Buserelin: Difference between revisions
m (Bot: Automated text replacement (-{{SIB}} + & -{{EH}} + & -{{EJ}} + & -{{Editor Help}} + & -{{Editor Join}} +)) |
No edit summary |
||
| Line 2: | Line 2: | ||
{{ | {{Drugbox2 | ||
| Verifiedfields = changed | |||
| verifiedrevid = 477165149 | |||
| image = Buserelin.svg | | image = Buserelin.svg | ||
<!--Clinical data--> | |||
| tradename = Etilamide | |||
| Drugs.com = {{drugs.com|CONS|buserelin}} | |||
| pregnancy_category = X | |||
| legal_status = ℞-only | |||
| routes_of_administration = [[Implant (medicine)|implant]], intranasal | |||
<!--Pharmacokinetic data--> | |||
| elimination_half-life = 72 to 80 minutes | |||
| excretion = [[Kidney|Renal]] | |||
<!--Identifiers--> | |||
| CASNo_Ref = {{cascite|correct|CAS}} | |||
| CAS_number_Ref = {{cascite|correct|??}} | |||
| CAS_number = 57982-77-1 | | CAS_number = 57982-77-1 | ||
| ATC_prefix = L02 | | ATC_prefix = L02 | ||
| ATC_suffix =AE01 | | ATC_suffix = AE01 | ||
| PubChem = 50225 | | ATC_supplemental = {{ATCvet|H01|CA90}} | ||
| DrugBank = | | PubChem = 50225 | ||
| C=62 | H=90| N=16| O=15 | | DrugBank_Ref = {{drugbankcite|changed|drugbank}} | ||
| DrugBank = DB06719 | |||
| ChEMBL_Ref = {{ebicite|changed|EBI}} | |||
| ChEMBL = 1909304 | |||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 45545 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = PXW8U3YXDV | |||
<!--Chemical data--> | |||
| C=62 | H=90 | N=16 | O=15 | |||
| molecular_weight = 1299.48 g/mol | | molecular_weight = 1299.48 g/mol | ||
| InChI = 1/C60H86N16O13/c1-7-64-57(87)48-15-11-23-76(48)58(88)41(14-10-22-65-59(61)62)69-51(81)42(24-33(2)3)70-56(86)47(31-89-60(4,5)6)75-52(82)43(25-34-16-18-37(78)19-17-34)71-55(85)46(30-77)74-53(83)44(26-35-28-66-39-13-9-8-12-38(35)39)72-54(84)45(27-36-29-63-32-67-36)73-50(80)40-20-21-49(79)68-40/h8-9,12-13,16-19,28-29,32-33,40-48,66,77-78H,7,10-11,14-15,20-27,30-31H2,1-6H3,(H,63,67)(H,64,87)(H,68,79)(H,69,81)(H,70,86)(H,71,85)(H,72,84)(H,73,80)(H,74,83)(H,75,82)(H4,61,62,65)/t40-,41-,42-,43-,44-,45-,46-,47+,48-/m0/s1 | |||
| InChIKey = CUWODFFVMXJOKD-UVLQAERKBG | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C60H86N16O13/c1-7-64-57(87)48-15-11-23-76(48)58(88)41(14-10-22-65-59(61)62)69-51(81)42(24-33(2)3)70-56(86)47(31-89-60(4,5)6)75-52(82)43(25-34-16-18-37(78)19-17-34)71-55(85)46(30-77)74-53(83)44(26-35-28-66-39-13-9-8-12-38(35)39)72-54(84)45(27-36-29-63-32-67-36)73-50(80)40-20-21-49(79)68-40/h8-9,12-13,16-19,28-29,32-33,40-48,66,77-78H,7,10-11,14-15,20-27,30-31H2,1-6H3,(H,63,67)(H,64,87)(H,68,79)(H,69,81)(H,70,86)(H,71,85)(H,72,84)(H,73,80)(H,74,83)(H,75,82)(H4,61,62,65)/t40-,41-,42-,43-,44-,45-,46-,47+,48-/m0/s1 | |||
| StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChIKey = CUWODFFVMXJOKD-UVLQAERKSA-N | |||
| synonyms = D-Ser(Tbu)<sup>6</sup>EA<sup>10</sup>LHRH | | synonyms = D-Ser(Tbu)<sup>6</sup>EA<sup>10</sup>LHRH | ||
}} | }} | ||
'''Buserelin''' ([[International Nonproprietary Name|INN]]) is a [[gonadotropin-releasing hormone agonist]] (GnRH agonist). The drug's effects are dependent on the frequency and time course of administration. GnRH is released in a pulsatile fashion in the postpubertal adult. Initial interaction of any GnRH agonist, such as buserelin, with the GnRH receptor induces release of FSH and LH by gonadotrophes. Long-term exposure to constant levels of buserelin, rather than endogenous pulses, leads to downregulation of the GnRH receptors and subsequent suppression of the pituitary release of LH and FSH. | |||
