
- On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with amyloid in a fibrovascular stroma, are characteristic findings of insulinoma.[15]
- It is also evaluated for the mitotic index(mitosis per 10 high-power field) and immunohistochemistry staining by Chromogranin A, synaptophysin, and Ki-67 index.[16]
- The structure of tumor cells observed under electron microscopy as Group A characterized by abundant well-granulated typical B cells with the trabecular arrangement and Group B as scarce well-granulated typical B cells and a medullary arrangement.
References
- ↑ Kuznetsova LA, Plesneva SA, Sharova TS, Pertseva MN, Shpakov AO (2013). "[Regulation of adenylyl cyclase signaling system by insulin, biogenic amines, and glucagon at their separate and combined action in the muscle membranes of the mollusc Anodonta cygnea]". Zh Evol Biokhim Fiziol. 49 (2): 111–7. PMID 23789396.
- ↑ Ahmad K (2014). "Insulin sources and types: a review of insulin in terms of its mode on diabetes mellitus". J Tradit Chin Med. 34 (2): 234–7. PMID 24783939.
- ↑ Dunning BE, Gerich JE (2007). "The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications". Endocr Rev. 28 (3): 253–83. doi:10.1210/er.2006-0026. PMID 17409288.
- ↑ Raju B, Cryer PE (2005). "Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans". Diabetes. 54 (3): 757–64. PMID 15734853.
- ↑ Dagogo-Jack SE, Craft S, Cryer PE (1993). "Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia". J Clin Invest. 91 (3): 819–28. doi:10.1172/JCI116302. PMC 288033. PMID 8450063.
- ↑ Geddes J, Schopman JE, Zammitt NN, Frier BM (2008). "Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes". Diabet Med. 25 (4): 501–4. doi:10.1111/j.1464-5491.2008.02413.x. PMID 18387080.
- ↑ Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER; et al. (2009). "Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline". J Clin Endocrinol Metab. 94 (3): 709–28. doi:10.1210/jc.2008-1410. PMID 19088155.
- ↑ 8.0 8.1 Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL; et al. (2013). "Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive". Endocr Rev. 34 (6): 798–826. doi:10.1210/er.2012-1033. PMID 23671155.
- ↑ Pociot F, Lernmark Å (2016). "Genetic risk factors for type 1 diabetes". Lancet. 387 (10035): 2331–9. doi:10.1016/S0140-6736(16)30582-7. PMID 27302272.
- ↑ Weksberg R, Shuman C, Smith AC (2005). "Beckwith-Wiedemann syndrome". Am J Med Genet C Semin Med Genet. 137C (1): 12–23. doi:10.1002/ajmg.c.30058. PMID 16010676.
- ↑ Murakami M, Mizuide M, Kashima K, Kojima A, Tomioka SI, Kohama T; et al. (2000). "Identification of monoclonal insulin autoantibodies in insulin autoimmune syndrome associated with HLA-DRB1*0401". Horm Res. 54 (1): 49–52. doi:63437 Check
|doi=value (help). PMID 11182636. - ↑ Mittendorf EA, Liu YC, McHenry CR (2005). "Giant insulinoma: case report and review of the literature". J Clin Endocrinol Metab. 90 (1): 575–80. doi:10.1210/jc.2004-0825. PMID 15522939.
- ↑ Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y; et al. (2013). "Diagnosis and management of insulinoma". World J Gastroenterol. 19 (6): 829–37. doi:10.3748/wjg.v19.i6.829. PMC 3574879. PMID 23430217.
- ↑ de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M; et al. (2006). "Well-differentiated pancreatic tumor/carcinoma: insulinoma". Neuroendocrinology. 84 (3): 183–8. doi:10.1159/000098010. PMID 17312378.
- ↑ Lloyd, Ricardo (2010). Endocrine pathology : differential diagnosis and molecular advances. New York London: Springer. ISBN 978-1441910684.
- ↑ de Herder, Wouter W.; Niederle, Bruno; Scoazec, Jean-Yves; Pauwels, Stanislas; Klöppel, Günter; Falconi, Massimo; Kwekkeboom, Dik J.; Öberg, Kjel; Eriksson, Barbro; Wiedenmann, Bertram; Rindi, Guido; O’Toole, Dermot; Ferone, Diego (2007). "Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma". Neuroendocrinology. 84 (3): 183–188. doi:10.1159/000098010. ISSN 0028-3835.