Disopyramide: Difference between revisions
Matt Pijoan (talk | contribs) m (Protected "Disopyramide": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
Adeel Jamil (talk | contribs) No edit summary |
||
| (16 intermediate revisions by 4 users not shown) | |||
| Line 1: | Line 1: | ||
{{DrugProjectFormSinglePage | |||
|authorTag={{Alonso}}; {{AJ}} | |||
|genericName=Disopyramide Phosphate | |||
|aOrAn=a | |||
|drugClass=[[Antiarrhythmic medication|antiarrhythmic Group IA]] , [[cardiovascular|cardiovascular agent]] | |||
|indicationType=treatment | |||
|indication=[[ventricular arrhythmias]] such as [[sustained ventricular tachycardia]], that, in the judgment of the physician, are life-threatening | |||
|hasBlackBoxWarning=Yes | |||
|adverseReactions=[[negative inotropic]] effect on [[myocardium]], [[constipation]], [[nausea]], [[xerostomia]], [[muscle weakness]], [[blurred vision]], delay when starting to pass [[urine]], [[urinary retention]], generalized [[aches]] and [[pains]], [[malaise]] and [[fatigue]] | |||
|blackBoxWarningTitle=Mortality | |||
|blackBoxWarningBody=In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-center, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had had a myocardial infarction more than 6 days but less than 2 years previously, an excessive mortality or non-fatal cardiac arrest rate (7.7%) was seen in patients treated with encainide or flecainide compared with that seen in patients assigned to carefully matched placebo-treated groups (3.0%). The average duration of treatment with encainide or flecainide in this study was 10 months. | |||
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) is uncertain. Considering the known proarrhythmic properties of Disopyramide Phosphate and the lack of evidence of improved survival for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of Disopyramide Phosphate as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmias. | |||
|fdaLIADAdult=The dosage of disopyramide phosphate must be individualized for each patient on the basis of response and tolerance. The usual adult dosage of disopyramide phosphate is 400 to 800 mg per day given in divided doses. The recommended dosage for most adults is 600 mg/day given in divided doses (150 mg every 6 hours). For patients whose body weight is less than 110 pounds (50 kg), the recommended dosage is 400 mg/day given in divided doses (100 mg every 6 hours). In the event of increased [[Anticholinergic syndrome|anticholinergic side effects]], plasma levels of disopyramide should be monitored and the dose of the drug adjusted accordingly. A reduction of the dose by one third, from the recommended 600 mg/day to 400 mg/day, would be reasonable, without changing the dosing interval. | |||
For patients with [[cardiomyopathy]] or possible [[cardiac]] decompensation, a loading dose, as discussed below, should not be given, and initial dosage should be limited to 100 mg every 6 to 8 hours. Subsequent dosage adjustments should be made gradually, with close monitoring for the possible development of [[hypotension]] and/or [[congestive heart failure]]. | |||
[[ | |||
[[ | |||
[[ | |||
[[ | |||
For patients with moderate renal insufficiency ([[creatinine clearance]] greater than 40 mL/min) or [[hepatic insufficiency]], the recommended dosage is 400 mg/day given in divided doses (100 mg every 6 hours). | |||
[[ | |||
[[ | |||
For patients with severe [[renal insufficiency]] (Ccr 40 mL/min or less), the recommended dosage regimen is 100 mg at intervals shown in the table below, with or without an initial loading dose of 150 mg. | |||
[ | |||
[[File:DisopyramideTable1.png|800px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
[ | {{clr}} | ||
For patients in whom rapid control of [[ventricular arrhythmia]] is essential, an initial loading dose of 300 mg of disopyramide phosphate (200 mg for patients whose body weight is less than 110 pounds) is recommended, followed by the appropriate maintenance dosage. [[Therapeutic]] effects are usually attained 30 minutes to 3 hours after administration of a 300 mg loading dose. If there is no response or evidence of [[toxicity]] within 6 hours of the loading dose, 200 mg of disopyramide phosphate every 6 hours may be prescribed instead of the usual 150 mg. If there is no response to this dosage within 48 hours, either disopyramide phosphate should then be discontinued or the physician should consider hospitalizing the patient for careful monitoring while subsequent disopyramide phosphate doses of 250 mg or 300 mg every 6 hours are given. A limited number of patients with severe refractory [[ventricular tachycardia]] have tolerated daily doses of disopyramide phosphate up to 1600 mg per day (400 mg every 6 hours), resulting in disopyramide plasma levels up to 9 mcg/mL. If such treatment is warranted, it is essential that patients be hospitalized for close evaluation and continuous monitoring. | |||
[ | |||
== | =====Transferring to Disopyramide Phosphate===== | ||
The following dosage schedule based on theoretical considerations rather than experimental data is suggested for transferring patients with normal [[renal function]] from either [[quinidine sulfate]] or [[procainamide]] therapy (Type 1 [[antiarrhythmic agents]]) to disopyramide phosphate therapy: | |||
* Disopyramide phosphate should be started using the regular maintenance schedule without a loading dose 6 to 12 hours after the last dose of [[quinidine sulfate]] or 3 to 6 hours after the last dose of [[procainamide]]. | |||
* In patients in whom withdrawal of [[quinidine sulfate]] or [[procainamide]] is likely to produce life-threatening [[arrhythmias]], the physician should consider hospitalization of the patient. | |||
|offLabelAdultGuideSupport=====Atrial Fibrillation===== | |||
[ | |||
[ | |||
[ | |||
== | * Developed by: AHA/ACC<ref name="pmid24685669">{{cite journal| author=January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE et al.| title=2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society. | journal=J Am Coll Cardiol | year= 2014 | volume= | issue= | pages= | pmid=24685669 | doi=10.1016/j.jacc.2014.03.022 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=24685669 }} </ref> | ||
[[Category:Drugs]] | * Class of Recommendation: [[ACC AHA guidelines classification scheme|Class IIb]] | ||
* Strength of Evidence: [[ACC AHA guidelines classification scheme|Level of Evidence C]] | |||
* Dosing Information | |||
:* Immediate release tablets: 100-200 mg four times a day. | |||
:* Extended release tablets: 200-400 mg bid. | |||
|offLabelAdultNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in adult patients. | |||
|fdaLIADPed=Controlled clinical studies have not been conducted in pediatric patients; however, the following suggested dosage table is based on published clinical experience. | |||
Total daily dosage should be divided and equal doses administered orally every 6 hours or at intervals according to individual patient needs. Disopyramide plasma levels and [[therapeutic]] response must be monitored closely. Patients should be hospitalized during the initial treatment period, and dose titration should start at the lower end of the ranges provided below. | |||
[[File:DisopyramideTable2.png|800px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clr}} | |||
|offLabelPedGuideSupport=There is limited information regarding <i>Off-Label Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|offLabelPedNoGuideSupport=There is limited information regarding <i>Off-Label Non–Guideline-Supported Use</i> of {{PAGENAME}} in pediatric patients. | |||
