Hypoglycemia pathophysiology: Difference between revisions
| (28 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
__NOTOC__ | __NOTOC__ | ||
{{Hypoglycemia}} | {{Hypoglycemia}} | ||
{{CMG}} {{AE}} {{MAD}} | {{CMG}} {{AE}} {{MAD}} {{ADS}} | ||
==Overview== | ==Overview== | ||
The pathophysiology of hypoglycemia depends on the failure of physiological defense mechanisms | The pathophysiology of hypoglycemia depends on the failure of physiological defense mechanisms and [[Hormone|hormones]] such as [[insulin]], [[Glucagon|glucagon,]] and [[epinephrine]] to correct hypoglycemia. Most of these defense mechanisms are hormones that control [[glycogenolysis]] and [[Gluconeogenesis|gluconeogenesis.]] [[Insulinoma]] is a rare benign [[pancreatic neuroendocrine tumor]] that arises from [[Islet cell|β islet cells]]. It is mediated by a mutation in [[Mammalian target of rapamycin|mTOR]]/P70S6K signaling pathway. Non-islet-cell [[tumors]] (NICTH) are large tumors of [[mesenchymal]] or [[Epithelial cells|epithelial cell]] types originate from the [[Pancreas|pancreas.]] Hypoglycemia due to NICTH appears to be related to increased [[glucose]] utilization and inhibition of glucose release from the [[liver]]. This happens as a result of tumor production of incompletely processed [[IGF2|IGF-2]]. On gross pathology [[Insulinoma|insulinomas]] have a gray to red-brown appearance, encapsulated and are usually small and solitary [[tumors]]. On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with [[amyloid]] in a fibrovascular [[stroma]], are characteristic findings of [[Insulinoma|insulinoma.]] It is also evaluated for the [[mitotic index]] and [[immunohistochemistry]] staining by [[Chromogranin A]], [[synaptophysin]], and [[Ki-67]] index. | ||
== | |||
=== Physiological effect of | == Hypoglycemia pathophysiology == | ||
[[Insulin]] binds to its receptor which | |||
[[File:Insulin | ==== Physiological effect of insulin ==== | ||
<br clear="left" /> | # [[Insulin]] binds to its [[receptor]] which involves many [[protein]] activation cascades.<ref name="pmid23789396" /> | ||
* [[Insulin]] decreases [[Blood sugar|blood glucose]] concentration by inducing | # Binding of [[insulin]] to the α-subunit results in changes which activate [[tyrosine kinase]] domains on each β-subunit. | ||
# The [[tyrosine kinase]] activity causes [[phosphorylation]] of intracellular [[enzymes]]. | |||
# The [[phosphorylation]] of MAP-Kinase leads to induction of [[gene expression]]. | |||
# [[Phosphorylation]] of PI-3K isolates the GLUT-4 Vesicle and sends the vesicles back to the cell membrane. | |||
# The [[GLUT4|GLUT-4]] vesicles fuse with the cellular membrane allowing [[glucose]] to be transported into the cell. | |||
[[File:Insulin-intracellular-signalings 1.gif|500px|center|thumb: Insulin cellular effect, source: Wikipedia]] | |||
<br clear="left" />[[Insulin]] is involved in many aspects of [[metabolism]] including:<ref name="pmid24783939">{{cite journal| author=Ahmad K| title=Insulin sources and types: a review of insulin in terms of its mode on diabetes mellitus. | journal=J Tradit Chin Med | year= 2014 | volume= 34 | issue= 2 | pages= 234-7 | pmid=24783939 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24783939 }}</ref> | |||
* [[Insulin]] decreases [[Blood sugar|blood glucose]] concentration by inducing uptake of the [[glucose]] by peripheral cells. This function is as a result of increased [[GLUT4]] transporter insertion in the [[cell membrane]] of [[Muscle|muscles]] and [[Adipose tissue|fat tissues]] which allows [[glucose]] to enter the cells. | |||
* Increase of [[DNA replication]] and [[protein synthesis]] via control of [[Amino acid|amino acids]] uptake. | * Increase of [[DNA replication]] and [[protein synthesis]] via control of [[Amino acid|amino acids]] uptake. | ||
* Induction of [[glycogen]] synthesis when [[glucose]] levels are high. | * Induction of [[glycogen]] synthesis when [[glucose]] levels are high. | ||
* Increase of cellular [[potassium]] uptake. | * Increase of cellular [[potassium]] uptake. | ||
* Decreased [[gluconeogenesis]] and [[glycogenolysis]] | * Decreased [[gluconeogenesis]] and [[glycogenolysis]]; decreased production of [[glucose]] from noncarbohydrate substrates, primarily in the [[liver]] (the vast majority of endogenous [[insulin]] arriving into the [[liver]] never leaves the [[Liver|liver)]]. | ||
* Increase of [[lipid]] synthesis: [[insulin]] forces [[fat cells]] to | * Increase of [[lipid]] synthesis: [[insulin]] forces [[fat cells]] to use [[blood glucose]], which is converted into [[triglycerides]]; a decrease of [[insulin]] causes the reverse. | ||
* | * Decrease in [[lipolysis]]: [[insulin]] forces reduction in conversion of [[Lipid|fat cell lipid]] stores into [[blood]] [[fatty acids]] and [[glycerol]]; a decrease of [[insulin]] causes the reverse. | ||
* | * Decrease in [[proteolysis]]: [[insulin]] decreases the breakdown of [[protein]]. | ||
* Decrease of renal [[sodium]] excretion. | * Decrease of renal [[sodium]] excretion. | ||
=== Pathogenesis of hypoglycemia in diabetics === | === Pathogenesis of hypoglycemia in diabetics === | ||
The pathophysiology of hypoglycemia mainly relies on the failure of physiological defense mechanisms and [[hormones]] such as [[insulin]], [[glucagon]] and [[epinephrine]] to correct hypoglycemia. Most of these hormones control [[glycogenolysis]] and [[gluconeogenesis]], including: | The pathophysiology of hypoglycemia mainly relies on the failure of physiological defense mechanisms and [[hormones]] such as [[insulin]], [[glucagon]] and [[epinephrine]] to correct hypoglycemia. Most of these hormones control [[glycogenolysis]] and [[gluconeogenesis]], including: | ||
