Tuberculosis medical therapy
|
Tuberculosis Microchapters |
|
Diagnosis |
|---|
|
Treatment |
|
Case Studies |
|
Tuberculosis medical therapy On the Web |
|
American Roentgen Ray Society Images of Tuberculosis medical therapy |
|
Risk calculators and risk factors for Tuberculosis medical therapy |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Charmaine Patel, M.D. [2]; Ahmed Zaghw, M.D. [3]; Ammu Susheela, M.D. [4]; Sara Mehrsefat, M.D. [5]
Overview
The treatment of tuberculosis with anti-TB drugs is divided mainly into two phases; the initiation phase and maintenance phase. If there is a high likelihood of infection, start anti-TB treatment the patient even if the AFB stain is negative, while waiting for the culture results. The patient should come back in few weeks. Patients usually feel better a few weeks post-treatment. Patients have to be monitored for side effects and treatment failure. In addition, all TB cases are tested for drug resistance in the U.S.
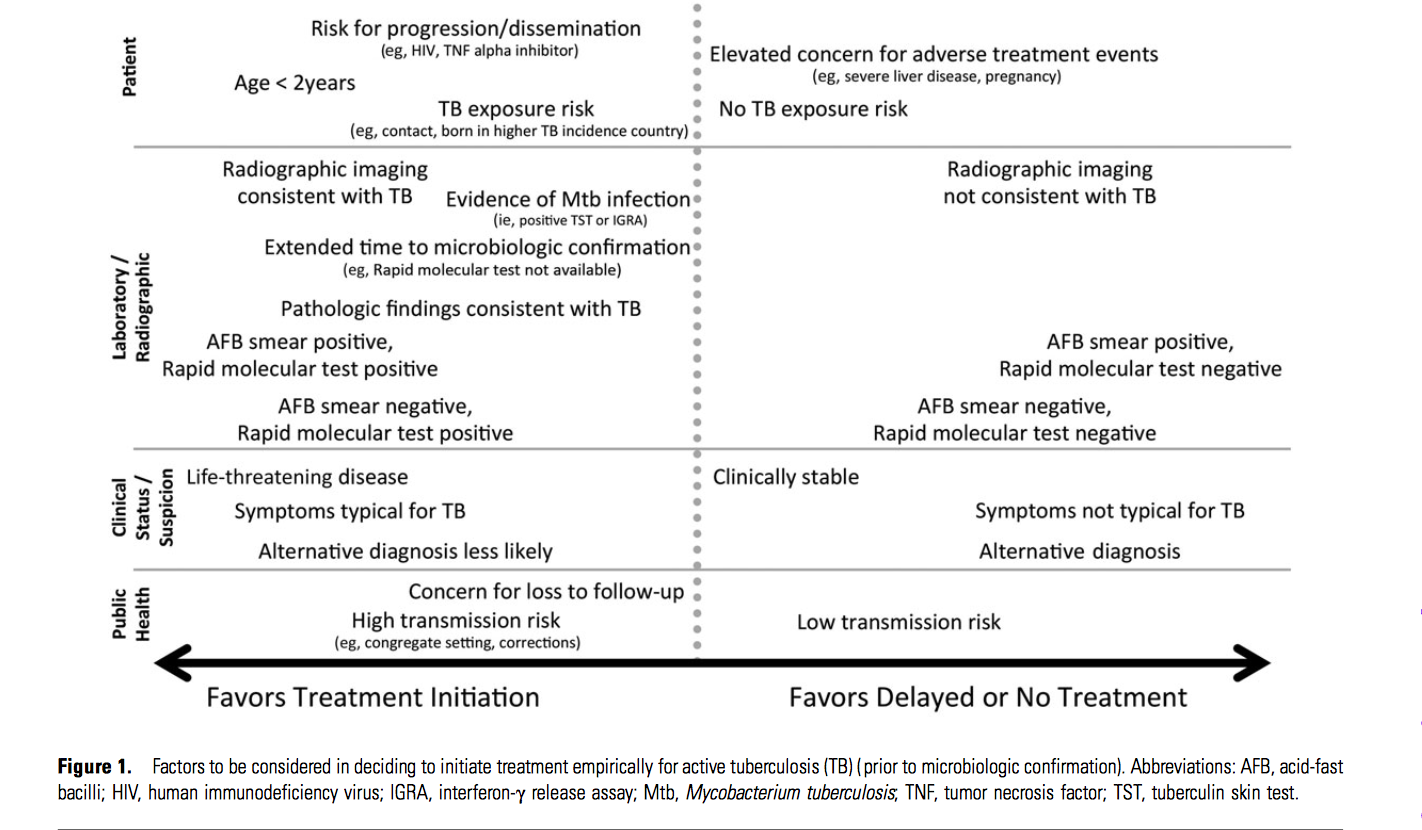
Deciding To Initiate Treatment
- The decision to initiate combination anti-tuberculous therapy is made according to the following:
- Epidemiological information
- Clinical evidence
- Pathological
- Radiographic findings
- Microscopic examination of acid-fast bacilli (AFB)--stained sputum (smears) and cultures for mycobacteria
- Positive PPD-tuberculin skin test
Drugs Used in the Treatment of Tuberculosis
| Groups | Drugs |
|---|---|
| Group 1: First-line oral drugs |
|
| Group 2: Injectable drugs |
|
| Group 3: Fluoroquinolones | |
| Group 4: Oral bacteriostatic second-line drugs |
|
| Group 5: Agents with unclear role in treatment of drug resistant-TB |
|
| Adapted from WHO 2013 Treatment of Tuberculosis: Guidelines – 4th ed.[2] | |
Standard Treatment Regimens
Empirical Anti-Tuberculosis Therapy
- In developing endemic countries and in cases with high clinical suspicion of tuberculous pericarditis, it is recommended to start with empiric antituberculous treatment before establishing a definitive diagnosis. In the clinical settings where the diagnosis cannot be confirmed according to bacteriology, histology, or pericardial fluid analysis, clinical response to antituberculous treatment can be suggestive of a diagnosis of tuberculous pericarditis.[3]
- In developed countries where TB is not endemic, antituberculous treatment should not be started empirically without a definitive diagnosis.[4]
