Cardiac action potential

Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
The cardiac action potential is a specialized action potential in the heart, with unique properties necessary for function of the electrical conduction system of the heart.
The cardiac action potential differs significantly in different portions of the heart. This differentiation of the action potentials allows the different electrical characteristics of the different portions of the heart. For instance, the specialized conduction tissue of the heart has the special property of depolarizing without any external influence. This is known as automaticity.
The electrical activity of the specialized conduction tissues are not apparent on the surface electrocardiogram (ECG). This is due to the relatively small mass of these tissues compared to the myocardium.
| Ion | Extracellular | Intracellular | Ratio |
|---|---|---|---|
| Na+ | 135 - 145 | 10 | 14:1 |
| K+ | 3.5 - 5.0 | 155 | 1:16 |
| Cl- | 95 - 110 | 20 - 30 | 4:1 |
| Ca2+ | 2 | 10-4 | 2 x 104 |
| Although intracellular Ca2+ content is about 2 mM, most of this is bound or sequestered in intracellular organelles (mitochondria and sarcoplasmic reticulum). | |||
Cardiac muscle has some similarities to neurons and skeletal muscle, as well as important unique properties. Like a neuron, a given myocardial cell has a negative membrane potential when at rest. Stimulation above a threshold value induces the opening of voltage-gated ion channels and a flood of cations into the cell. When the threshold is met, an action potential initiates. This causes the positively charged ions to enter the cell [depolarization]. Like skeletal muscle, depolarization causes the opening of voltage-gated calcium channels and entry of Ca2+ from the t-tubules. This influx of calcium causes calcium-induced calcium release from the sarcoplasmic reticulum, and the increase in myoplasmic free Ca2+ concentration causes muscle contraction. After a delay (the absolute refractory period), Potassium channels reopen and the resulting flow of K+ out of the cell causes repolarization to the resting state.
Note that there are important physiological differences between nodal cells and ventricular cells; the specific differences in ion channels and mechanisms of polarization give rise to unique properties of SA node cells, most importantly the spontaneous depolarizations (automaticity) necessary for the SA node's pacemaker activity.
Major currents
| Ion | Current | α subunit protein | α subunit gene | Phase / role |
|---|---|---|---|---|
| Na+ | INa | NaV1.5 | SCN5A | 0 |
| Ca2+ | ICa(L) | CaV1.2 | CACNA1C | 0-2 |
| K+ | Ito1 | KV4.2/4.3 | KCND2/KCND3 | 1, notch |
| K+ | IKs | KV7.1 | KCNQ1 | 2,3 |
| K+ | IKr | KV11.1 (hERG) | KCNH2 | 3 |
| K+ | IK1 | Kir2.1/2.2/2.3 | KCNJ2/KCNJ12/KCNJ4 | 3,4 |
| Na+, Ca2+ | INaCa | 3Na+-1Ca2+-exchanger | NCX1 (SLC8A1) | ion homeostasis |
| Na+, K+ | INaK | 3Na+-2K+-ATPase | ATP1A | ion homeostasis |
| Ca2+ | IpCa | Ca2+-transporting ATPase | ATP1B | ion homeostasis |
Calcium channels
Two voltage-dependent calcium channels play critical roles in the physiology of cardiac muscle: L-type calcium channel ('L' for Long-lasting) and T-type calcium channels ('T' for Transient) voltage-gated calcium channels.
These channels respond differently to voltage changes across the membrane: L-type channels respond to higher membrane potentials, open more slowly, and remain open longer than T-type channels.
Because of these properties, L-type channels are important in sustaining an action potential, while T-type channels are important in initiating them.
Because of their rapid kinetics, T-type channels respond better to rhythmic stimulation and are also found in some neuron cell bodies, where they play an important role in rhythmic processes such as heartbeat, breathing, and spinal cord pattern generators used in walking.
L-type channels are selectively blocked by dihydropyridines.
Resting membrane potential
The resting membrane potential is caused by the difference in ionic concentrations and conductances across the membrane of the cell during phase 4 of the action potential. The normal resting membrane potential in the ventricular myocardium is about -85 to -95 mV. This potential is determined by the selective permeability of the cell membrane to various ions. The membrane is most permeable to K+ and relatively impermeable to other ions. The resting membrane potential is therefore dominated by the K+ equilibrium potential according to the K+ gradient across the cell membrane. The membrane potential can be calculated using the Goldman equation|Goldman-Hodgkin-Katz voltage equation. The maintenance of this electrical gradient is due to various ion pumps and exchange mechanisms, including the Na+-K+ ion exchange pump, the Na+-Ca2+ exchanger current and the IK1 inwardly rectifying K+ current.
Intracellularly (within the cell), K+ is the principal cation, and phosphate and the conjugate bases of organic acids are the dominant anions. Extracellularly (outside the cell), Na+ and Cl- predominate.
Phases of the cardiac action potential
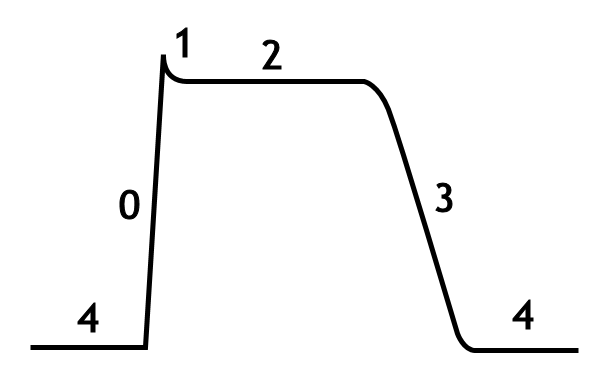
The standard model used to understand the cardiac action potential is the action potential of the ventricular myocyte. The action potential has 5 phases (numbered 0-4). Phase 4 is the resting membrane potential, and describes the membrane potential when the cell is not being stimulated.