* ''' | * '''Insulin''' | ||
The most important and the first mechanism to counter-regulate hypoglycemia is the ability to suppress insulin release. This happens early when blood glucose level is | The most important and the first mechanism to counter-regulate hypoglycemia is the ability to suppress insulin release. This happens early when blood [[glucose]] level is between 80–85 mmHg. This can not occur in patients with absolute [[Beta cell|beta-cell]] failure, [[Diabetes mellitus type 1|type 1 diabetes mellitus]], and long-standing [[Diabetes mellitus type 2|type 2 diabetes]].<ref name="pmid174092882">{{cite journal| author=Dunning BE, Gerich JE| title=The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications. | journal=Endocr Rev | year= 2007 | volume= 28 | issue= 3 | pages= 253-83 | pmid=17409288 | doi=10.1210/er.2006-0026 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17409288 }}</ref> High [[insulin]] levels inhibit [[Glycogenlysis|hepatic]] [[glycogenolysis]] causing more hypoglycemia. | ||
* ''' | * '''Glucagon''' | ||
Hypoglycemia stimulates secretion of [[glucagon]]. This happens when blood glucose level falls | Hypoglycemia stimulates secretion of [[glucagon]]. This happens when blood [[glucose]] level falls between 65–70 mmHg. Failure to secrete [[glucagon]] may be the result of [[Beta cell|beta-cell]] failure and high [[insulin]] level that inhibits [[glucagon]] secretion.<ref name="pmid157348532">{{cite journal| author=Raju B, Cryer PE| title=Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans. | journal=Diabetes | year= 2005 | volume= 54 | issue= 3 | pages= 757-64 | pmid=15734853 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15734853 }}</ref> | ||
* ''' | * '''Epinephrine''' | ||
[[Epinephrine]] response to hypoglycemia | [[Epinephrine]] response to hypoglycemia becomes suppressed in many patients.<ref name="pmid8450063">{{cite journal| author=Dagogo-Jack SE, Craft S, Cryer PE| title=Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia. | journal=J Clin Invest | year= 1993 | volume= 91 | issue= 3 | pages= 819-28 | pmid=8450063 | doi=10.1172/JCI116302 | pmc=288033 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=8450063 }}</ref> This happens when [[blood glucose]] level falls between 65–70mmHg. A suppressed [[epinephrine]] response causes defective [[glucose]] counter-regulation and hypoglycemia unawareness occurs.<ref name="pmid18387080">{{cite journal| author=Geddes J, Schopman JE, Zammitt NN, Frier BM| title=Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes. | journal=Diabet Med | year= 2008 | volume= 25 | issue= 4 | pages= 501-4 | pmid=18387080 | doi=10.1111/j.1464-5491.2008.02413.x | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18387080 }}</ref> This may be due to shifting the [[glycemic]] threshold for the [[Adrenal|sympathoadrenal]] response to a lower [[plasma glucose]] concentration. [[Brain|The brain]] is the first organ to be affected by decreased [[blood glucose]] level. Impairment of judgment and [[Seizure]]s may occur resulting in [[coma]]. | ||
=== Pathogenesis of hypoglycemia in | === Pathogenesis of hypoglycemia in insulinoma: === | ||
* [[Insulinoma]] is a rare benign [[pancreatic neuroendocrine tumor]] that arises from [[Islet cell|β islet cells]].<sup>[[Insulinoma pathophysiology#cite note-AlJadir2015-1|[1]]]</sup> | * [[Insulinoma]] is a rare benign [[pancreatic neuroendocrine tumor]] that arises from [[Islet cell|β islet cells]].<sup>[[Insulinoma pathophysiology#cite note-AlJadir2015-1|[1]]]</sup><ref name="pmid6262168">{{cite journal| author=Rizza RA, Haymond MW, Verdonk CA, Mandarino LJ, Miles JM, Service FJ et al.| title=Pathogenesis of hypoglycemia in insulinoma patients: suppression of hepatic glucose production by insulin. | journal=Diabetes | year= 1981 | volume= 30 | issue= 5 | pages= 377-81 | pmid=6262168 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=6262168 }}</ref> | ||
* It usually occurs sporadically but 10% are found to be associated with [[MEN 1]] syndrome.<sup>[[Insulinoma pathophysiology#cite note-pmid18672144-2|[2]]]</sup> | * It usually occurs sporadically but 10% are found to be associated with [[MEN 1]] syndrome.<sup>[[Insulinoma pathophysiology#cite note-pmid18672144-2|[2]]]</sup> | ||
* It is thought that [[insulinoma]] is mediated by mTOR/P70S6K signaling pathway. An oral mTOR inhibitor | * It is thought that [[insulinoma]] is mediated by a mutation in [[Mammalian target of rapamycin|mTOR]]/P70S6K signaling pathway. An oral [[Mammalian target of rapamycin|mTOR]] inhibitor ([[Everolimus]]) may make better [[Glycemic control|glycemic control]]<nowiki/> in people having an [[insulinoma]].<sup>[[Insulinoma pathophysiology#cite note-pmid19129539-4|[4]]]</sup> | ||
* [[Mitochondria]] | * [[Mitochondria]] plays a key role in [[glucose]] and [[insulin]] coupling to assure [[insulin]] secretion after [[glucose]] stimulation in [[pancreatic]] [[Beta cells|β cells]]. Coupling is impaired due to abnormal [[mitochondrial]] function in [[Beta cells|β cells]] causes the death of the cell.<sup>[[Insulinoma pathophysiology#cite note-pmid22766318-6|[6]]]</sup> | ||