Like other GnRH agonists, buserelin may be used in the treatment of hormone-responsive cancers such as [[prostate cancer]] or [[breast cancer]], estrogen-dependent conditions (such as [[endometriosis]] or [[uterine fibroids]]), and in [[Reproductive technology|assisted reproduction]]. | |||
It is normally delivered via a [[nasal spray]], but is also available as an injection. | |||
{{ | Buserelin acetate is marketed by [[Sanofi-Aventis]] under the brand name '''Suprefact''' and a generic form of Buserelin is now produced by [[CinnaGen]] under the brand name '''CinnaFact'''. | ||
Buserelin is also marketed under the brand name '''Metrelef'''. '''Metrelef''' is approved to treat patients with [[endometriosis]] by suppression of ovarian hormone production. In [[ovulation induction]] '''Metrelef''' is used as a [[pituitary]] blockade as an adjunct to [[gonadotrophin]] administration. | |||
==See also== | |||
* [[Leuprorelin]] | |||
==References== | |||
{{Reflist|2}} | |||
{{Gonadotropins and GnRH}} | |||
{{Peptidergics}} | |||
[[Category:GnRH agonists]] | [[Category:GnRH agonists]] | ||
{{pharmacology-stub}} | |||
{{ | |||
Revision as of 19:28, 3 February 2015
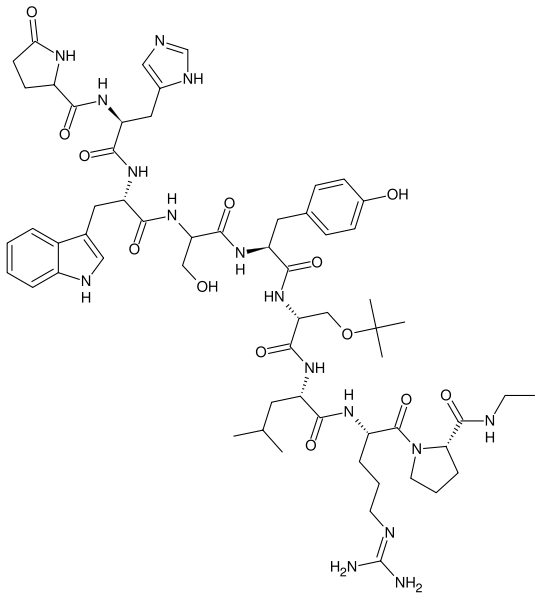
| |
Buserelin
| |
| Systematic (IUPAC) name | |
| ? | |
| Identifiers | |
| CAS number | |
| ATC code | L02 Template:ATCvet |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 1299.48 g/mol |
| Synonyms | D-Ser(Tbu)6EA10LHRH |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | 72 to 80 minutes |
| Excretion | Renal |
| Therapeutic considerations | |
| Pregnancy cat. |
X |
| Legal status |
℞-only |
| Routes | implant, intranasal |
Buserelin (INN) is a gonadotropin-releasing hormone agonist (GnRH agonist). The drug's effects are dependent on the frequency and time course of administration. GnRH is released in a pulsatile fashion in the postpubertal adult. Initial interaction of any GnRH agonist, such as buserelin, with the GnRH receptor induces release of FSH and LH by gonadotrophes. Long-term exposure to constant levels of buserelin, rather than endogenous pulses, leads to downregulation of the GnRH receptors and subsequent suppression of the pituitary release of LH and FSH.
Like other GnRH agonists, buserelin may be used in the treatment of hormone-responsive cancers such as prostate cancer or breast cancer, estrogen-dependent conditions (such as endometriosis or uterine fibroids), and in assisted reproduction.
It is normally delivered via a nasal spray, but is also available as an injection.
Buserelin acetate is marketed by Sanofi-Aventis under the brand name Suprefact and a generic form of Buserelin is now produced by CinnaGen under the brand name CinnaFact.
Buserelin is also marketed under the brand name Metrelef. Metrelef is approved to treat patients with endometriosis by suppression of ovarian hormone production. In ovulation induction Metrelef is used as a pituitary blockade as an adjunct to gonadotrophin administration.