Standard Regimens for New Patients
|
Adults ▸ Preferred regimen ▸ Alternate regimen 1 ▸ Alternate regimen 2 Children ▸ Preferred regimen |
|
Standard Regimens for Previously Treated Patients
The previously treated patients should receive the 8-months regimen with first-line drugs.
|
Extrapulmonary Tuberculosis Treatment
The principles underlying the treatment of pulmonary tuberculosis can also be applied to extrapulmonary disease. Increasing evidence, including randomized controlled trials, reports that 6–9 month isoniazid and rifampicin containing regimens are effective for most of extrapulmonary sites of disease.[5]
| Type of Extrapulmonary Tuberculosis | Treatment |
|---|---|
| Lymph Node Tuberculosis |
|
| Bone, Joint, and Spinal Tuberculosis |
|
| Pericardial Tuberculosis |
|
| Pleural Tuberculosis |
|
| Tuberculous Meningitis |
|
| Disseminated Tuberculosis (miliary tuberculosis) |
|
| Genitourinary Tuberculosis |
|
| Abdominal Tuberculosis |
|
Monitoring during treatment
Directly observed treatment, short-course (DOTS) strategy
| 5 components of DOTS strategy |
|---|
| Government committed to sustained TB control and activities |
| Case detection by sputum smear microscopy among symptomatic patients self reporting to health services |
| Standardized treatment, with supervision and patient support |
| An effective drug supply and management system |
| Monitoring and evaluation system, and impact measurement |
Monitoring the Patients and Baseline Evaluations
- Tuberculosis patients should have the following:
- Microscopic examination of sputum specimens and culture. It is recommended to obtain three sputum specimens. Induction of sputum with hypertonic saline may be required to obtain specimens and bronchoscopy is considered for certain patients who are unable to produce sputum, according to the clinical evaluation.
- Drug susceptibility testing for INH, RIF, and EMB should be done once the initial positive culture is obtained, regardless of the source of the specimen. Second-line drug susceptibility testing should be performed only in reference laboratories and only for specimens from those patients who had previous treatment, who are contacts of patients with multi-drug resistant tuberculosis, who have shown resistance to rifampin or to other first-line drugs, or who have positive cultures after more than 3 months of therapy.