* [[YY1]] regulates this [[mitochondria]]<nowiki/>l function.<sup>[[Insulinoma pathophysiology#cite note-pmid18046414-7|[7]]]</sup>T372R mutation | * [[YY1]] regulates this [[mitochondria]]<nowiki/>l function.<sup>[[Insulinoma pathophysiology#cite note-pmid18046414-7|[7]]]</sup> T372R mutation increases the [[transcription]] of YY1. The understanding of role and functions of [[YY1]] in [[Beta cells|β cells]] in near future might prove to be therapeutic potentials.<sup>[[Insulinoma pathophysiology#cite note-CaoGao2013-8|[8]]]</sup> | ||
* The progression to [[hypoglycemia]] is actually because of decreased [[glucose]] synthesis rather than increased use due to the direct effect of [[insulin]] on the [[liver]].<sup>[[Insulinoma pathophysiology#cite note-RizzaHaymond1981-9|[9]]]</sup> | * The progression to [[hypoglycemia]] is actually because of decreased [[glucose]] synthesis rather than increased use due to the direct effect of [[insulin]] on the [[liver]].<sup>[[Insulinoma pathophysiology#cite note-RizzaHaymond1981-9|[9]]]</sup> | ||
* The neuroglycopenic symptoms appear eventually due to decreased [[blood glucose]]. [[Hypoglycemia]] stimulates [[catecholamine]] release which produces [[adrenergic]] symptoms.<sup>[[Insulinoma pathophysiology#cite note-pmid1305178-10|[10]]]</sup> | * The neuroglycopenic symptoms appear eventually due to decreased [[blood glucose]]. [[Hypoglycemia]] stimulates [[catecholamine]] release which produces [[adrenergic]] symptoms.<sup>[[Insulinoma pathophysiology#cite note-pmid1305178-10|[10]]]</sup> | ||
=== Pathogenesis of hypoglycemia in non-islet-cell tumors hypoglycemia(NICTH): === | === Pathogenesis of hypoglycemia in non-islet-cell tumors hypoglycemia (NICTH): === | ||
* Non-islet-cell tumors are large tumors of [[mesenchymal]] or [[Epithelial cells|epithelial cell]] types originate from the [[Pancreas|pancreas.]] | * Non-islet-cell [[tumors]] are large tumors of [[mesenchymal]] or [[Epithelial cells|epithelial cell]] types originate from the [[Pancreas|pancreas.]] | ||
* NICTH appears to be increased [[glucose]] utilization and inhibition of glucose release from the [[liver]]. | * NICTH appears to be increased [[glucose]] utilization and inhibition of [[glucose]] release from the [[liver]]. | ||
* This happens as a result of tumor production of incompletely processed [[IGF2|IGF-2]].<ref name="pmid19088155">{{cite journal| author=Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER et al.| title=Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 3 | pages= 709-28 | pmid=19088155 | doi=10.1210/jc.2008-1410 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19088155 }}</ref> | * This happens as a result of tumor production of incompletely processed [[IGF2|IGF-2]].<ref name="pmid19088155">{{cite journal| author=Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER et al.| title=Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline. | journal=J Clin Endocrinol Metab | year= 2009 | volume= 94 | issue= 3 | pages= 709-28 | pmid=19088155 | doi=10.1210/jc.2008-1410 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=19088155 }}</ref> | ||
* Incompletely processed [[IGF2|IGF-2]] also suppresses [[glucagon]] and [[growth hormone]] release.<ref name="pmid23671155">{{cite journal| author=Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL et al.| title=Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. | journal=Endocr Rev | year= 2013 | volume= 34 | issue= 6 | pages= 798-826 | pmid=23671155 | doi=10.1210/er.2012-1033 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23671155 }}</ref> | * Incompletely processed [[IGF2|IGF-2]] also suppresses [[glucagon]] and [[growth hormone]] release.<ref name="pmid23671155">{{cite journal| author=Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL et al.| title=Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive. | journal=Endocr Rev | year= 2013 | volume= 34 | issue= 6 | pages= 798-826 | pmid=23671155 | doi=10.1210/er.2012-1033 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23671155 }}</ref> | ||
* The net result is continued [[glucose]] utilization by skeletal muscle and inhibition of [[glucose]] release, [[glycogenolysis]], and [[gluconeogenesis]] in the [[liver]].<ref name="pmid23671155" /> | * The net result is continued [[glucose]] utilization by [[skeletal muscle]] and inhibition of [[glucose]] release, [[glycogenolysis]], and [[gluconeogenesis]] in the [[liver]].<ref name="pmid23671155" /> | ||
== Genetics == | == Genetics == | ||
==== Genes associated with diabetes include the following:<ref name="pmid27302272">{{cite journal| author=Pociot F, Lernmark Å| title=Genetic risk factors for type 1 diabetes. | journal=Lancet | year= 2016 | volume= 387 | issue= 10035 | pages= 2331-9 | pmid=27302272 | doi=10.1016/S0140-6736(16)30582-7 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27302272 }}</ref> ==== | ==== Genes associated with diabetes include the following:<ref name="pmid27302272">{{cite journal| author=Pociot F, Lernmark Å| title=Genetic risk factors for type 1 diabetes. | journal=Lancet | year= 2016 | volume= 387 | issue= 10035 | pages= 2331-9 | pmid=27302272 | doi=10.1016/S0140-6736(16)30582-7 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=27302272 }}</ref><ref name="pmid15161766">{{cite journal| author=Højlund K, Hansen T, Lajer M, Henriksen JE, Levin K, Lindholm J et al.| title=A novel syndrome of autosomal-dominant hyperinsulinemic hypoglycemia linked to a mutation in the human insulin receptor gene. | journal=Diabetes | year= 2004 | volume= 53 | issue= 6 | pages= 1592-8 | pmid=15161766 | doi= | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15161766 }}</ref> ==== | ||