|contraindications=* [[Cardiogenic shock]] | |||
* Preexisting [[second-degree AV block]] or [[third-degree AV block]] (if no [[pacemaker]] is present) | |||
* [[Congenital]] [[Q-T prolongation]] | |||
* [[Hypersensitivity]] to the drug | |||
|warnings=====Negative Inotropic Properties===== | |||
=====Heart Failure/Hypotension===== | |||
Disopyramide phosphate may cause or worsen [[congestive heart failure]] or produce severe [[hypotension]] as a consequence of its [[negative inotropic]] properties. [[Hypotension]] has been observed primarily in patients with primary [[cardiomyopathy]] or inadequately compensated [[congestive heart failure]]. Disopyramide phosphate should not be used in patients with uncompensated or marginally compensated [[congestive heart failure]] or [[hypotension]] unless the [[congestive heart failure]] or [[hypotension]] is secondary to cardiac [[arrhythmia]]. Patients with a history of [[heart failure]] may be treated with Disopyramide phosphate, but careful attention must be given to the maintenance of cardiac function, including optimal [[digitalization]]. If [[hypotension]] occurs or congestive [[heart failure]] worsens, disopyramide phosphate should be discontinued and, if necessary, restarted at a lower dosage only after adequate cardiac compensation has been established. | |||
======QRS Widening====== | |||
Although it is unusual, significant widening (greater than 25%) of the [[QRS complex]] may occur during Disopyramide phosphate administration; in such cases disopyramide phosphate should be discontinued. | |||
======Q-T Prolongation====== | |||
As with other [[antiarrhythmic drugs|Type 1 antiarrhythmic drugs]], [[QT prolongation|prolongation of the Q-T interval]] (corrected) and worsening of the [[arrhythmia]], including [[ventricular tachycardia]] and [[ventricular fibrillation]], may occur. Patients who have evidenced [[QT prolongation|prolongation of the Q-T interval]] in response to [[quinidine]] may be at particular risk. As with other [[antiarrhythmics drugs|Type 1A antiarrhythmics]], disopyramide phosphate has been associated with [[torsade de pointes]]. | |||
If a [[Q-T prolongation]] of greater than 25% is observed and if ectopy continues, the patient should be monitored closely, and consideration given to discontinuing disopyramide phosphate. | |||
=====Hypoglycemia===== | |||
In rare instances significant lowering of blood-glucose values has been reported during disopyramide phosphate administration. The physician should be alert to this possibility, especially in patients with [[congestive heart failure]], [[malnutrition|chronic malnutrition]], [[hepatic]], [[renal]] or other diseases, or drugs (e.g., [[beta blockers]], [[alcohol]]) which could compromise preservation of the normal glucoregulatory mechanisms in the absence of food. In these patients, the [[Blood glucose|blood-glucose levels]] should be carefully followed. | |||
=====Concomitant Antiarrhythmic Therapy===== | |||
The concomitant use of disopyramide phosphate with other [[antiarrhythmic agents|Type 1A antiarrhythmic agents]] (such as [[quinidine]] or [[procainamide]]), [[antiarrhythmic agents|Type 1C antiarrhythmics]] (such as [[encainide]], [[flecainide]] or [[propafenone]]), and/or [[propranolol]] should be reserved for patients with life-threatening [[arrhythmias]] who are demonstrably unresponsive to single-agent [[antiarrhythmic]] therapy. Such use may produce serious [[negative inotropic]] effects, or may excessively prolong conduction. This should be considered particularly in patients with any degree of cardiac decompensation or those with a prior history thereof. Patients receiving more than one [[antiarrhythmic]] drug must be carefully monitored. | |||
=====Heart Block===== | |||
If [[first-degree heart block]] develops in a patient receiving disopyramide phosphate, the dosage should be reduced. If the block persists despite reduction of dosage, continuation of the drug must depend upon weighing the benefit being obtained against the risk of higher degrees of [[heart block]]. Development of [[second-degree AV block]] or [[third-degree AV block]] or unifascicular, [[Bifascicular block|bifascicular]], or [[trifascicular block]] requires discontinuation of disopyramide phosphate therapy, unless the ventricular rate is adequately controlled by a [[ventricular pacemaker|temporary or implanted ventricular pacemaker]]. | |||
=====Anticholinergic Activity===== | |||
Because of its [[anticholinergic]] activity, disopyramide phosphate should not be used in patients with [[glaucoma]], [[myasthenia gravis]] or [[urinary retention]] unless adequate overriding measures are taken; these consist of the topical application of potent [[miotics]] (e.g., [[pilocarpine]]) for patients with [[glaucoma]], and [[catheter]] drainage or operative relief for patients with [[urinary retention]]. [[Urinary retention]] may occur in patients of either sex as a consequence of disopyramide phosphate administration, but males with [[benign prostatic hypertrophy]] are at particular risk. In patients with a family history of [[glaucoma]], [[intraocular pressure]] should be measured before initiating disopyramide phosphate therapy. Disopyramide phosphate should be used with special care in patients with [[myasthenia gravis]] since its [[anticholinergic]] properties could precipitate a [[myasthenic crisis]] in such patients. | |||
===Precautions=== | |||
====General==== | |||
=====Atrial Tachyarrhythmias===== | |||
Patients with [[atrial flutter]] or [[atrial fibrillation]] should be [[Digitalis|digitalized]] prior to disopyramide phosphate administration to ensure that drug-induced enhancement of AV conduction does not result in an increase of [[ventricular rate]] beyond [[physiologically]] acceptable limits. | |||
=====Conduction Abnormalities===== | |||
Care should be taken when prescribing disopyramide phosphate for patients with [[sick sinus syndrome]] ([[Bradycardia-tachycardia syndrome|bradycardia-tachycardia syndrome]]), [[Wolff-Parkinson-White syndrome]] ([[WPW]]), or [[bundle branch block]]. The effect of disopyramide phosphate in these conditions is uncertain at present. | |||
=====Cardiomyopathy===== | |||
Patients with [[myocarditis]] or other [[cardiomyopathy]] may develop significant [[hypotension]] in response to the usual dosage of disopyramide phosphate, probably due to cardiodepressant mechanisms. Therefore, a loading dose of disopyramide phosphate should not be given to such patients, and initial dosage and subsequent dosage adjustments should be made under close supervision. | |||
|clinicalTrials=The adverse reactions which were reported in disopyramide phosphate clinical trials encompass observations in 1,500 patients, including 90 patients studied for at least 4 years. The most serious adverse reactions are [[hypotension]] and [[congestive heart failure]]. The most common adverse reactions, which are dose dependent, are associated with the [[anticholinergic]] properties of the drug. These may be transitory, but may be persistent or can be severe. [[Urinary retention]] is the most serious [[anticholinergic]] effect. | |||
=====The following reactions were reported in 10% to 40% of patients===== | |||
* '''Anticholinergic:''' [[Dry mouth]], [[urinary hesitancy]], [[constipation]]. | |||
=====The following reactions were reported in 3% to 9% of patients===== | |||
* '''Anticholinergic:''' [[Blurred vision]], dry nose/eyes/throat. | |||
* '''Genitourinary:''' [[Urinary retention]], [[urinary frequency]] and [[urinary urgency]]. | |||
* '''Gastrointestinal:''' [[Nausea]], [[abdominal pain]]/[[bloating]]/[[gas]]. | |||
