Kanamycin
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Black Box Warning
|
Boxed warning
See full prescribing information for complete Boxed Warning.
* Patients treated with aminoglycosides by any route should be under close clinical observation because of the potential toxicity associated with their use.
|
Overview
Kanamycin is an antibiotic that is FDA approved for the treatment of shortterm treatment of serious infections caused by susceptible strains of the designated microorganisms like E. coli, Proteus species (both indole-positive and indole-negative), Enterobacter aerogenes, Klebsiella pneumoniae, Serratia marcescens, Acinetobacter species. There is a Black Box Warning for this drug as shown here. Common adverse reactions include ototoxicity, nephrotoxicity and neuromuscular blockade.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- To reduce the development of drug-resistant bacteria and maintain the effectiveness of Kanamycin Injection and other antibacterial drugs, Kanamycin Injection should be used only to treat or prevent infections that are proven or strongly suspected to be caused by susceptible bacteria. When culture and susceptibility information are available, they should be considered in selecting or modifying antibacterial therapy.
- In the absence of such data, local epidemiology and susceptibility patterns may contribute to the empiric selection of therapy.
- Kanamycin injection is indicated in the short term treatment of serious infections caused by susceptible strains of the designated microorganisms below. Bacteriological studies to identify the causative organisms and to determine their susceptibility to kanamycin should be performed. Therapy may be instituted prior to obtaining the results of susceptibility testing.
- Kanamycin may be considered as initial therapy in the treatment of infections where one or more of the following are the known or suspected pathogens: E. coli, Proteus species (both indole-positive and indole-negative), Enterobacter aerogenes, Klebsiella pneumoniae, Serratia marcescens, Acinetobacter species. The decision to continue therapy with the drug should be based on results of the susceptibility tests, the response of the infection to therapy.
- In serious infections when the causative organisms are unknown, kanamycin injection, may be administered as initial therapy in conjunction with a penicillin- or cephalosporin-type drug before obtaining results of susceptibility testing. If anaerobic organisms are suspected, consideration should be given to using other suitable antimicrobial therapy in conjunction with kanamycin.
- Although kanamycin is not the drug of choice for staphylococcal infections, it may be indicated under certain conditions for the treatment of known or suspected staphylococcal disease. These situations include the initial therapy of severe infections where the organism is thought to be either a Gram-negative bacterium or a staphylococcus, infections due to susceptible strains of staphylococci in patients allergic to other antibiotics, and mixed staphylococcal/Gram-negative infections.
- Kanamycin injection may be given intramuscularly or intravenously. The patient’s pretreatment body weight should be obtained for calculation of the correct dosage. The dosage of an aminoglycoside in obese patients should be based on an estimate of the lean body mass. The status of renal function should be determined by measurement of serum creatinine concentration or calculation of the endogenous creatinine clearance rate. The blood urea nitrogen (BUN) level is much less reliable for this purpose. Renal function should be reassessed frequently during therapy.
- It is desirable to measure both peak and trough serum concentrations intermittently during therapy since both concentrations are used to determine the adequacy and safety of the dose and to adjust the dosage during treatment. Peak serum concentrations (30 to 90 minutes after injection) above 35 mcg per mL and trough concentrations (just prior to the next dose) above 10 mcg per mL should be avoided.
Intramuscular Route
- Inject deeply into the upper outer quadrant of the gluteal muscle. The recommended dose for adults or children is 15 mg/kg/day in two equally divided dosages administered at equally divided intervals; i.e., 7.5 mg/kg q12h. If continuously high blood levels are desired, the daily dose of 15 mg/kg may be given in equally divided doses every 6 or 8 hours. Treatment of patients in the heavier weight classes, i.e., 100 kg, should not exceed 1.5 g/day.
- In patients with impaired renal function, it is desirable to follow therapy by appropriate serum assays. If this is not feasible, a suggested method is to reduce the frequency of administration in patients with renal dysfunction. The interval between doses may be calculated with the following formula:
- Serum creatinine (mg/100 mL) x 9 = Dosage Interval (in hours); e.g., if the serum creatinine is 2 mg, the recommended dose (7.5 mg/kg) should be administered every 18 hours. Changes in creatinine concentration during therapy would, of course, necessitate changes in the dosage frequency.