*Currently, 58 [[Genomics|genomic regions]] are found to be associated | *Currently, 58 [[Genomics|genomic regions]] are found to be associated with [[Diabetes mellitus type 1|type 1 diabetes mellitus]] ([[DM1|DM]]). | ||
*The major susceptibility gene for [[DM1|type1 DM]] is located on [[HLA]] region of [[Chromosome 6 (human)|chromosome 6]]. It accounts for 40-50% of the genetic risk for [[DM1|type1 DM]]. This region encodes for class II [[major histocompatibility complex]] (MHC) molecules. [[Major histocompatibility complex 2|Class II major histocompatibility complex]] (MHC) molecules play an important role in presenting antigen to helper [[T cell|T cells]] and initiating an immune response. | *The major susceptibility gene for [[DM1|type1 DM]] is located on [[HLA]] region of [[Chromosome 6 (human)|chromosome 6]]. It accounts for 40-50% of the genetic risk for [[DM1|type1 DM]]. This region encodes for class II [[major histocompatibility complex]] (MHC) molecules. [[Major histocompatibility complex 2|Class II major histocompatibility complex]] (MHC) molecules play an important role in presenting antigen to helper [[T cell|T cells]] and initiating an immune response. | ||
*Other major susceptibility genes which were associated with [[DM1|Type1 DM]] include [[polymorphisms]] in the [[promoter region]] of the insulin gene, the [[CTLA-4]] gene, [[IL-2|interleukin 2 receptor]], [[CTLA4]], and [[PTPN22]]. | *Other major susceptibility genes which were associated with [[DM1|Type1 DM]] include [[polymorphisms]] in the [[promoter region]] of the insulin gene, the [[CTLA-4]] gene, [[IL-2|interleukin 2 receptor]], [[CTLA4]], and [[PTPN22]]. | ||
*Presence of certain genes confers protection against the development of the disease. Haplotype DQA1*0102, DQB1*0602 | *Presence of certain [[genes]] confers protection against the development of the disease. [[Haplotype|Haplotypes]] DQA1*0102, DQB1*0602 are extremely rare in individuals with [[DM1|type1 DM]] (<1%) and appears to provide protection from [[DM1|type1 DM]]. | ||
==== Genetics associated with | ==== Genetics associated with:<ref name="pmid16010676">{{cite journal| author=Weksberg R, Shuman C, Smith AC| title=Beckwith-Wiedemann syndrome. | journal=Am J Med Genet C Semin Med Genet | year= 2005 | volume= 137C | issue= 1 | pages= 12-23 | pmid=16010676 | doi=10.1002/ajmg.c.30058 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16010676 }}</ref> ==== | ||
*Deregulation of imprinted gene expression in the chromosome 11p15.5 region can result in the [[Beckwith-Wiedemann syndrome|BWS]] phenotype. | *Deregulation of imprinted [[gene]] expression in the [[Chromosome 11 (human)|chromosome 11p15.5]] region can result in the [[Beckwith-Wiedemann syndrome|BWS]] phenotype. | ||
*The critical [[Beckwith-Wiedemann syndrome|BWS]] genes in that region include [[insulin-like growth factor 2]] (''IGF2''), ''[[H19 (gene)|H19]]'', [[cyclin-dependent kinase inhibitor 1C]] (''[[CDKN1C]]''), potassium channel voltage-gated KQT-like subfamily member 1 (''KCNQ1''), and ''KCNQ1''-overlapping transcript 1 (''KCNQ1OT1'', or long QT intronic transcript 1). | *The critical [[Beckwith-Wiedemann syndrome|BWS]] genes in that region include [[insulin-like growth factor 2]] (''IGF2''), ''[[H19 (gene)|H19]]'', [[cyclin-dependent kinase inhibitor 1C]] (''[[CDKN1C]]''), [[potassium channel]] [[Voltage-gated ion channel|voltage-gated]] KQT-like subfamily member 1 [[KvLQT1|(''KCNQ1'']]), and ''[[KvLQT1|KCNQ1]]''-overlapping [[Transcription factors|transcript]] 1 [[KCNQ1OT1|(''KCNQ1OT1'']], or long QT intronic transcript 1). | ||
==== | == Gross pathology == | ||
One of the causes of hypoglycemia is [[insulinoma]]. The gross pathology of insulinoma is described below: | |||
On gross pathology insulinomas have a | * On gross pathology, [[Insulinoma|insulinomas]] have a grey to red-brown appearance, encapsulated and are usually small and solitary [[tumors]]. | ||
* [[Insulinoma]] is firm, [[Homogeneous|homogeneous,]] [[pedunculated]] and rarely weighing more than 100g.<ref name="pmid15522939">{{cite journal| author=Mittendorf EA, Liu YC, McHenry CR| title=Giant insulinoma: case report and review of the literature. | journal=J Clin Endocrinol Metab | year= 2005 | volume= 90 | issue= 1 | pages= 575-80 | pmid=15522939 | doi=10.1210/jc.2004-0825 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=15522939 }} </ref> | |||
* | * Almost all [[Insulinoma|insulinomas]] arises from [[pancreas]], extrapancreatic ones causing hypoglycemia are rare.<ref name="pmid23430217">{{cite journal| author=Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y et al.| title=Diagnosis and management of insulinoma. | journal=World J Gastroenterol | year= 2013 | volume= 19 | issue= 6 | pages= 829-37 | pmid=23430217 | doi=10.3748/wjg.v19.i6.829 | pmc=3574879 | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=23430217 }}</ref> | ||
* [[ | * [[Tumors]] may have a [[cystic]] component. | ||
* | * [[Lipid]]-rich [[Insulinoma|insulinomas]] mimic [[Adrenocortical carcinoma|adrenal cortical neoplasia.]] | ||
* | * Features of [[malignancy]]: Large size, invasion to fibro-[[adipose tissue]], invasion to adjacent organs, and invasion to large vessels.<ref name="pmid17312378">{{cite journal| author=de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M et al.| title=Well-differentiated pancreatic tumor/carcinoma: insulinoma. | journal=Neuroendocrinology | year= 2006 | volume= 84 | issue= 3 | pages= 183-8 | pmid=17312378 | doi=10.1159/000098010 | pmc= | url=https://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=17312378 }}</ref> | ||