* '''General:''' [[Dizziness]], [[fatigue]]/[[muscle weakness]], [[headache]], [[malaise]], [[aches]]/[[pains]]. | |||
=====The following reactions were reported in 1% to 3% of patients===== | |||
* '''Genitourinary:''' [[Impotence]]. | |||
* '''Cardiovascular:''' [[Hypotension]] with or without [[congestive heart failure]], increased [[congestive heart failure]], cardiac conduction disturbances]], [[edema]]/weight gain, [[shortness of breath]], [[syncope]], [[chest pain]]. | |||
* '''Gastrointestinal:''' [[Anorexia]], [[diarrhea]], [[vomiting]]. | |||
* '''Dermatologic:''' Generalized [[rash]]/[[dermatoses]], [[itching]]. | |||
* '''Central nervous system:''' [[Nervousness]]. | |||
* '''Other:''' [[Hypokalemia]], [[Hypercholesteremia|elevated cholesterol]]/[[Hypertriglyceridemia|triglycerides]] | |||
=====The following reactions were reported in less than 1%===== | |||
* [[Depression]]. | |||
* [[Insomnia]]. | |||
* [[Dysuria]]. | |||
* [[Numbness]]/[[tingling]]. | |||
* [[Abnormal liver function test|Elevated liver enzymes]]. | |||
* [[AV block]]. | |||
* Elevated [[BUN]]. | |||
* Elevated [[creatinine]]. | |||
* Decreased [[hemoglobin]]/[[hematocrit]]. | |||
* [[Hypoglycemia]]. | |||
* [[Cholestatic jaundice|Reversible cholestatic jaundice]]. | |||
* [[Fever]]. | |||
* [[Thrombocytopenia]]. | |||
* Reversible [[agranulocytosis]]. | |||
* [[Gynecomastia]]. | |||
* Some cases of [[SLE]] ([[systmic lupus erythematosus]]) symptoms have been reported; most cases occurred in patients who had been switched to disopyramide from [[procainamide]] following the development of [[SLE]] symptoms. | |||
* Rarely, [[psychosis|acute psychosis]] has been reported following disopyramide phosphate therapy, with prompt return to normal mental status when therapy was stopped. | |||
|drugInteractions=* If [[phenytoin]] or other hepatic enzyme inducers are taken concurrently with disopyramide phosphate, lower plasma levels of disopyramide may occur. Monitoring of disopyramide plasma levels is recommended in such concurrent use to avoid ineffective therapy. | |||
* Other [[antiarrhythmic drugs]] (e.g., [[quinidine]], [[procainamide]], [[lidocaine]], [[propranolol]]) have occasionally been used concurrently with disopyramide phosphate. Excessive widening of the [[QRS complex]] and/or prolongation of the [[Q-T interval]] may occur in these situations. In healthy subjects, no significant drug-drug interaction was observed when disopyramide phosphate was coadministered with either [[propranolol]] or [[diazepam]]. Concomitant administration of Disopyramide Phosphate and [[quinidine]] resulted in slight increases in plasma disopyramide levels and slight decreases in plasma [[quinidine]] levels. | |||
* Disopyramide Phosphate does not increase serum [[digoxin]] levels. | |||
* Until data on possible interactions between [[verapamil]] and disopyramide phosphate are obtained, disopyramide should not be administered within 48 hours before or 24 hours after [[verapamil]] administration. | |||
* Although potent inhibitors of [[cytochrome P450]] 3A4 (e.g., [[ketoconazole]]) have not been studied clinically, in vitro studies have shown that [[erythromycin]] and [[oleandomycin]] inhibit the metabolism of disopyramide. Cases of life-threatening interactions have been reported for disopyramide when given with [[clarithromycin]] and [[erythromycin]] indicating that coadministration of disopyramide with inhibitors of [[cytochrome P450]] 3A4 could result in potentially fatal interaction. | |||
|FDAPregCat=C | |||
|useInPregnancyFDA=====Teratogenic Effeects===== | |||
Disopyramide Phosphate was associated with decreased numbers of [[implantation]] sites and decreased growth and survival of pups when administered to [[pregnant]] rats at 250 mg/kg/day (20 or more times the usual daily human dose of 12 mg/kg, assuming a patient weight of at least 50 kg), a level at which weight gain and food consumption of dams were also reduced. Increased resorption rates were reported in rabbits at 60 mg/kg/day (5 or more times the usual daily human dose). Effects on implantation, pup growth, and survival were not evaluated in rabbits. There are no adequate and well-controlled studies in pregnant women. Disopyramide Phosphate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus. | |||
=====Nonteratogenic Effects===== | |||
Disopyramide phosphate has been reported to stimulate contractions of the [[pregnant]] uterus. Disopyramide has been found in human [[fetal]] [[blood]]. | |||
|useInLaborDelivery=It is not known whether the use of disopyramide phosphate during [[labor]] or [[delivery]] has immediate or delayed adverse effects on the fetus, or whether it prolongs the duration of labor or increases the need for [[forceps]] [[delivery]] or other [[obstetric]] intervention. | |||
|useInNursing=Studies in rats have shown that the concentration of disopyramide and its metabolites is between one and three times greater in milk than it is in [[plasma]]. Following oral administration, disopyramide has been detected in human milk at a concentration not exceeding that in [[plasma]]. Because of the potential for serious adverse reactions in nursing infants from disopyramide phosphate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother. | |||
|useInPed=Safety and effectiveness in pediatric patients have not been established. | |||
|useInGeri=Clinical studies of disopyramide phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased [[hepatic]], [[renal]], or [[cardiac]] function, and of concomitant disease or other drug therapy. | |||
Because of its [[anticholinergic]] activity, disopyramide phosphate should not be used in patients with [[glaucoma]], [[urinary retention]], or [[benign prostatic hypertrophy]] (medical conditions commonly associated with the elderly) unless adequate overriding measures are taken (see WARNINGS: [[Anticholinergic]] Activity). In the event of increased [[anticholinergic]] side effects, [[plasma]] levels of disopyramide should be monitored and the dose of the drug adjusted accordingly. A reduction of the dose by one third, from the recommended 600 mg/day to 400 mg/day, would be reasonable, without changing the dosing interval. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with [[impaired renal function]]. Because elderly patients are more likely to have decreased [[renal function]], care should be taken in dose selection, and it may be useful to monitor [[renal function]]. | |||
|useInGender=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific gender populations. | |||
|useInRace=There is no FDA guidance on the use of {{PAGENAME}} with respect to specific racial populations. | |||
|useInRenalImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with renal impairment. | |||
|useInHepaticImpair=There is no FDA guidance on the use of {{PAGENAME}} in patients with hepatic impairment. | |||
|useInReproPotential=There is no FDA guidance on the use of {{PAGENAME}} in women of reproductive potentials and males. | |||
|useInImmunocomp=There is no FDA guidance one the use of {{PAGENAME}} in patients who are immunocompromised. | |||
<!--Administration and Monitoring--> | |||
|administration=* Oral | |||
|monitoring=For patients with [[cardiomyopathy]] or possible [[cardiac]] decompensation, a loading dose, as discussed below, should not be given, and initial dosage should be limited to 100 mg every 6 to 8 hours. Subsequent dosage adjustments should be made gradually, with close monitoring for the possible development of [[hypotension]] and/or [[congestive heart failure]] | |||
|overdose=====Symptoms===== | |||