- It is desirable to limit the duration of treatment with kanamycin to short-term. The usual duration of treatment is 7 to 10 days. Total daily dose by all routes of administration should not exceed 1.5 g/day. If longer therapy is required, measurement of kanamycin peak and trough serum concentrations is particularly important as a basis for determining the adequacy and safety of the dose. These patients should be carefully monitored for changes in renal, auditory, and vestibular function. Dosage should be adjusted as needed. The risks of toxicity multiply as the length of treatment increases.
- At the recommended dosage level, uncomplicated infections due to kanamycin-susceptible organisms should respond to therapy in 24 to 48 hours. If definite clinical response does not occur within 3 to 5 days, therapy should be stopped and the antibiotic susceptibility pattern of the invading organism should be rechecked. Failure of the infection to respond may be due to resistance of the organism or to the presence of septic foci requiring surgical drainage.
Intravenous Administration
- The dose should not exceed 15 mg/kg per day and must be administered slowly. The solution for intravenous use is prepared by adding the contents of a 500 mg vial to 100 to 200 mL of sterile diluent such as Normal Saline or 5% Dextrose in Water, or the contents of a 1g vial to 200 to 400 mL of sterile diluent. The appropriate dose is administered over a 30- to 60-minute period. The total daily dose should be divided into 2 or 3 equally divided doses.
- Kanamycin Injection, USP should not be physically mixed with other antibacterial agents but each should be administered separately in accordance with its recommended route of administration and dosage schedule.
Intraperitoneal Use
- (Following exploration for established peritonitis or after peritoneal contamination due to fecal spill during surgery.)
Adults
- 500 mg diluted in 20 mL sterile distilled water may be instilled through a polyethylene catheter sutured into the wound at closure. If possible, instillation should be postponed until the patient has fully recovered from the effects of anesthesia and muscle-relaxing drugs. Serum levels should be carefully monitored during treatment.
- NOTE: The pediatric dosage form “75 mg/2 mL” should be used for pediatric patients.
Aerosol Treatment
- 250 mg two to four times a day. Withdraw 250 mg (1mL) from a 500 mg vial and dilute it with 3 mL Physiological Saline and nebulize. Serum levels should be carefully monitored during treatment.
Other Routes of Administration
- Kanamycin injection in concentrations of 0.25 percent (2.5 mg/mL) has been used as an irrigating solution in abscess cavities, pleural space, peritoneal and ventricular cavities. Possible absorption of kanamycin by such routes must be taken into account and dosage adjustments should be arranged so that a maximum total dose of 1.5 g/day by all routes of administration is not exceeded. Serum levels should be carefully monitored during treatment.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Kanamycin in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Kanamycin in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding FDA-Labeled Use of Kanamycin in pediatric patients.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Kanamycin in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Kanamycin in pediatric patients.
Contraindications
- A history of hypersensitivity or toxic reaction to one aminoglycoside may also contraindicate the use of any other aminoglycoside, because of the known cross-sensitivity and cumulative effects of drugs in this category.
- THIS DRUG IS NOT INDICATED IN LONG-TERM THERAPY (e.g., Tuberculosis) BECAUSE OF THE TOXIC HAZARD ASSOCIATED WITH EXTENDED ADMINISTRATION.
Warnings
|
Boxed warning
See full prescribing information for complete Boxed Warning.
* Patients treated with aminoglycosides by any route should be under close clinical observation because of the potential toxicity associated with their use.
|
- Aminoglycosides can cause fetal harm when administered to pregnant women. Aminoglycoside antibiotics cross the placenta and there have been several reports of total, irreversible, bilateral congenital deafness in children whose mothers received streptomycin during pregnancy.
- Although serious side effects to fetus or newborn have not been reported in treatment of pregnant women with other aminoglycosides, the potential for harm exists.