[[File:Insulinoma.jpg|center|300px|thumb|Gross pathology of insulinoma, source: By Edward Alabraba et al. - Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World Journal of Surgical Oncology 2009, 7:18doi:10.1186/1477-7819-7-18, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=6686376]] | [[File:Insulinoma.jpg|center|300px|thumb|Gross pathology of insulinoma, source: By Edward Alabraba et al. - Pancreatic insulinoma co-existing with gastric GIST in the absence of neurofibromatosis-1. World Journal of Surgical Oncology 2009, 7:18doi:10.1186/1477-7819-7-18, CC BY 2.0, https://commons.wikimedia.org/w/index.php?curid=6686376]] | ||
== Microscopic pathology == | == Microscopic pathology == | ||
* On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with [[amyloid]] in a fibrovascular [[stroma]], are characteristic findings of [[Insulinoma|insulinoma.]]<ref>{{cite book | last = Lloyd | first = Ricardo | title = Endocrine pathology : differential diagnosis and molecular advances | publisher = Springer | location = New York London | year = 2010 | isbn = 978-1441910684 }}</ref> | * On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with [[amyloid]] in a fibrovascular [[stroma]], are characteristic findings of [[Insulinoma|insulinoma.]]<ref>{{cite book | last = Lloyd | first = Ricardo | title = Endocrine pathology : differential diagnosis and molecular advances | publisher = Springer | location = New York London | year = 2010 | isbn = 978-1441910684 }}</ref> | ||
* It is also evaluated for the [[mitotic index]](mitosis per 10 high power | * It is also evaluated for the [[mitotic index]] (mitosis per 10 high-power fields) and [[immunohistochemistry]] staining by [[Chromogranin A]], [[synaptophysin]], and [[Ki-67]] index.<ref name="de HerderNiederle2007">{{cite journal|last1=de Herder|first1=Wouter W.|last2=Niederle|first2=Bruno|last3=Scoazec|first3=Jean-Yves|last4=Pauwels|first4=Stanislas|last5=Klöppel|first5=Günter|last6=Falconi|first6=Massimo|last7=Kwekkeboom|first7=Dik J.|last8=Öberg|first8=Kjel|last9=Eriksson|first9=Barbro|last10=Wiedenmann|first10=Bertram|last11=Rindi|first11=Guido|last12=O’Toole|first12=Dermot|last13=Ferone|first13=Diego|title=Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma|journal=Neuroendocrinology|volume=84|issue=3|year=2007|pages=183–188|issn=0028-3835|doi=10.1159/000098010}}</ref> | ||
* The structure of tumor cells observed under [[Electron microscope|electron microscopy]] as | * The structure of [[Tumor cell|tumor cells]] observed under [[Electron microscope|electron microscopy]] as group A characterized by abundant well-granulated typical B cells with the [[Trabeculae|trabecular]] arrangement and group B as scarce well-granulated typical B cells and a medullary arrangement. | ||
<gallery widths="250px"> | |||
Pancreatic insulinoma histology 2.JPG|Histopathology of a pancreatic endocrine tumor (insulinoma). ''Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas''<ref name=aaa> Neuroendocrine tumor of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas</ref> | |||
Pancreatic insulinoma histopathology 3.JPG|Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. ''Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas''<ref name=aaa> Neuroendocrine tumour of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas</ref> | |||
Pancreatic insulinoma histology 4.JPG|Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. ''Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas''<ref name=aaa> Neuroendocrine tumour of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas</ref> | |||
< | </gallery> | ||
==References== | ==References== | ||
{{Reflist|2}} | {{Reflist|2}} | ||
Latest revision as of 21:27, 13 November 2017
|
Hypoglycemia Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Hypoglycemia pathophysiology On the Web |
|
American Roentgen Ray Society Images of Hypoglycemia pathophysiology |
|
Risk calculators and risk factors for Hypoglycemia pathophysiology |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1] Associate Editor(s)-in-Chief: Mohammed Abdelwahed M.D[2] Amandeep Singh M.D.[3]
Overview
The pathophysiology of hypoglycemia depends on the failure of physiological defense mechanisms and hormones such as insulin, glucagon, and epinephrine to correct hypoglycemia. Most of these defense mechanisms are hormones that control glycogenolysis and gluconeogenesis. Insulinoma is a rare benign pancreatic neuroendocrine tumor that arises from β islet cells. It is mediated by a mutation in mTOR/P70S6K signaling pathway. Non-islet-cell tumors (NICTH) are large tumors of mesenchymal or epithelial cell types originate from the pancreas. Hypoglycemia due to NICTH appears to be related to increased glucose utilization and inhibition of glucose release from the liver. This happens as a result of tumor production of incompletely processed IGF-2. On gross pathology insulinomas have a gray to red-brown appearance, encapsulated and are usually small and solitary tumors. On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with amyloid in a fibrovascular stroma, are characteristic findings of insulinoma. It is also evaluated for the mitotic index and immunohistochemistry staining by Chromogranin A, synaptophysin, and Ki-67 index.