* Deliberate or accidental [[overdosage]] of oral disopyramide may be followed by [[apnea]], [[loss of consciousness]], cardiac [[arrhythmias]], and loss of spontaneous [[respiration]]. Death has occurred following [[overdosage]]. | |||
* Toxic plasma levels of disopyramide produce excessive widening of the [[QRS complex]] and [[Q-T interval]], worsening of [[congestive heart failure]], [[hypotension]], varying kinds and degrees of conduction disturbance, [[bradycardia]], and finally [[asystole]]. Obvious [[anticholinergic]] effects are also observed. | |||
* The approximate oral LD50 of disopyramide phosphate is 580 and 700 mg/kg for rats and mice, respectively. | |||
=====Treatment===== | |||
* Experience indicates that prompt and vigorous treatment of [[overdosage]] is necessary, even in the absence of symptoms. Such treatment may be life-saving. No specific [[antidote]] for disopyramide phosphate has been identified. Treatment should be symptomatic and may include induction of [[emesis]] or [[gastric lavage]], administration of a [[cathartic]] followed by [[activated charcoal]] by mouth or stomach tube, intravenous administration of [[isoproterenol]] and [[dopamine]], insertion of an [[intra-aortic balloon]] for[[counterpulsation]], and [[mechanical ventilation]]. [[Hemodialysis]] or, preferably, [[hemoperfusion]] with charcoal may be employed to lower [[serum]] concentration of the drug. | |||
* The [[electrocardiogram]] should be monitored, and supportive therapy with [[cardiac glycosides]] and [[diuretics]] should be given as required. | |||
* If progressive [[AV block]] should develop, [[endocardial]] pacing should be implemented. In case of any [[impaired renal function]], measures to increase the [[glomerular filtration rate]] may reduce the toxicity (disopyramide is excreted primarily by the [[kidney]]). | |||
* The anticholinergic effects can be reversed with [[neostigmine]] at the discretion of the physician. | |||
* Altering the urinary pH in humans does not affect the plasma half-life or the amount of disopyramide excreted in the urine. | |||
|drugBox=<!--Mechanism of Action--> | |||
|mechAction=Disopyramide phosphate is a Type 1 [[antiarrhythmic drug]] (i.e., similar to [[procainamide]] and [[quinidine]]). In animal studies, disopyramide phosphate decreases the rate of diastolic depolarization (phase 4) in cells with augmented [[automaticity]], decreases the upstroke velocity (phase 0) and increases the [[action potential]] duration of normal [[cardiac]] cells, decreases the disparity in refractoriness between [[infarction|infarcted]] and adjacent normally perfused [[myocardium]], and has no effect on [[Alpha-adrenergic receptor|alpha]]- or [[Beta-adrenergic receptor|beta-adrenergic receptors]]. | |||
|structure=Disopyramide Phosphate is an [[antiarrhythmic drug]] available for oral administration in capsules containing 100 mg or 150 mg of disopyramide base, present as the phosphate. The base content of the phosphate salt is 77.6%. The structural formula of Disopyramide Phosphate is: | |||
[[File:DisopyramideStructure.png|600px|thumbnail|left|This image is provided by the National Library of Medicine.]] | |||
{{clr}} | |||
C21H29N3O·H3PO4 M.W. 437.47 | |||
α-[2-(diisopropylamino) ethyl]-α-phenyl-2-pyridineacetamide phosphate | |||
Disopyramide Phosphate is freely soluble in water, and the free base (pKa 10.4) has an aqueous solubility of 1 mg/mL. The chloroform:water partition coefficient of the base is 3.1 at pH 7.2. | |||
Disopyramide Phosphate is a racemic mixture of d- and l-isomers. This drug is not chemically related to other [[antiarrhythmic drugs]]. | |||
Inactive Ingredients: Capsules: Lactose Monohydrate, Magnesium Stearate and Sodium Starch Glycolate. | |||
Capsule Print and Shell Constituents: Black Iron Oxide, D&C Red #28, D&C Red #33, D&C Yellow #10, D&C Yellow #10 Aluminum Lake, FD&C Blue #1, FD&C Blue #1 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, Gelatin, Propylene Glycol, Shellac, Sodium Lauryl Sulfate, Sorbitan Monolaurate and Titanium Dioxide. | |||
|PD=====Electrophysiology===== | |||
In man, disopyramide phosphate at [[therapeutic]] plasma levels shortens the sinus node recovery time, lengthens the effective refractory period of the [[atrium]], and has a minimal effect on the effective refractory period of the [[AV node]]. Little effect has been shown on [[AV-nodal]] and [[His-Purkinje]] conduction times or [[QRS duration]]. However, prolongation of conduction in [[accessory pathways]] occurs. | |||
=====Hemodynamics===== | |||
At recommended oral doses, disopyramide phosphate rarely produces significant alterations of [[blood pressure]] in patients without [[congestive heart failure]]. With intravenous disopyramide phosphate, either increases in [[systolic]]/[[diastolic]] or decreases in [[systolic blood pressure]] have been reported, depending on the [[infusion]] rate and the patient population. [[Intravenous]] disopyramide phosphate may cause [[cardiac depression]] with an approximate mean 10% reduction of [[cardiac output]], which is more pronounced in patients with [[cardiac dysfunction]]. | |||
=====Anticholinergic Activity===== | |||
The in vitro [[anticholinergic]] activity of disopyramide phosphate is approximately 0.06% that of [[atropine]]; however, the usual dose for disopyramide phosphate is 150 mg every 6 hours compared to 0.4 to 0.6 mg for [[atropine]]. | |||
|PK=Following oral administration of disopyramide phosphate, disopyramide phosphate is rapidly and almost completely absorbed, and peak plasma levels are usually attained within 2 hours. The usual [[therapeutic]] plasma levels of disopyramide base are 2 to 4 mcg/mL, and at these concentrations protein binding varies from 50% to 65%. Because of concentration-dependent [[protein]] binding, it is difficult to predict the concentration of the free drug when total drug is measured. | |||
The mean plasma half-life of disopyramide in healthy humans is 6.7 hours (range of 4 to 10 hours). In six patients with impaired renal function ([[creatinine clearance]] less than 40 mL/min), disopyramide half-life values were 8 to 18 hours. | |||
After the oral administration of 200 mg of disopyramide to 10 cardiac patients with borderline to moderate [[heart failure]], the time to peak serum concentration of 2.3 ± 1.5 hours (mean ± SD) was increased, and the mean peak serum concentration of 4.8 ± 1.6 mcg/mL was higher than in healthy volunteers. After [[intravenous]] administration in these same patients, the mean elimination half-life was 9.7 ± 4.2 hours (range in healthy volunteers of 4.4 to 7.8 hours). In a second study of the oral administration of disopyramide to 7 patients with heart disease, including [[left ventricular dysfunction]], the mean plasma half-life was slightly prolonged to 7.8 ± 1.9 hours (range of 5 to 9.5 hours). | |||
In healthy men, about 50% of a given dose of disopyramide is excreted in the urine as the unchanged drug, about 20% as the mono-N-dealkylated metabolite, and 10% as the other metabolites. The [[plasma]] concentration of the major metabolite is approximately one tenth that of disopyramide. Altering the urinary pH in man does not affect the [[plasma]] half-life of disopyramide. | |||
|nonClinToxic=Eighteen months of Disopyramide Phosphate administration to rats, at oral doses up to 400 mg/kg/day (about 30 times the usual daily human dose of 600 mg/day, assuming a patient weight of at least 50 kg), revealed no evidence of carcinogenic potential. An evaluation of mutagenic potential by Ames test was negative. Disopyramide Phosphate, at doses up to 250 mg/kg/day, did not adversely affect fertility of rats. | |||