Once the cell is electrically stimulated (typically by an electric current from an adjacent cell), it begins a sequence of actions involving the influx and efflux of multiple cations and anions that together produce the action potential of the cell, propagating the electrical stimulation to the cells that lie adjacent to it. In this fashion, an electrical stimulation is conducted from one cell to all the cells that are adjacent to it, to all the cells of the heart.
Phase 4
Phase 4 is the resting membrane potential. This is the period that the cell remains in until it is stimulated by an external electrical stimulus (typically an adjacent cell). This phase of the action potential is associated with diastole of the chamber of the heart.
Certain cells of the heart have the ability to undergo spontaneous depolarization, in which an action potential is generated without any influence from nearby cells. This is also known as automaticity. The cells that can undergo spontaneous depolarization the fastest are the primary pacemaker cells of the heart, and set the heart rate. Usually, these are cells in the SA node of the heart. Electrical activity that originates from the SA node is propagated to the rest of the heart. The fastest conduction of electrical activity is via the electrical conduction system of the heart.
In cases of heart block, in which the activity of the primary pacemaker does not propagate to the rest of the heart, a latent pacemaker (also known as an escape pacemaker) will undergo spontaneous depolarization and create an action potential.
The mechanism of automaticity involves the so-called pacemaker channels of the HCN family, Hyperpolarization-gated, Cyclic Nucleotide-gated channels. These poorly selective cation channels conduct more current as the membrane potential becomes more negative, or hyperpolarized. They conduct both potassium and sodium. The activity of these channels in the SA node cells causes the membrane potential to slowly become more positive (depolarized) until, eventually, calcium channels are activated and an action potential is initiated.
Phase 0
Phase 0 is the rapid depolarization phase. The slope of phase 0 represents the maximum rate of depolarization of the cell and is known as Vmax. This phase is due to the opening of the fast Na+ channels causing a rapid increase in the membrane conductance to Na+ (GNa) and thus a rapid influx of Na+ ions (INa) into the cell; a Na+ current.
The ability of the cell to open the fast Na+ channels during phase 0 is related to the membrane potential at the moment of excitation. If the membrane potential is at its baseline (about -85 mV), all the fast Na+ channels are closed, and excitation will open them all, causing a large influx of Na+ ions. If, however, the membrane potential is less negative, some of the fast Na+ channels will be in an inactivated state insensitive to opening, thus causing a lesser response to excitation of the cell membrane and a lower Vmax. For this reason, if the resting membrane potential becomes too positive, the cell may not be excitable, and conduction through the heart may be delayed, increasing the risk for arrhythmias.
The fast Na+ channel
The fast sodium channel can be modeled as being controlled by a number of gates. Each gate (or gating variable) can attain a value between 1 (fully open) and 0 (fully closed). The product of all the gates denotes the percentage of channels available to conduct Na+. Following the model of Hodgkin and Huxley, the sodium channel contains three gates: m, h, and j. In the resting state, the m gate is closed (zero) and the h and j gates are open (one). Hence, the product denoting the percentage of conducting channels is also zero. Upon electrical stimulation of the cell, the m gate opens quickly while simultaneously the h and j gates close more slowly. For a brief period of time, all gates are open (i.e. non-zero) and Na+ can enter the cell following its electrochemical gradient. If, as above, the resting membrane potential is too positive, the h or j gates may be considerably less than one, such that the product of m, h and j becomes too small upon depolarization.
Phase 1
Phase 1 of the action potential occurs with the inactivation of the fast Na+ channels. The transient net outward current causing the small downward deflection of the action potential is due to the movement of K+ and Cl- ions, carried by the Ito1 and Ito2 currents, respectively. Particularly the Ito1 contributes to the "notch" of some ventricular cardiomyocyte action potentials.
It has been suggested that Cl- ions movement across the cell membrane during Phase I is as a result of the change in membrane potential, from K+ efflux, and is not a contributory factor to the initial repolarisation ("notch").
Phase 2
This "plateau" phase of the cardiac action potential is sustained by a balance between inward movement of Ca2+ (ICa) through L-type calcium channels and outward movement of K+ through the slow delayed rectifier potassium channels, IKs. The sodium-calcium exchanger current, INa,Ca and the sodium/potassium pump current, INa,K also play minor roles during phase 2.
Phase 3
During phase 3 of the action potential, the L-type Ca2+ channels close, while the slow delayed rectifier (IKs) K+ channels are still open. This ensures a net outward current, corresponding to negative change in membrane potential, thus allowing more types of K+ channels to open. These are primarily the rapid delayed rectifier K+ channels (IKr) and the inwardly rectifiyng K+ current, IK1. This net outward, positive current (equal to loss of positive charge from the cell) causes the cell to repolarize. The delayed rectifier K+ channels close when the membrane potential is restored to about -80 to -85 mV, while IK1 remains conducting throughout phase 4, contributing to set the resting membrane potential.
Abnormal automaticity
The normal activity of the pacemaker cells of the heart is to spontaneously depolarize at a regular rhythm, generating the normal heart rate. Abnormal automaticity involves the abnormal spontaneous depolarization of cells of the heart. This typically causes arrhythmias (irregular rhythms) in the heart.
See also
- Electrical conduction system of the heart
- Action potential
- Antiarrhythmic agents
- Cardiac arrhythmia
- Cardiac pacemaker
- Resting membrane potential
- Ventricular action potential
External links
- Interactive animation illustrating the generation of a cardiac action potential.
- Interactive mathematical models of cardiac action potential and other generic action potentials.