Hypoglycemia pathophysiology
Physiological effect of insulin
- Insulin binds to its receptor which involves many protein activation cascades.[1]
- Binding of insulin to the α-subunit results in changes which activate tyrosine kinase domains on each β-subunit.
- The tyrosine kinase activity causes phosphorylation of intracellular enzymes.
- The phosphorylation of MAP-Kinase leads to induction of gene expression.
- Phosphorylation of PI-3K isolates the GLUT-4 Vesicle and sends the vesicles back to the cell membrane.
- The GLUT-4 vesicles fuse with the cellular membrane allowing glucose to be transported into the cell.
Insulin is involved in many aspects of metabolism including:[2]
- Insulin decreases blood glucose concentration by inducing uptake of the glucose by peripheral cells. This function is as a result of increased GLUT4 transporter insertion in the cell membrane of muscles and fat tissues which allows glucose to enter the cells.
- Increase of DNA replication and protein synthesis via control of amino acids uptake.
- Induction of glycogen synthesis when glucose levels are high.
- Increase of cellular potassium uptake.
- Decreased gluconeogenesis and glycogenolysis; decreased production of glucose from noncarbohydrate substrates, primarily in the liver (the vast majority of endogenous insulin arriving into the liver never leaves the liver).
- Increase of lipid synthesis: insulin forces fat cells to use blood glucose, which is converted into triglycerides; a decrease of insulin causes the reverse.
- Decrease in lipolysis: insulin forces reduction in conversion of fat cell lipid stores into blood fatty acids and glycerol; a decrease of insulin causes the reverse.
- Decrease in proteolysis: insulin decreases the breakdown of protein.
- Decrease of renal sodium excretion.
Pathogenesis of hypoglycemia in diabetics
The pathophysiology of hypoglycemia mainly relies on the failure of physiological defense mechanisms and hormones such as insulin, glucagon and epinephrine to correct hypoglycemia. Most of these hormones control glycogenolysis and gluconeogenesis, including:
- Insulin
The most important and the first mechanism to counter-regulate hypoglycemia is the ability to suppress insulin release. This happens early when blood glucose level is between 80–85 mmHg. This can not occur in patients with absolute beta-cell failure, type 1 diabetes mellitus, and long-standing type 2 diabetes.[3] High insulin levels inhibit hepatic glycogenolysis causing more hypoglycemia.
- Glucagon
Hypoglycemia stimulates secretion of glucagon. This happens when blood glucose level falls between 65–70 mmHg. Failure to secrete glucagon may be the result of beta-cell failure and high insulin level that inhibits glucagon secretion.[4]
- Epinephrine
Epinephrine response to hypoglycemia becomes suppressed in many patients.[5] This happens when blood glucose level falls between 65–70mmHg. A suppressed epinephrine response causes defective glucose counter-regulation and hypoglycemia unawareness occurs.[6] This may be due to shifting the glycemic threshold for the sympathoadrenal response to a lower plasma glucose concentration. The brain is the first organ to be affected by decreased blood glucose level. Impairment of judgment and Seizures may occur resulting in coma.
Pathogenesis of hypoglycemia in insulinoma:
- Insulinoma is a rare benign pancreatic neuroendocrine tumor that arises from β islet cells.[1][7]
- It usually occurs sporadically but 10% are found to be associated with MEN 1 syndrome.[2]
- It is thought that insulinoma is mediated by a mutation in mTOR/P70S6K signaling pathway. An oral mTOR inhibitor (Everolimus) may make better glycemic control in people having an insulinoma.[4]
- Mitochondria plays a key role in glucose and insulin coupling to assure insulin secretion after glucose stimulation in pancreatic β cells. Coupling is impaired due to abnormal mitochondrial function in β cells causes the death of the cell.[6]
- YY1 regulates this mitochondrial function.[7] T372R mutation increases the transcription of YY1. The understanding of role and functions of YY1 in β cells in near future might prove to be therapeutic potentials.[8]
- The progression to hypoglycemia is actually because of decreased glucose synthesis rather than increased use due to the direct effect of insulin on the liver.[9]
- The neuroglycopenic symptoms appear eventually due to decreased blood glucose. Hypoglycemia stimulates catecholamine release which produces adrenergic symptoms.[10]
Pathogenesis of hypoglycemia in non-islet-cell tumors hypoglycemia (NICTH):
- Non-islet-cell tumors are large tumors of mesenchymal or epithelial cell types originate from the pancreas.
- NICTH appears to be increased glucose utilization and inhibition of glucose release from the liver.
- This happens as a result of tumor production of incompletely processed IGF-2.[8]
- Incompletely processed IGF-2 also suppresses glucagon and growth hormone release.[9]
- The net result is continued glucose utilization by skeletal muscle and inhibition of glucose release, glycogenolysis, and gluconeogenesis in the liver.[9]
Genetics
Genes associated with diabetes include the following:[10][11]
- Currently, 58 genomic regions are found to be associated with type 1 diabetes mellitus (DM).
- The major susceptibility gene for type1 DM is located on HLA region of chromosome 6. It accounts for 40-50% of the genetic risk for type1 DM. This region encodes for class II major histocompatibility complex (MHC) molecules. Class II major histocompatibility complex (MHC) molecules play an important role in presenting antigen to helper T cells and initiating an immune response.
- Other major susceptibility genes which were associated with Type1 DM include polymorphisms in the promoter region of the insulin gene, the CTLA-4 gene, interleukin 2 receptor, CTLA4, and PTPN22.