Pregnancy | |||
|clinicalStudies=There is limited information regarding <i>Clinical Studies</i> of {{PAGENAME}} in the drug label. | |||
|howSupplied=Disopyramide Phosphate is supplied as: | |||
* 100 mg - hard gelatin capsule with a light-blue body imprinted "93-3127" and a scarlet cap imprinted "93-3127", containing 100 mg of disopyramide base present as the phosphate, in bottles of 100. | |||
* 150 mg - hard gelatin capsule with a scarlet body imprinted "93-3129" and a buff cap imprinted "93-3129", containing 150 mg of disopyramide base present as the phosphate, in bottles of 100. | |||
|storage=Store at 20º to 25º (68º to77ºF). | |||
|fdaPatientInfo=There is limited information regarding <i>Patient Counseling Information</i> of {{PAGENAME}} in the drug label. | |||
|alcohol=In rare instances significant lowering of blood-glucose values has been reported during disopyramide phosphate administration. The physician should be alert to this possibility, especially in patients with [[congestive heart failure]], chronic malnutrition, [[hepatic]], [[renal]] or other diseases, or drugs (e.g., [[beta blockers]], [[alcohol]]) which could compromise preservation of the normal glucoregulatory mechanisms in the absence of food. In these patients, the [[Blood glucose|blood-glucose levels]] should be carefully followed. | |||
|brandNames=* Norpace | |||
* Norpace CR | |||
|lookAlike=* Disopyramide - Desipramine | |||
|drugShortage= | |||
}} | |||
{{LabelImage | |||
|fileName=DisopyramidePackage1.png | |||
}} | |||
{{LabelImage | |||
|fileName=DisopyramidePackage2.png | |||
}} | |||
<!--Pill Image--> | |||
<!--Label Display Image--> | |||
<!--Category--> | |||
[[Category:Antiarrhythmic agents]] | |||
[[Category:Cardiovascular Drugs]] | |||
[[Category:Drug]] | |||
Latest revision as of 15:29, 24 March 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Alonso Alvarado, M.D. [2]; Adeel Jamil, M.D. [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Mortality
See full prescribing information for complete Boxed Warning.
In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-center, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had had a myocardial infarction more than 6 days but less than 2 years previously, an excessive mortality or non-fatal cardiac arrest rate (7.7%) was seen in patients treated with encainide or flecainide compared with that seen in patients assigned to carefully matched placebo-treated groups (3.0%). The average duration of treatment with encainide or flecainide in this study was 10 months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) is uncertain. Considering the known proarrhythmic properties of Disopyramide Phosphate and the lack of evidence of improved survival for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of Disopyramide Phosphate as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmias.
|
Overview
Disopyramide is a antiarrhythmic Group IA , cardiovascular agent that is FDA approved for the treatment of ventricular arrhythmias such as sustained ventricular tachycardia, that, in the judgment of the physician, are life-threatening. There is a Black Box Warning for this drug as shown here. Common adverse reactions include negative inotropic effect on myocardium, constipation, nausea, xerostomia, muscle weakness, blurred vision, delay when starting to pass urine, urinary retention, generalized aches and pains, malaise and fatigue.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
The dosage of disopyramide phosphate must be individualized for each patient on the basis of response and tolerance. The usual adult dosage of disopyramide phosphate is 400 to 800 mg per day given in divided doses. The recommended dosage for most adults is 600 mg/day given in divided doses (150 mg every 6 hours). For patients whose body weight is less than 110 pounds (50 kg), the recommended dosage is 400 mg/day given in divided doses (100 mg every 6 hours). In the event of increased anticholinergic side effects, plasma levels of disopyramide should be monitored and the dose of the drug adjusted accordingly. A reduction of the dose by one third, from the recommended 600 mg/day to 400 mg/day, would be reasonable, without changing the dosing interval.
For patients with cardiomyopathy or possible cardiac decompensation, a loading dose, as discussed below, should not be given, and initial dosage should be limited to 100 mg every 6 to 8 hours. Subsequent dosage adjustments should be made gradually, with close monitoring for the possible development of hypotension and/or congestive heart failure.
For patients with moderate renal insufficiency (creatinine clearance greater than 40 mL/min) or hepatic insufficiency, the recommended dosage is 400 mg/day given in divided doses (100 mg every 6 hours).
For patients with severe renal insufficiency (Ccr 40 mL/min or less), the recommended dosage regimen is 100 mg at intervals shown in the table below, with or without an initial loading dose of 150 mg.
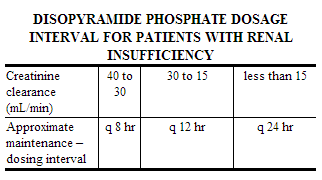
For patients in whom rapid control of ventricular arrhythmia is essential, an initial loading dose of 300 mg of disopyramide phosphate (200 mg for patients whose body weight is less than 110 pounds) is recommended, followed by the appropriate maintenance dosage. Therapeutic effects are usually attained 30 minutes to 3 hours after administration of a 300 mg loading dose. If there is no response or evidence of toxicity within 6 hours of the loading dose, 200 mg of disopyramide phosphate every 6 hours may be prescribed instead of the usual 150 mg. If there is no response to this dosage within 48 hours, either disopyramide phosphate should then be discontinued or the physician should consider hospitalizing the patient for careful monitoring while subsequent disopyramide phosphate doses of 250 mg or 300 mg every 6 hours are given. A limited number of patients with severe refractory ventricular tachycardia have tolerated daily doses of disopyramide phosphate up to 1600 mg per day (400 mg every 6 hours), resulting in disopyramide plasma levels up to 9 mcg/mL. If such treatment is warranted, it is essential that patients be hospitalized for close evaluation and continuous monitoring.
Transferring to Disopyramide Phosphate
The following dosage schedule based on theoretical considerations rather than experimental data is suggested for transferring patients with normal renal function from either quinidine sulfate or procainamide therapy (Type 1 antiarrhythmic agents) to disopyramide phosphate therapy:
- Disopyramide phosphate should be started using the regular maintenance schedule without a loading dose 6 to 12 hours after the last dose of quinidine sulfate or 3 to 6 hours after the last dose of procainamide.
- In patients in whom withdrawal of quinidine sulfate or procainamide is likely to produce life-threatening arrhythmias, the physician should consider hospitalization of the patient.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
Atrial Fibrillation=
- Developed by: AHA/ACC[1]
- Class of Recommendation: Class IIb
- Strength of Evidence: Level of Evidence C
- Dosing Information
- Immediate release tablets: 100-200 mg four times a day.
- Extended release tablets: 200-400 mg bid.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Disopyramide in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
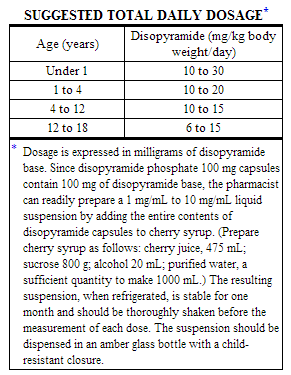
Controlled clinical studies have not been conducted in pediatric patients; however, the following suggested dosage table is based on published clinical experience.