- Counseling and testing for HIV infection is important. In patients with HIV infection, a CD4+ lymphocyte count should be ordered. In Patients with risk factors for hepatitis B or C viruses (e.g., injection drug use, HIV infection) serologic tests for these viruses should be performed.
- Baseline measurements of liver enzymes (aspartate aminotransferase [AST], alanine aminotransferase [ALT]), alkaline phosphatase, bilirubin and serum creatinine and a platelet count should be performed.
- If a patient is treated with EMB, visual acuity and red-green color discrimination should be tested.
- During and following pulmonary tuberculosis therapy, the following should be performed:
- Microscopic examination of a sputum specimen and culture should be performed at a minimum of monthly intervals until two consecutive specimens are negative on culture.
- AFB smear can assess the early response to TB therapy and inform of the infectiousness of the patient
- In case of extrapulmonary tuberculosis, the frequency and types of evaluations will be based on the site involved.
- At least monthly clinical evaluation to detect possible side effects of the antituberculosis medications and to assess compliance.
- Patients do not need follow-up after completion of treatment but should be instructed to seek medical care promptly in case of recurrence of signs or symptoms.
- Routine measurements of hepatic and renal function and platelet count are not indicated during the course of treatment unless patients have abnormal baseline measurements or are at high risk of hepatotoxicity (e.g., hepatitis B or C virus infection, alcoholics).
- Patients who are hepatitis virus carriers, have a past history of acute hepatitis, or current excessive alcohol consumption can be given the usual TB treatment regimens unless there is clinical evidence of chronic liver disease. However, hepatotoxic adverse effects of anti-TB drugs may be more common among these patients, so regular follow-up is highly recommended.
Assessment of Treatment Response in New and Previously Treated Pulmonary TB
Definition of Treatment Response₳
| Outcome | Definition |
| Cure | A patient with positive sputum smear/positive culture at the beginning of the therapy that are converted into smear-negative/culture-negative in the last month of therapy and on at least one previous occasion. |
| Treatment completed | A patient who completed treatment but who does not have a negative sputum smear or culture result in the last month of therapy and on at least one previous occasionb ( Two consecutive negative specimens ) |
| Treatment failure | A patient with positive sputum smear or culture at 5 months or later during treatment course or has a multidrug-resistant (MDR) strain at any point of time during the course of treatment, whether they are smear-positive or smear-negative. |
| Died | A patient who dies for any cause during the treatment course. |
| Default | A patient whose course of treatment was interrupted for ≥ 2 months. |
| Transfer out | A patient who was transferred to another recording and reporting unit and whose outcome of treatment is unknown. |
| Treatment success | A sum of cured and completed treatmentc |
| |
Identification and Management of Patients at Increased Risk of Treatment Failure and Relapse
- Approximately 80% of patients with pulmonary tuberculosis caused by drug-susceptible organisms who are started on standard four-drug therapy will have negative sputum cultures at this time. Patients with positive cultures after 2 months of treatment should undergo a careful evaluation to determine the cause.
- The risk factors for adverse outcomes (treatment failure or relapse) include:
- The initial chest X-ray showed cavitation in addition to having a positive sputum culture at the time of the initial phase.
- Nonadherence to prescribed drugs (particularly for patients not receiving DOT)
- Extensive cavitary disease at the time of diagnosis
- Drug resistance (especially for patients receiving DOT)
- Malabsorption of drugs
- Laboratory error
- Biological variation in response
Prevention of Adverse Effects of Drugs
Isoniazid-induced peripheral neuropathy manifests as:
- Numbness
- Tingling or burning of the hands or feet
- This adverse effect is commonly occur in pregnant women in addition to the following conditions: diabetes, HIV infection, chronic liver disease, chronic renal failure, malnutrition, or alcohol abuse.