- Reproductive studies have been performed in rats and rabbits and have revealed no evidence of impaired fertility or teratogenic effects. Dosages of 200 mg/kg/day in pregnant rats and pregnant guinea pigs led to hearing impairment in the off-spring. There are no well-controlled studies in pregnant women but clinical experience does not include any positive evidence of adverse effects on the fetus. However, if the drug is used during pregnancy, or if the patient becomes pregnant while taking this drug, the patient should be apprised of the potential hazard on the fetus.
- Contains sodium bisulfite, a sulfite that may cause allergic-type reactions including anaphylactic symptoms and life-threatening or less severe asthmatic episodes in certain susceptible people. The overall prevalence of sulfite sensitivity in the general population is unknown and probably low. Sulfite sensitivity is seen more frequently in asthmatic than in nonasthmatic people.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Kanamycin in the drug label.
Postmarketing Experience
- Kanamycin has the potential to induce auditory and sometimes vestibular toxicity, renal toxicity, and neuromuscular blockade.
- The risks are higher for patients with a present or past history of renal impairment (especially if hemodialysis is required): for those receiving concomitant or sequential treatment with other ototoxic or nephrotoxic drugs or rapid acting diuretic agents given intravenously (ethacrynic acid, furosemide, and mannitol), and for patients treated for longer periods and/or with higher doses than recommended.
Ototoxicity
- Toxic effects of kanamycin on the eighth cranial nerve can result in partially reversible or irreversible bilateral loss of hearing, loss of balance, or both.
- Tinnitus or vertigo may or may not be experienced. Cochlear damage is usually manifested initially by small changes in audiometric test results at the high frequencies and may not be associated with subjective hearing loss.
- Vestibular dysfunction is usually manifested by nystagmus, vertigo, nausea, vomiting, or acute Meniere’s syndrome.
Nephrotoxicity
- Albuminuria, presence of red and white cells, and granular casts; azotemia and oliguria have been reported.
- Renal function changes are usually reversible when the drug is discontinued. Renal impairment may be characterized by a rise in serum creatinine and may be accompanied by oliguria, presence of casts, cells, and protein in the urine, by rising levels of BUN or by decrease in creatinine clearance.
Neuromuscular Blockage
- Acute muscular paralysis and apnea can occur following treatment with aminoglycoside antibiotics.
- Neurotoxicity can occur after intrapleural and interperitoneal instillation of large doses of an aminoglycoside; however, the reaction has followed intravenous, intramuscular, and even the oral administration of these agents.
Other
- Some local irritation or pain may follow the intramuscular injection of kanamycin. Other adverse reactions of the drug reported on rare occasions are skin rash, drug fever, headache, paresthesia, nausea, vomiting, and diarrhea.
- The “malabsorption syndrome” characterized by an increase in fecal fat, decrease in serum carotene, and fall in xylose absorption, reportedly has occurred with prolonged therapy.
Drug Interactions
There is limited information regarding Kanamycin Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Pregnancy Category
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Kanamycin in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Kanamycin during labor and delivery.
Nursing Mothers
- Kanamycin is excreted in minute amounts in human milk. Because of the potential for serious adverse reactions from aminoglycosides in nursing infants, a decision should be made whether to discontinue nursing or to discontinue the drug taking into account the importance of the drug to the mother
Pediatric Use
- Aminoglycosides should be used with caution in prematures and neonates because of the renal immaturity of these patients and the resulting prolongation of serum half-life of these drugs.
Geriatic Use
There is no FDA guidance on the use of Kanamycin with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Kanamycin with respect to specific gender populations.
Race
There is no FDA guidance on the use of Kanamycin with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Kanamycin in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Kanamycin in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Kanamycin in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Kanamycin in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intramuscular.
- Intraperitoneal.
- Intravenous.
- Aerosol.
- An irrigating solution in abscess cavities, pleural space, peritoneal and ventricular cavities.