Total daily dosage should be divided and equal doses administered orally every 6 hours or at intervals according to individual patient needs. Disopyramide plasma levels and therapeutic response must be monitored closely. Patients should be hospitalized during the initial treatment period, and dose titration should start at the lower end of the ranges provided below.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Disopyramide in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Disopyramide in pediatric patients.
Contraindications
- Cardiogenic shock
- Preexisting second-degree AV block or third-degree AV block (if no pacemaker is present)
- Congenital Q-T prolongation
- Hypersensitivity to the drug
Warnings
|
Mortality
See full prescribing information for complete Boxed Warning.
In the National Heart, Lung and Blood Institute’s Cardiac Arrhythmia Suppression Trial (CAST), a long-term, multi-center, randomized, double-blind study in patients with asymptomatic non-life-threatening ventricular arrhythmias who had had a myocardial infarction more than 6 days but less than 2 years previously, an excessive mortality or non-fatal cardiac arrest rate (7.7%) was seen in patients treated with encainide or flecainide compared with that seen in patients assigned to carefully matched placebo-treated groups (3.0%). The average duration of treatment with encainide or flecainide in this study was 10 months.
The applicability of the CAST results to other populations (e.g., those without recent myocardial infarction) is uncertain. Considering the known proarrhythmic properties of Disopyramide Phosphate and the lack of evidence of improved survival for any antiarrhythmic drug in patients without life-threatening arrhythmias, the use of Disopyramide Phosphate as well as other antiarrhythmic agents should be reserved for patients with life-threatening ventricular arrhythmias.
|
Negative Inotropic Properties=
Heart Failure/Hypotension
Disopyramide phosphate may cause or worsen congestive heart failure or produce severe hypotension as a consequence of its negative inotropic properties. Hypotension has been observed primarily in patients with primary cardiomyopathy or inadequately compensated congestive heart failure. Disopyramide phosphate should not be used in patients with uncompensated or marginally compensated congestive heart failure or hypotension unless the congestive heart failure or hypotension is secondary to cardiac arrhythmia. Patients with a history of heart failure may be treated with Disopyramide phosphate, but careful attention must be given to the maintenance of cardiac function, including optimal digitalization. If hypotension occurs or congestive heart failure worsens, disopyramide phosphate should be discontinued and, if necessary, restarted at a lower dosage only after adequate cardiac compensation has been established.
QRS Widening
Although it is unusual, significant widening (greater than 25%) of the QRS complex may occur during Disopyramide phosphate administration; in such cases disopyramide phosphate should be discontinued.
Q-T Prolongation
As with other Type 1 antiarrhythmic drugs, prolongation of the Q-T interval (corrected) and worsening of the arrhythmia, including ventricular tachycardia and ventricular fibrillation, may occur. Patients who have evidenced prolongation of the Q-T interval in response to quinidine may be at particular risk. As with other Type 1A antiarrhythmics, disopyramide phosphate has been associated with torsade de pointes. If a Q-T prolongation of greater than 25% is observed and if ectopy continues, the patient should be monitored closely, and consideration given to discontinuing disopyramide phosphate.
Hypoglycemia
In rare instances significant lowering of blood-glucose values has been reported during disopyramide phosphate administration. The physician should be alert to this possibility, especially in patients with congestive heart failure, chronic malnutrition, hepatic, renal or other diseases, or drugs (e.g., beta blockers, alcohol) which could compromise preservation of the normal glucoregulatory mechanisms in the absence of food. In these patients, the blood-glucose levels should be carefully followed.
Concomitant Antiarrhythmic Therapy
The concomitant use of disopyramide phosphate with other Type 1A antiarrhythmic agents (such as quinidine or procainamide), Type 1C antiarrhythmics (such as encainide, flecainide or propafenone), and/or propranolol should be reserved for patients with life-threatening arrhythmias who are demonstrably unresponsive to single-agent antiarrhythmic therapy. Such use may produce serious negative inotropic effects, or may excessively prolong conduction. This should be considered particularly in patients with any degree of cardiac decompensation or those with a prior history thereof. Patients receiving more than one antiarrhythmic drug must be carefully monitored.
Heart Block
If first-degree heart block develops in a patient receiving disopyramide phosphate, the dosage should be reduced. If the block persists despite reduction of dosage, continuation of the drug must depend upon weighing the benefit being obtained against the risk of higher degrees of heart block. Development of second-degree AV block or third-degree AV block or unifascicular, bifascicular, or trifascicular block requires discontinuation of disopyramide phosphate therapy, unless the ventricular rate is adequately controlled by a temporary or implanted ventricular pacemaker.
Anticholinergic Activity
Because of its anticholinergic activity, disopyramide phosphate should not be used in patients with glaucoma, myasthenia gravis or urinary retention unless adequate overriding measures are taken; these consist of the topical application of potent miotics (e.g., pilocarpine) for patients with glaucoma, and catheter drainage or operative relief for patients with urinary retention. Urinary retention may occur in patients of either sex as a consequence of disopyramide phosphate administration, but males with benign prostatic hypertrophy are at particular risk. In patients with a family history of glaucoma, intraocular pressure should be measured before initiating disopyramide phosphate therapy. Disopyramide phosphate should be used with special care in patients with myasthenia gravis since its anticholinergic properties could precipitate a myasthenic crisis in such patients.
Precautions
General
Atrial Tachyarrhythmias
Patients with atrial flutter or atrial fibrillation should be digitalized prior to disopyramide phosphate administration to ensure that drug-induced enhancement of AV conduction does not result in an increase of ventricular rate beyond physiologically acceptable limits.
Conduction Abnormalities
Care should be taken when prescribing disopyramide phosphate for patients with sick sinus syndrome (bradycardia-tachycardia syndrome), Wolff-Parkinson-White syndrome (WPW), or bundle branch block. The effect of disopyramide phosphate in these conditions is uncertain at present.
Cardiomyopathy
Patients with myocarditis or other cardiomyopathy may develop significant hypotension in response to the usual dosage of disopyramide phosphate, probably due to cardiodepressant mechanisms. Therefore, a loading dose of disopyramide phosphate should not be given to such patients, and initial dosage and subsequent dosage adjustments should be made under close supervision.
Adverse Reactions
Clinical Trials Experience
The adverse reactions which were reported in disopyramide phosphate clinical trials encompass observations in 1,500 patients, including 90 patients studied for at least 4 years. The most serious adverse reactions are hypotension and congestive heart failure. The most common adverse reactions, which are dose dependent, are associated with the anticholinergic properties of the drug. These may be transitory, but may be persistent or can be severe. Urinary retention is the most serious anticholinergic effect.
The following reactions were reported in 10% to 40% of patients
- Anticholinergic: Dry mouth, urinary hesitancy, constipation.
The following reactions were reported in 3% to 9% of patients
- Anticholinergic: Blurred vision, dry nose/eyes/throat.
- Genitourinary: Urinary retention, urinary frequency and urinary urgency.
- Gastrointestinal: Nausea, abdominal pain/bloating/gas.
- General: Dizziness, fatigue/muscle weakness, headache, malaise, aches/pains.
The following reactions were reported in 1% to 3% of patients
- Genitourinary: Impotence.