- Presence of certain genes confers protection against the development of the disease. Haplotypes DQA1*0102, DQB1*0602 are extremely rare in individuals with type1 DM (<1%) and appears to provide protection from type1 DM.
Genetics associated with:[12]
- Deregulation of imprinted gene expression in the chromosome 11p15.5 region can result in the BWS phenotype.
- The critical BWS genes in that region include insulin-like growth factor 2 (IGF2), H19, cyclin-dependent kinase inhibitor 1C (CDKN1C), potassium channel voltage-gated KQT-like subfamily member 1 (KCNQ1), and KCNQ1-overlapping transcript 1 (KCNQ1OT1, or long QT intronic transcript 1).
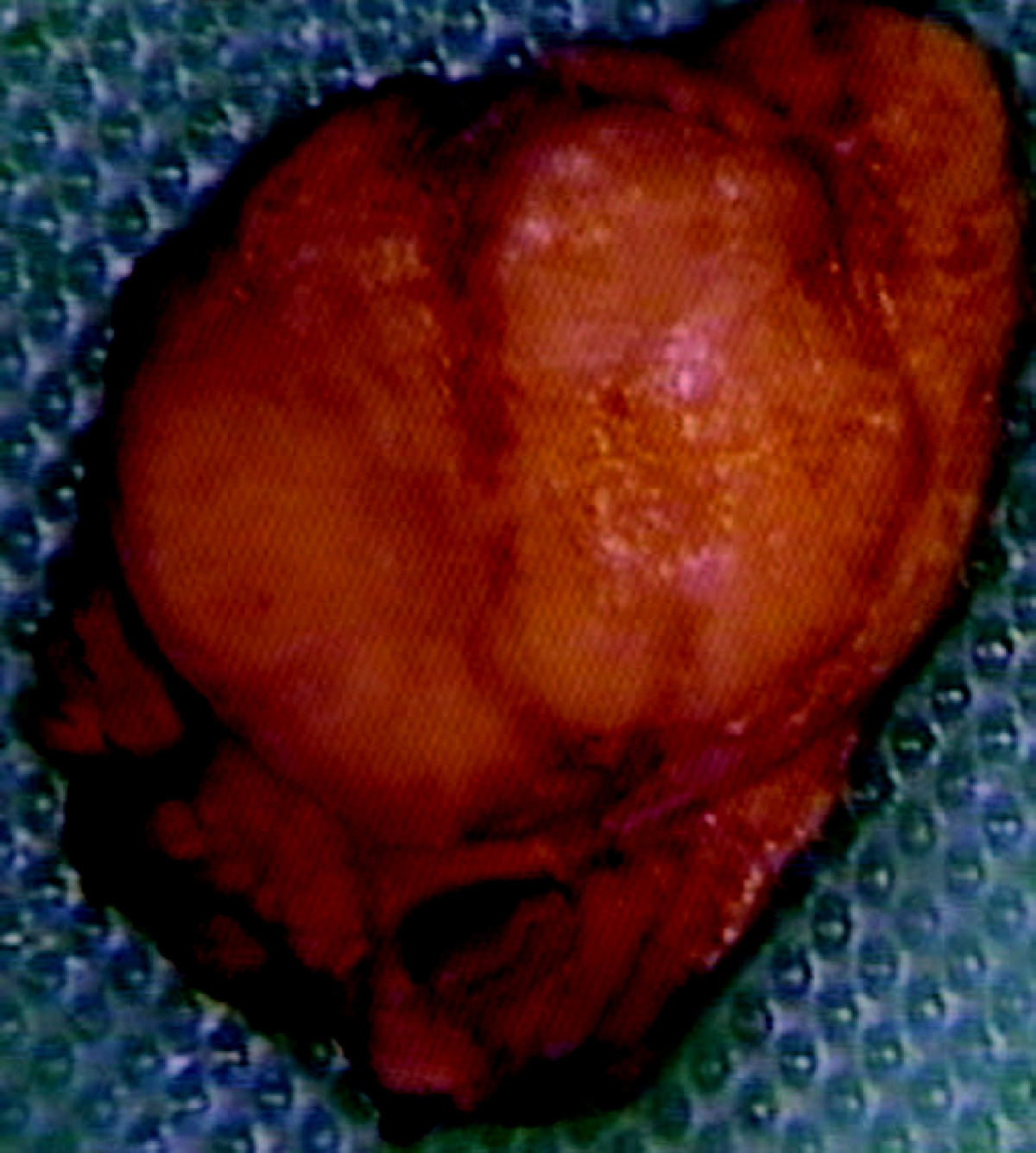
Gross pathology
One of the causes of hypoglycemia is insulinoma. The gross pathology of insulinoma is described below:
- On gross pathology, insulinomas have a grey to red-brown appearance, encapsulated and are usually small and solitary tumors.
- Insulinoma is firm, homogeneous, pedunculated and rarely weighing more than 100g.[13]
- Almost all insulinomas arises from pancreas, extrapancreatic ones causing hypoglycemia are rare.[14]
- Tumors may have a cystic component.
- Lipid-rich insulinomas mimic adrenal cortical neoplasia.
- Features of malignancy: Large size, invasion to fibro-adipose tissue, invasion to adjacent organs, and invasion to large vessels.[15]
Microscopic pathology
- On microscopic histopathological analysis, patterns like trabecular, gyriform, lobular and solid structures, particularly with amyloid in a fibrovascular stroma, are characteristic findings of insulinoma.[16]
- It is also evaluated for the mitotic index (mitosis per 10 high-power fields) and immunohistochemistry staining by Chromogranin A, synaptophysin, and Ki-67 index.[17]
- The structure of tumor cells observed under electron microscopy as group A characterized by abundant well-granulated typical B cells with the trabecular arrangement and group B as scarce well-granulated typical B cells and a medullary arrangement.