Monitoring
- Renal and eighth nerve function should be closely monitored, especially in patients with known or suspected reduced renal function at the onset of therapy, and also in those whose renal function is initially normal but who develop signs of renal dysfunction during therapy.
- Serum concentrations of parenterally administered aminoglycosides should be monitored when feasible to assure adequate levels and to avoid potentially toxic levels.
- Monitoring of renal function during treatment with kanamycin, as with other aminoglycosides, is particularly important in such patients.
- If longer therapy is required, measurement of kanamycin peak and trough serum concentrations is particularly important as a basis for determining the adequacy and safety of the dose. These patients should be carefully monitored for changes in renal, auditory, and vestibular function. Dosage should be adjusted as needed. The risks of toxicity multiply as the length of treatment increases.
IV Compatibility
There is limited information regarding IV Compatibility of Kanamycin in the drug label.
Overdosage
- In the event of overdosage or toxic reaction, hemodialysis or peritoneal dialysis will aid in the removal of kanamycin from the blood. In the newborn infant, exchange transfusion may also be considered.
Pharmacology
Mechanism of Action
- Kanamycin is a bactericidal antibiotic which acts by inhibiting the synthesis of protein in susceptible microorganisms.
- Kanamycin sulfate is active in vitro against many strains of Staphylococcus aureus (including penicillinase and non penicillinase-producing strains), Staphylococcus epidermidis, N. gonorrhoeae, H. influenzae, E. coli, Enterobactor aerogenes, Shigella and Salmonella species, K. pneumoniae, Serratia marcescens, Providencia species, Acinetobacter species and Citrobacter freundii and Citrobacter species, and many strains of both indole-positive and indole-negative Proteus strains that are frequently resistant to other antibiotics.
- Aminoglycosides have a low order of activity against most gram-positive organisms including Streptococcus pyogenes, Streptococcus pneumoniae and enterococci. In vitro studies have demonstrated that an aminoglycoside combined with an antibiotic which interferes with cell wall synthesis (i.e., Penicillin G or ampicillin) affects some Group D streptococcal strains synergistically.
- Bacteriological testing and tests for antibiotic synergism are necessary.
Structure
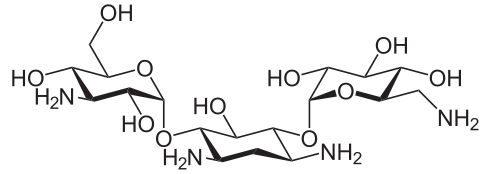
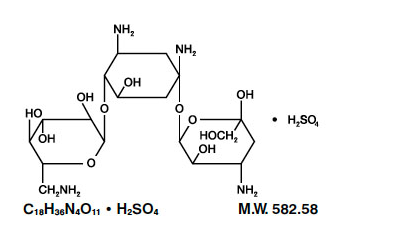
- Kanamycin sulfate is an aminoglycoside antibiotic produced by Streptomyces kanamyceticus. It is D-Streptamine, 0-3-amino-3-deoxy-α-D-glucopyranosyl - (1→6)-0- [6-amino-6-deoxy-α-D-glucopyranosyl - (1→4)]-2-deoxy, sulfate 1:1 (salt). It consists of two amino sugars glycosidically linked to deoxystreptamine.
- Kanamycin Injection, USP, sterile solution for parenteral administration, contains respectively; kanamycin sulfate equivalent to 500 mg and 1g kanamycin; sodium bisulfite, an antioxidant, 0.66% and 0.45%; and sodium citrate, 2.2% and 2.2% with pH of each dosage form adjusted to 4.5 with sulfuric acid.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Kanamycin in the drug label.
Pharmacokinetics
- The drug is rapidly absorbed after intramuscular injection and peak serum levels are generally reached within approximately one hour.
- Doses of 7.5 mg/kg give mean peak levels of 22 mcg/mL. At 8 hours following a 7.5 mg/kg dose, mean serum levels are 3.2 mcg/mL. The serum half-life is 2 1/2 hours.
- Intravenous administration of kanamycin over a period of one hour resulted in serum concentrations similar to those obtained by intramuscular administration.