- Cardiovascular: Hypotension with or without congestive heart failure, increased congestive heart failure, cardiac conduction disturbances]], edema/weight gain, shortness of breath, syncope, chest pain.
- Gastrointestinal: Anorexia, diarrhea, vomiting.
- Dermatologic: Generalized rash/dermatoses, itching.
- Central nervous system: Nervousness.
- Other: Hypokalemia, elevated cholesterol/triglycerides
The following reactions were reported in less than 1%
- Depression.
- Insomnia.
- Dysuria.
- Numbness/tingling.
- Elevated liver enzymes.
- AV block.
- Elevated BUN.
- Elevated creatinine.
- Decreased hemoglobin/hematocrit.
- Hypoglycemia.
- Reversible cholestatic jaundice.
- Fever.
- Thrombocytopenia.
- Reversible agranulocytosis.
- Gynecomastia.
- Some cases of SLE (systmic lupus erythematosus) symptoms have been reported; most cases occurred in patients who had been switched to disopyramide from procainamide following the development of SLE symptoms.
- Rarely, acute psychosis has been reported following disopyramide phosphate therapy, with prompt return to normal mental status when therapy was stopped.
Postmarketing Experience
There is limited information regarding Disopyramide Postmarketing Experience in the drug label.
Drug Interactions
- If phenytoin or other hepatic enzyme inducers are taken concurrently with disopyramide phosphate, lower plasma levels of disopyramide may occur. Monitoring of disopyramide plasma levels is recommended in such concurrent use to avoid ineffective therapy.
- Other antiarrhythmic drugs (e.g., quinidine, procainamide, lidocaine, propranolol) have occasionally been used concurrently with disopyramide phosphate. Excessive widening of the QRS complex and/or prolongation of the Q-T interval may occur in these situations. In healthy subjects, no significant drug-drug interaction was observed when disopyramide phosphate was coadministered with either propranolol or diazepam. Concomitant administration of Disopyramide Phosphate and quinidine resulted in slight increases in plasma disopyramide levels and slight decreases in plasma quinidine levels.
- Disopyramide Phosphate does not increase serum digoxin levels.
- Until data on possible interactions between verapamil and disopyramide phosphate are obtained, disopyramide should not be administered within 48 hours before or 24 hours after verapamil administration.
- Although potent inhibitors of cytochrome P450 3A4 (e.g., ketoconazole) have not been studied clinically, in vitro studies have shown that erythromycin and oleandomycin inhibit the metabolism of disopyramide. Cases of life-threatening interactions have been reported for disopyramide when given with clarithromycin and erythromycin indicating that coadministration of disopyramide with inhibitors of cytochrome P450 3A4 could result in potentially fatal interaction.
Use in Specific Populations
Pregnancy
Teratogenic Effeects=
Disopyramide Phosphate was associated with decreased numbers of implantation sites and decreased growth and survival of pups when administered to pregnant rats at 250 mg/kg/day (20 or more times the usual daily human dose of 12 mg/kg, assuming a patient weight of at least 50 kg), a level at which weight gain and food consumption of dams were also reduced. Increased resorption rates were reported in rabbits at 60 mg/kg/day (5 or more times the usual daily human dose). Effects on implantation, pup growth, and survival were not evaluated in rabbits. There are no adequate and well-controlled studies in pregnant women. Disopyramide Phosphate should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
Nonteratogenic Effects
Disopyramide phosphate has been reported to stimulate contractions of the pregnant uterus. Disopyramide has been found in human fetal blood.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Disopyramide in women who are pregnant.
Labor and Delivery
It is not known whether the use of disopyramide phosphate during labor or delivery has immediate or delayed adverse effects on the fetus, or whether it prolongs the duration of labor or increases the need for forceps delivery or other obstetric intervention.
Nursing Mothers
Studies in rats have shown that the concentration of disopyramide and its metabolites is between one and three times greater in milk than it is in plasma. Following oral administration, disopyramide has been detected in human milk at a concentration not exceeding that in plasma. Because of the potential for serious adverse reactions in nursing infants from disopyramide phosphate, a decision should be made whether to discontinue nursing or to discontinue the drug, taking into account the importance of the drug to the mother.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Clinical studies of disopyramide phosphate did not include sufficient numbers of subjects aged 65 and over to determine whether they respond differently from younger subjects. Other reported clinical experience has not identified differences in responses between the elderly and younger patients. In general, dose selection for an elderly patient should be cautious, usually starting at the low end of the dosing range, reflecting the greater frequency of decreased hepatic, renal, or cardiac function, and of concomitant disease or other drug therapy. Because of its anticholinergic activity, disopyramide phosphate should not be used in patients with glaucoma, urinary retention, or benign prostatic hypertrophy (medical conditions commonly associated with the elderly) unless adequate overriding measures are taken (see WARNINGS: Anticholinergic Activity). In the event of increased anticholinergic side effects, plasma levels of disopyramide should be monitored and the dose of the drug adjusted accordingly. A reduction of the dose by one third, from the recommended 600 mg/day to 400 mg/day, would be reasonable, without changing the dosing interval. This drug is known to be substantially excreted by the kidney, and the risk of toxic reactions to this drug may be greater in patients with impaired renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection, and it may be useful to monitor renal function.
Gender
There is no FDA guidance on the use of Disopyramide with respect to specific gender populations.
Race
There is no FDA guidance on the use of Disopyramide with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Disopyramide in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Disopyramide in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Disopyramide in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Disopyramide in patients who are immunocompromised.
Administration and Monitoring
Administration
- Oral
Monitoring
For patients with cardiomyopathy or possible cardiac decompensation, a loading dose, as discussed below, should not be given, and initial dosage should be limited to 100 mg every 6 to 8 hours. Subsequent dosage adjustments should be made gradually, with close monitoring for the possible development of hypotension and/or congestive heart failure
IV Compatibility
There is limited information regarding the compatibility of Disopyramide and IV administrations.
Overdosage
Symptoms=
- Deliberate or accidental overdosage of oral disopyramide may be followed by apnea, loss of consciousness, cardiac arrhythmias, and loss of spontaneous respiration. Death has occurred following overdosage.
- Toxic plasma levels of disopyramide produce excessive widening of the QRS complex and Q-T interval, worsening of congestive heart failure, hypotension, varying kinds and degrees of conduction disturbance, bradycardia, and finally asystole. Obvious anticholinergic effects are also observed.
- The approximate oral LD50 of disopyramide phosphate is 580 and 700 mg/kg for rats and mice, respectively.
Treatment
- Experience indicates that prompt and vigorous treatment of overdosage is necessary, even in the absence of symptoms. Such treatment may be life-saving. No specific antidote for disopyramide phosphate has been identified. Treatment should be symptomatic and may include induction of emesis or gastric lavage, administration of a cathartic followed by activated charcoal by mouth or stomach tube, intravenous administration of isoproterenol and dopamine, insertion of an intra-aortic balloon forcounterpulsation, and mechanical ventilation. Hemodialysis or, preferably, hemoperfusion with charcoal may be employed to lower serum concentration of the drug.
- The electrocardiogram should be monitored, and supportive therapy with cardiac glycosides and diuretics should be given as required.
- If progressive AV block should develop, endocardial pacing should be implemented. In case of any impaired renal function, measures to increase the glomerular filtration rate may reduce the toxicity (disopyramide is excreted primarily by the kidney).