- Adding Pyridoxine is recommended as a preventive treatment (10 mg/day with anti-TB drugs). Other guidelines recommend 25 mg/day.[6]
Symptom-Based Approach for Side-Effects of Anti-tuberculous Drugs
The Role of Drug-Susceptibility Testing (DST)
- Initial Phase:
- DST is performed for all TB patients at the initiation of treatment to determine most appropriate therapy for each patient. However, the target of universal access to DST has not yet been recognized for most of the worldwide TB patients. Although countries are increasing laboratory capacity and implementing new rapid tests, WHO recommends that sputum specimens for testing susceptibility to isoniazid and rifampicin be performed for the following patient groups at the start of treatment:
- All previously treated patients. The highest levels of MDR are detected in patients whose previous course of treatment has failed.
- All individuals living with HIV plus they are diagnosed with active TB, particularly if they live in areas of high MDR prevalence. It is necessary to identify MDR as soon as possible in patients living with HIV because of their high risk of mortality.
- Continuation Phase:
- If rapid molecular-based DST is available, the results of MDR can be confirmed or excluded within 1-2 days, hence it can guide the choice of treatment regimen.
- If DST is not available, the first-line drugs include 2HRZES/1HRZE/5HRE if country-specific data reports low or medium levels of MDR in its patients or if such data are not available
- When DST results become available, treatment regimens should be adjusted accordingly.
Recommendations For New Patients
- In new patients, if the specimen performed at the end of the intensive phase second month is smear-positive, sputum smear microscopy should be done at the end of the third month (strong/High grade of evidence).
- In new patients, if the specimen performed at the end of third month is smear-positive, sputum culture and drug susceptibility testing (DST) should be done (strong/High grade of evidence)
- For smear-positive pulmonary TB patients treated with first-line drugs, sputum smear microscopy may be performed at the completion of the intensive phase of treatment (conditional/High or moderate grade of evidence).
- Sputum should be collected after the 1st dose of the intensive phase treatment. The end of the intensive phase is defined as the end of the 2nd month in new patients and the end of the 3rd month in previously treated patients receiving the 8-month regimen of first-line agents. This recommendation also applies to smear-negative patients.
- Sputum specimens must be collected for smear examination at every follow-up sputum check. They must be collected without interrupting the treatment course and transported to the laboratory urgently.
- The status of sputum smear at the end of the intensive phase is a poor predictor of relapse. However, identification of a positive sputum smear is still an essential way of the patient assessment.
- The number of sputum smear-positive patients converted to negative at the end of the intensive phase is considered a good indicator of TB program performance.
Management of Treatment Interruption[1]
| Time Point of Interruption | Details of Interruption | Approach | |
|---|---|---|---|
| During intensive phase | *Lapse is <14 d in duration | Lapse is <14 d in duration Continue treatment to complete planned total number of doses (as long as all doses are completed within 3 mo) | |
| Lapse is ≥14 d in duration | Restart treatment from the beginning | ||
| During continuation phase | Received ≥80% of doses and sputum was AFB smear negative on initial testing | Additional therapy may not be necessary | |
| *Received ≥80% of doses and sputum was AFB smear positive on initial testing | Continue the treatment until all doses are completed | ||