- Kanamycin diffuses rapidly into most body fluids including synovial and peritoneal fluids and bile. Significant levels of the drug appear in cord blood and amniotic fluid following intramuscular administration to pregnant patients. Spinal fluid concentrations in normal infants are approximately 10 to 20 percent of serum levels and may reach 50 percent when the meninges are inflamed.
- Studies in normal adult patients have shown only trace levels of kanamycin in spinal fluid. No data are available on adults with meningitis.
- The drug is excreted almost entirely by glomerular filtration and is not reabsorbed by the renal tubules. Hence, high concentrations are attained in the nephron, and the urine may contain levels 10 to 20 times higher than those in serum. Little, if any, metabolic transformation occurs.
- Renal excretion is extremely rapid. In patients with normal renal function, approximately one-half of the administered dose is cleared within 4 hours and excretion is complete within 24 to 48 hours.
- Patients with impaired renal function or with diminished glomerular filtration pressure excrete kanamycin more slowly. Such patients may build up excessively high blood levels which greatly increase the risk of ototoxic reactions.
- In severely burned patients the half-life may be significantly decreased and resulting serum concentrations may be lower than anticipated from the mg per kg dose.
Nonclinical Toxicology
- Quantitative methods for susceptibility testing that require measurement of zone diameters give the most precise estimates of antibiotic susceptibility.
- One such procedure has been recommended for use with discs to test susceptibility to kanamycin. Interpretation involves correlation of the diameters obtained in the disc test with minimal inhibitory concentration (MIC) values for kanamycin.
- Reports from the laboratory give results of the standardized single disc susceptibility test (Bauer, et al., Am J Clin Path 1966;45:493 and Federal Register 37:20525-20529, 1972), using a 30 mcg kanamycin disc should be interpreted according to the following criteria:
- Organisms producing zones of 18 mm or greater, or MIC’s of 16 mcg or less are considered susceptible, indicating that the test organism is likely to respond to therapy.
- Resistant organisms produce zones of 14 mm or less or MIC’s of 16 mcg or greater. A report of “resistant” from the laboratory indicates that the infecting organism is not likely to respond to therapy.
- Zones greater than 14 mm and less than 18 mm, or MIC’s of greater than 16 mcg and less than 65 mcg, indicate intermediate susceptibility. A report of “intermediate” susceptibility suggests that the organism would be susceptible if the infection is confined to tissues and fluids (e.g., urine), in which high antibiotic levels are attained.
- Control organisms are recommended for susceptibility testing. Each time the test is performed one or more of the following organisms should be included: Escherichia coli ATCC 15922, Staphylococcus aureus ATCC 25923 and Pseudomonas aeruginosa ATCC 27853. The control organisms should produce zones of inhibition within the following ranges:
Clinical Studies
There is limited information regarding Clinical Studies of Kanamycin in the drug label.
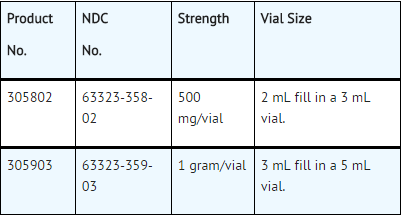
How Supplied
- Kanamycin Injection, USP, is supplied in packages of 10 vials.
- Vial stoppers do not contain natural rubber latex.
Storage
- Store at 20° to 25°C (68° to 77°F)
Images
Drug Images
{{#ask: Page Name::Kanamycin |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Kanamycin |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
There is limited information regarding Patient Counseling Information of Kanamycin in the drug label.
Precautions with Alcohol
- Alcohol-Kanamycin interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- KANAMYCIN ®[1]
Look-Alike Drug Names
There is limited information regarding Kanamycin Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Kanamycin |Label Name=Kanamycin 04.jpg
}}
{{#subobject:
|Label Page=Kanamycin |Label Name=Kanamycin 05.jpg
}}
{{#subobject:
|Label Page=Kanamycin |Label Name=Kanamycin 06.png
}}