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[18]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[18]
-
Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[18]
References
- ↑
- ↑ Ahmad K (2014). "Insulin sources and types: a review of insulin in terms of its mode on diabetes mellitus". J Tradit Chin Med. 34 (2): 234–7. PMID 24783939.
- ↑ Dunning BE, Gerich JE (2007). "The role of alpha-cell dysregulation in fasting and postprandial hyperglycemia in type 2 diabetes and therapeutic implications". Endocr Rev. 28 (3): 253–83. doi:10.1210/er.2006-0026. PMID 17409288.
- ↑ Raju B, Cryer PE (2005). "Loss of the decrement in intraislet insulin plausibly explains loss of the glucagon response to hypoglycemia in insulin-deficient diabetes: documentation of the intraislet insulin hypothesis in humans". Diabetes. 54 (3): 757–64. PMID 15734853.
- ↑ Dagogo-Jack SE, Craft S, Cryer PE (1993). "Hypoglycemia-associated autonomic failure in insulin-dependent diabetes mellitus. Recent antecedent hypoglycemia reduces autonomic responses to, symptoms of, and defense against subsequent hypoglycemia". J Clin Invest. 91 (3): 819–28. doi:10.1172/JCI116302. PMC 288033. PMID 8450063.
- ↑ Geddes J, Schopman JE, Zammitt NN, Frier BM (2008). "Prevalence of impaired awareness of hypoglycaemia in adults with Type 1 diabetes". Diabet Med. 25 (4): 501–4. doi:10.1111/j.1464-5491.2008.02413.x. PMID 18387080.
- ↑ Rizza RA, Haymond MW, Verdonk CA, Mandarino LJ, Miles JM, Service FJ; et al. (1981). "Pathogenesis of hypoglycemia in insulinoma patients: suppression of hepatic glucose production by insulin". Diabetes. 30 (5): 377–81. PMID 6262168.
- ↑ Cryer PE, Axelrod L, Grossman AB, Heller SR, Montori VM, Seaquist ER; et al. (2009). "Evaluation and management of adult hypoglycemic disorders: an Endocrine Society Clinical Practice Guideline". J Clin Endocrinol Metab. 94 (3): 709–28. doi:10.1210/jc.2008-1410. PMID 19088155.
- ↑ 9.0 9.1 Dynkevich Y, Rother KI, Whitford I, Qureshi S, Galiveeti S, Szulc AL; et al. (2013). "Tumors, IGF-2, and hypoglycemia: insights from the clinic, the laboratory, and the historical archive". Endocr Rev. 34 (6): 798–826. doi:10.1210/er.2012-1033. PMID 23671155.
- ↑ Pociot F, Lernmark Å (2016). "Genetic risk factors for type 1 diabetes". Lancet. 387 (10035): 2331–9. doi:10.1016/S0140-6736(16)30582-7. PMID 27302272.
- ↑ Højlund K, Hansen T, Lajer M, Henriksen JE, Levin K, Lindholm J; et al. (2004). "A novel syndrome of autosomal-dominant hyperinsulinemic hypoglycemia linked to a mutation in the human insulin receptor gene". Diabetes. 53 (6): 1592–8. PMID 15161766.
- ↑ Weksberg R, Shuman C, Smith AC (2005). "Beckwith-Wiedemann syndrome". Am J Med Genet C Semin Med Genet. 137C (1): 12–23. doi:10.1002/ajmg.c.30058. PMID 16010676.
- ↑ Mittendorf EA, Liu YC, McHenry CR (2005). "Giant insulinoma: case report and review of the literature". J Clin Endocrinol Metab. 90 (1): 575–80. doi:10.1210/jc.2004-0825. PMID 15522939.
- ↑ Okabayashi T, Shima Y, Sumiyoshi T, Kozuki A, Ito S, Ogawa Y; et al. (2013). "Diagnosis and management of insulinoma". World J Gastroenterol. 19 (6): 829–37. doi:10.3748/wjg.v19.i6.829. PMC 3574879. PMID 23430217.
- ↑ de Herder WW, Niederle B, Scoazec JY, Pauwels S, Kloppel G, Falconi M; et al. (2006). "Well-differentiated pancreatic tumor/carcinoma: insulinoma". Neuroendocrinology. 84 (3): 183–8. doi:10.1159/000098010. PMID 17312378.
- ↑ Lloyd, Ricardo (2010). Endocrine pathology : differential diagnosis and molecular advances. New York London: Springer. ISBN 978-1441910684.
- ↑ de Herder, Wouter W.; Niederle, Bruno; Scoazec, Jean-Yves; Pauwels, Stanislas; Klöppel, Günter; Falconi, Massimo; Kwekkeboom, Dik J.; Öberg, Kjel; Eriksson, Barbro; Wiedenmann, Bertram; Rindi, Guido; O’Toole, Dermot; Ferone, Diego (2007). "Well-Differentiated Pancreatic Tumor/Carcinoma: Insulinoma". Neuroendocrinology. 84 (3): 183–188. doi:10.1159/000098010. ISSN 0028-3835.
- ↑ 18.0 18.1 18.2 Neuroendocrine tumor of the pancreas. Libre Pathology. http://librepathology.org/wiki/index.php/Neuroendocrine_tumour_of_the_pancreas
![Histopathology of a pancreatic endocrine tumor (insulinoma). Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[18]](/images/2/2f/Pancreatic_insulinoma_histology_2.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Chromogranin A immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[18]](/images/a/a3/Pancreatic_insulinoma_histopathology_3.JPG)
![Histopathology of a pancreatic endocrine tumor (insulinoma). Insulin immunostain. Source:https://librepathology.org/wiki/Neuroendocrine_tumour_of_the_pancreas[18]](/images/d/d5/Pancreatic_insulinoma_histology_4.JPG)