| Received <80% of doses and accumulative lapse is <3 mo in duration | Continue therapy until all doses are completed (full course), unless consecutive lapse is >2 mo
If treatment regimen cannot be completed within the recommended time frame, restart therapy (ie, restart intensive phase then the continuation phase) | ||
| *Received <80% of doses and lapse is ≥3 mo in duration | Restart therapy with new intensive and continuation phases (ie, restart intensive phase then the continuation phase) | ||
Managing Side-Effects of Anti-TB Drugs[7]
| ||||||||||||||||||||||||||||||||||||||||||||||||||||||
Treatment Failure
- Failure to respond to anti-TB drugs means;
- Treatment failure necessitate to step-wise approach to identify the causes of failure which could be due to any of the following features[8]
- Poor supervision of the initial phase
- Poor patient adherence
- Poor quality of anti-TB drugs
- Inappropriate doses of anti-TB medications (below than recommended range)
- Slow resolution due to progressive cavitation and a initial heavy mycobacterial load
- Co-morbidities that interfere with the response or adherence to treatment
- MDR M. tuberculosis with no response to the first-line treatment
- Non-viable mycobacteria remain visible by microscopy
Treatment Regimen
- 1. Standard regimens for new patients [9]
- 1.1. Adult
- 1.1.1. Initial phase
- Preferred regimen: Isoniazid 300 mg PO (5 mg/kg/day) qd for 8 weeks AND Rifampicin 600 mg PO (10 mg/kg/day) qd for 8 weeks AND Pyrazinamide 2 g PO (25 mg/kg/day) qd for 8 weeks AND Ethambutol 1.6 g PO (15 mg/kg/day) qd for 8 weeks
- Alternative regimen (1): Isoniazid 300 mg/day PO for 2 weeks (5 mg/kg/day) AND Rifampicin 600 mg/day PO for 2 weeks (10 mg/kg/day) AND Pyrazinamide 2 g/day PO for 2 weeks (25 mg/kg/day) AND Ethambutol 1.6 g PO for 2 weeks (15 mg/kg/day), followed by Isoniazid 300 mg/day PO twice weekly for 6 weeks (5 mg/kg/day) AND Rifampicin 600 mg/day PO twice weekly for 6 weeks (10 mg/kg/day) AND Pyrazinamide 2 g/day PO twice weekly for 6 weeks AND Ethambutol 1.6 g PO for 2 weeks (15 mg/kg/day)
- Alternative regimen (2): Isoniazid 300 mg/day PO thrice weekly for 8 weeks (5 mg/kg/day) AND Rifampicin 600 mg/day PO thrice weekly for 8 weeks (10 mg/kg/day) AND Pyrazinamide 2g/day PO thrice weekly for 8 week (25 mg/kg/day) AND Ethambutol 1.6 g PO thrice weekly for 8 weeks (15 mg/kg/day)
- 1.1.2 Continuation phase
- Preferred regimen (1): Isoniazid 300 mg PO (5 mg/kg/day) qd AND Rifampicin 600 mg PO (10 mg/kg/day) qd for 18 weeks
- Preferred regimen (2): Isoniazid 300 mg PO twice weekly (5 mg/kg/day) AND Rifampicin 600 mg/day PO twice weekly (10 mg/kg/day) for 18 weeks
- Alternative regimen (1): Isoniazid 300 mg/day PO biweekly for 18 weeks (5 mg/kg/day) AND Rifampicin 600 mg/day PO biweekly for 18 weeks (10 mg/kg/day)
- Alternative regimen (2): Isoniazid 300 mg/day PO thrice weekly for 18 weeks (5 mg/kg/day) AND Rifampicin 600 mg/day PO thrice weekly for 18 weeks (10 mg/kg/day)
- 1.2 Pediatric
- 1.2.1 Initial phase
- Preferred regimen: Isoniazid 10 mg/kg PO (Maximum, 300 mg/day) AND Rifampicin 15 mg/kg PO (Maximum, 600 mg/day) AND Pyrazinamide 35 mg/kg PO (Maximum, 2 g/day) AND Ethambutol 20 mg/kg PO (Maximum, 1.6 g/day), each for 8 weeks
- 1.2.2 Continuation phase
- Preferred regimen: Isoniazid 10 mg/kg PO (Maximum, 300 mg/day) AND Rifampicin 15 mg/kg PO (Maximum, 600 mg/day), each drug daily for 18 weeks
- 2. RR-TB or MDR-TB Tuberculosis[10]
- 2.1 Adult
- Preferred regimen: At least 5 agents combination
- Agent 1: Pyrazinamide 20–30 mg/kg
- Agent 2: Levofloxacin 500-1000 mg OR Moxifloxacin 400 mg OR Gatifloxacin 400mg
- Agent 3: Amikacin 7.5-10 mg/kg OR Capreomycin 15 mg/kg OR Kanamycin 15 mg/kg OR Streptomycin 12–18 mg/kg
- Agent 4: [Ethionamide]] 15-20 mg/kg OR Protionamide 15-20 mg/kg OR Cycloserine 10-15 mg/kg OR Terizidone 10-20 mg/kg OR Clofazimine 100mg