- The anticholinergic effects can be reversed with neostigmine at the discretion of the physician.
- Altering the urinary pH in humans does not affect the plasma half-life or the amount of disopyramide excreted in the urine.
Pharmacology
There is limited information regarding Disopyramide Pharmacology in the drug label.
Mechanism of Action
Disopyramide phosphate is a Type 1 antiarrhythmic drug (i.e., similar to procainamide and quinidine). In animal studies, disopyramide phosphate decreases the rate of diastolic depolarization (phase 4) in cells with augmented automaticity, decreases the upstroke velocity (phase 0) and increases the action potential duration of normal cardiac cells, decreases the disparity in refractoriness between infarcted and adjacent normally perfused myocardium, and has no effect on alpha- or beta-adrenergic receptors.
Structure
Disopyramide Phosphate is an antiarrhythmic drug available for oral administration in capsules containing 100 mg or 150 mg of disopyramide base, present as the phosphate. The base content of the phosphate salt is 77.6%. The structural formula of Disopyramide Phosphate is:
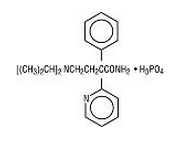
C21H29N3O·H3PO4 M.W. 437.47 α-[2-(diisopropylamino) ethyl]-α-phenyl-2-pyridineacetamide phosphate Disopyramide Phosphate is freely soluble in water, and the free base (pKa 10.4) has an aqueous solubility of 1 mg/mL. The chloroform:water partition coefficient of the base is 3.1 at pH 7.2. Disopyramide Phosphate is a racemic mixture of d- and l-isomers. This drug is not chemically related to other antiarrhythmic drugs. Inactive Ingredients: Capsules: Lactose Monohydrate, Magnesium Stearate and Sodium Starch Glycolate. Capsule Print and Shell Constituents: Black Iron Oxide, D&C Red #28, D&C Red #33, D&C Yellow #10, D&C Yellow #10 Aluminum Lake, FD&C Blue #1, FD&C Blue #1 Aluminum Lake, FD&C Blue #2 Aluminum Lake, FD&C Red #40 Aluminum Lake, Gelatin, Propylene Glycol, Shellac, Sodium Lauryl Sulfate, Sorbitan Monolaurate and Titanium Dioxide.
Pharmacodynamics
Electrophysiology=
In man, disopyramide phosphate at therapeutic plasma levels shortens the sinus node recovery time, lengthens the effective refractory period of the atrium, and has a minimal effect on the effective refractory period of the AV node. Little effect has been shown on AV-nodal and His-Purkinje conduction times or QRS duration. However, prolongation of conduction in accessory pathways occurs.
Hemodynamics
At recommended oral doses, disopyramide phosphate rarely produces significant alterations of blood pressure in patients without congestive heart failure. With intravenous disopyramide phosphate, either increases in systolic/diastolic or decreases in systolic blood pressure have been reported, depending on the infusion rate and the patient population. Intravenous disopyramide phosphate may cause cardiac depression with an approximate mean 10% reduction of cardiac output, which is more pronounced in patients with cardiac dysfunction.
Anticholinergic Activity
The in vitro anticholinergic activity of disopyramide phosphate is approximately 0.06% that of atropine; however, the usual dose for disopyramide phosphate is 150 mg every 6 hours compared to 0.4 to 0.6 mg for atropine.
Pharmacokinetics
Following oral administration of disopyramide phosphate, disopyramide phosphate is rapidly and almost completely absorbed, and peak plasma levels are usually attained within 2 hours. The usual therapeutic plasma levels of disopyramide base are 2 to 4 mcg/mL, and at these concentrations protein binding varies from 50% to 65%. Because of concentration-dependent protein binding, it is difficult to predict the concentration of the free drug when total drug is measured.
The mean plasma half-life of disopyramide in healthy humans is 6.7 hours (range of 4 to 10 hours). In six patients with impaired renal function (creatinine clearance less than 40 mL/min), disopyramide half-life values were 8 to 18 hours.
After the oral administration of 200 mg of disopyramide to 10 cardiac patients with borderline to moderate heart failure, the time to peak serum concentration of 2.3 ± 1.5 hours (mean ± SD) was increased, and the mean peak serum concentration of 4.8 ± 1.6 mcg/mL was higher than in healthy volunteers. After intravenous administration in these same patients, the mean elimination half-life was 9.7 ± 4.2 hours (range in healthy volunteers of 4.4 to 7.8 hours). In a second study of the oral administration of disopyramide to 7 patients with heart disease, including left ventricular dysfunction, the mean plasma half-life was slightly prolonged to 7.8 ± 1.9 hours (range of 5 to 9.5 hours).
In healthy men, about 50% of a given dose of disopyramide is excreted in the urine as the unchanged drug, about 20% as the mono-N-dealkylated metabolite, and 10% as the other metabolites. The plasma concentration of the major metabolite is approximately one tenth that of disopyramide. Altering the urinary pH in man does not affect the plasma half-life of disopyramide.
Nonclinical Toxicology
Eighteen months of Disopyramide Phosphate administration to rats, at oral doses up to 400 mg/kg/day (about 30 times the usual daily human dose of 600 mg/day, assuming a patient weight of at least 50 kg), revealed no evidence of carcinogenic potential. An evaluation of mutagenic potential by Ames test was negative. Disopyramide Phosphate, at doses up to 250 mg/kg/day, did not adversely affect fertility of rats. Pregnancy
Clinical Studies
There is limited information regarding Clinical Studies of Disopyramide in the drug label.
How Supplied
Disopyramide Phosphate is supplied as:
- 100 mg - hard gelatin capsule with a light-blue body imprinted "93-3127" and a scarlet cap imprinted "93-3127", containing 100 mg of disopyramide base present as the phosphate, in bottles of 100.
- 150 mg - hard gelatin capsule with a scarlet body imprinted "93-3129" and a buff cap imprinted "93-3129", containing 150 mg of disopyramide base present as the phosphate, in bottles of 100.
Storage
Store at 20º to 25º (68º to77ºF).
Images
Drug Images
{{#ask: Page Name::Disopyramide |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Disopyramide |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Disopyramide in the drug label.
Precautions with Alcohol
In rare instances significant lowering of blood-glucose values has been reported during disopyramide phosphate administration. The physician should be alert to this possibility, especially in patients with congestive heart failure, chronic malnutrition, hepatic, renal or other diseases, or drugs (e.g., beta blockers, alcohol) which could compromise preservation of the normal glucoregulatory mechanisms in the absence of food. In these patients, the blood-glucose levels should be carefully followed.
Brand Names
- Norpace
- Norpace CR
Look-Alike Drug Names
- Disopyramide - Desipramine
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ January CT, Wann LS, Alpert JS, Calkins H, Cleveland JC, Cigarroa JE; et al. (2014). "2014 AHA/ACC/HRS Guideline for the Management of Patients With Atrial Fibrillation: A Report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines and the Heart Rhythm Society". J Am Coll Cardiol. doi:10.1016/j.jacc.2014.03.022. PMID 24685669.
{{#subobject:
|Label Page=Disopyramide |Label Name=DisopyramidePackage1.png
}}
{{#subobject:
|Label Page=Disopyramide |Label Name=DisopyramidePackage2.png
}}