- Agent 5: Bedaquiline 200-400mg OR Delamanid
- Agent 6: Para-aminosalicylic acid 150 mg/kg/day q8-12h OR Imipenem/Cilastatin 250mg/250mg-750mg/750mg {{or} Meropenem 20-40mg/kg OR Amoxicillin clavulanate 500mg-125mg OR Thioacetazone 150mg
- Note: Pyrazinamide and four core second-line TB medicines (one chosen from Group A, one from Group B, and at least two from Group C2)
- Note: If the minimum number of effective TB medicines cannot be composed as given above, an agent from Group D2 and other agents from Group D3 may be added to bring the total to five
- 2.2 Pediatric
- Preferred regimen: At least 5 agents combination
- Agent 1: Pyrazinamide 20-30 mg/kg (Maximum: 600 mg)
- Agent 2 (Group A): Levofloxacin 7.5-10mg/kg OR Moxifloxacin 7.5-10mg/kg OR Gatifloxacin 10 mg/kg (maximum 600 mg)
- Agent 3 (Group B): Amikacin 7.5-10 mg/kg OR Capreomycin 15 mg/kg OR Kanamycin 15 mg/kg OR Streptomycin 12–18 mg/kg
- Agent 4 (Group C): [Ethionamide]] 15-20 mg/kg/day q12h (Maximum: 1000 mg)OR Protionamide 15-20 mg/kg OR Cycloserine 10-15 mg/kg (Maximum: 1000 mg) OR Terizidone 10-20 mg/kg Maximum: 1000 mg) OR Clofazimine 100mg
- Agent 5: (Group D3): Para-aminosalicylic acid 150 mg/kg/day q8-12h(Maximum: 12,000 mg) OR Imipenem/Cilastatin 250mg/250mg-750mg/750mg {{or} Meropenem 20-40mg/kg OR Amoxicillin clavulanate 500mg-125mg OR Thioacetazone 50mg
- Note: Pyrazinamide and four core second-line TB medicines (one chosen from Group A, one from Group B, and at least two from Group C2)
- Note: If the minimum number of effective TB medicines cannot be composed as given above, an agent from Group D2 and other agents from Group D3 may be added to bring the total to five
- 3. XDR Tuberculosis [11]
- 3.1 Adult
- Preferred regimen: 3 agents combination
- Agent 1: Pyrazinamide 20–30 mg/kg OR Ethambutol 15–25 mg/kg OR Rifabutin 5 mg/kg
- Agent 2: Ethionamide 15-20 mg/kg OR Protionamide 15-20 mg/kg OR Cycloserine 10-15 mg/kg OR Terizidone 10-20 kg/mg OR Para-aminosalicylic acid 8-12 g/day q8-12h
- Agent 3: Clofazimine 50 mg/d AND 300 mg once a month OR Amoxicillin/clavulanate 500 mg/125 mg q12h OR Linezolid 300-600 mg OR Imipenem 500mg q6h OR Clarithromycin 500-1000 mg q12h OR Thioacetazone 2.5 mg/kg OR Isoniazid (high-dose) 16–20 mg/kg
- 3.2 Pediatric
- Preferred regimen: 3 agents combination
- Agent 1: Pyrazinamide 20-30 mg/kg (Maximum: 600 mg) OR Ethambutol 15 mg/kg OR Rifabutin 5 mg/kg
- Agent 2: Ethionamide 15-20 mg/kg (Maximum: 1000 mg) OR Protionamide 15-20 mg/kg (Maximum: 1000 mg) OR Cycloserine 10-20 mg/kg (Maximum: 1000 mg) OR Terizidone 10-20 mg/kg (Maximum: 1000 mg) OR Para-aminosalicylic acid 150 mg/kg/day q8-12h
- Agent 3: Clofazimine 50 mg/d AND 300 mg once a month OR Amoxicillin/clavulanate OR Linezolid 300-600 mg OR Imipenem 500mg q6h OR Clarithromycin 500-1000 mg q12h OR Thioacetazone 2.5 mg/kg OR Isoniazid (high-dose) 16–20 mg/kg
Ocular tuberculosis
- 1. Adult patients
- 1.1 Intensive phase
- Preferred regimen: Isoniazid 5 mg/kg (max: 300 mg) PO qd for 2 months AND Rifampin 10 mg/kg (max: 600 mg) PO qd for 2 months AND Pyrazinamide 15–30 mg/kg (max: 2 g) PO qd for 2 months AND Ethambutol 15-20 mg/kg (max: 1 g) PO qd for 2 months
- 1.2 Continuation phase
- 2. Pediatric patients
- 2.1 Intensive phase
- Preferred regimen: Isoniazid 10-15 mg/kg (max: 300 mg) PO qd for 2 months AND Rifampin 10-20 mg/kg (max: 600 mg) PO qd for 2 months AND Pyrazinamide 15-30 mg/kg (max: 2 g) PO qd for 2 months AND Ethambutol
- 2.2 Continuation phase
- Note (1): Ethambutol may be administered at a dose of 15-20 mg/kg (max: 1 g) PO qd for 2 months but is generally avoided because of potential ocular toxicity.[14]
- Note (2): A short course of systemic corticosteroids may be necessary initially if there is sight-threatening inflammation.
Contraindicated medications
Active tuberculosis is considered an absolute contraindication to the use of the following medications:
References
- ↑ 1.0 1.1 Clinical Infectious Diseases. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. (2016) http://cid.oxfordjournals.org/content/63/7/e147.full.pdf+html Accessed on October 14, 2016
- ↑ 2.0 2.1 "2013 WHO Treatment of Tuberculosis: Guidelines for National Programmes (4th Edition)".
- ↑ Mayosi, BM.; Burgess, LJ.; Doubell, AF. (2005). "Tuberculous pericarditis". Circulation. 112 (23): 3608–16. doi:10.1161/CIRCULATIONAHA.105.543066. PMID 16330703. Unknown parameter
|month=ignored (help) - ↑ Soler-Soler, J.; Sagristà-Sauleda, J.; Permanyer-Miralda, G. (2001). "Management of pericardial effusion". Heart. 86 (2): 235–40. PMID 11454853. Unknown parameter
|month=ignored (help) - ↑ Clinical Infectious Diseases. Official American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America Clinical Practice Guidelines: Treatment of Drug-Susceptible Tuberculosis. (2016) http://cid.oxfordjournals.org/content/63/7/e147.full.pdf+html Accessed on October 14, 2016
- ↑ "Treatment of tuberculosis". MMWR Recomm Rep. 52 (RR-11): 1–77. 2003. PMID 12836625. Unknown parameter
|month=ignored (help) - ↑ "http://whqlibdoc.who.int/publications/2004/9241546034.pdf" (PDF). External link in
|title=(help) - ↑ "http://whqlibdoc.who.int/publications/2004/9241546034.pdf" (PDF). External link in
|title=(help) - ↑ Treatment of tuberculosis guidelines. Geneva: World Health Organization. 2010. ISBN 9789241547833.
- ↑ WHO treatment guidelines for drug- resistant tuberculosis 2016 update. http://apps.who.int/iris/bitstream/10665/250125/1/9789241549639-eng.pdf?ua=1Accessed on October 14, 2016
- ↑ "WHO".
- ↑ Blumberg, Henry M.; Burman, William J.; Chaisson, Richard E.; Daley, Charles L.; Etkind, Sue C.; Friedman, Lloyd N.; Fujiwara, Paula; Grzemska, Malgosia; Hopewell, Philip C.; Iseman, Michael D.; Jasmer, Robert M.; Koppaka, Venkatarama; Menzies, Richard I.; O'Brien, Richard J.; Reves, Randall R.; Reichman, Lee B.; Simone, Patricia M.; Starke, Jeffrey R.; Vernon, Andrew A.; American Thoracic Society, Centers for Disease Control and Prevention and the Infectious Diseases Society (2003-02-15). "American Thoracic Society/Centers for Disease Control and Prevention/Infectious Diseases Society of America: treatment of tuberculosis". American Journal of Respiratory and Critical Care Medicine. 167 (4): 603–662. doi:10.1164/rccm.167.4.603. ISSN 1073-449X. PMID 12588714.
- ↑ American Thoracic Society; CDC; Infectious Diseases Society of America (2003-06-20). "Treatment of tuberculosis". MMWR. Recommendations and reports: Morbidity and mortality weekly report. Recommendations and reports / Centers for Disease Control. 52 (RR-11): 1–77. ISSN 1057-5987. PMID 12836625.
- ↑ Bennett, John (2015). Mandell, Douglas, and Bennett's principles and practice of infectious diseases. Philadelphia, PA: Elsevier/Saunders. ISBN 978-1455748013.