Ceftazidime and avibactam
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Allison Tu [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Ceftazidime and avibactam is a combination of a cephalosporin and a beta-lactamase inhibitor that is FDA approved for the treatment of complicated intra-abdominal infections and complicated urinary tract infections. Common adverse reactions include diarrhea and nausea.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Complicated Intra-abdominal Infections (cIAI)
Ceftazidime and avibactam in combination with metronidazole, is indicated for the treatment of complicated intra-abdominal infections (cIAI) caused by the following susceptible Gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Proteus mirabilis, Enterobacter cloacae, Klebsiella oxytoca, Citrobacter freundii complex, and Pseudomonas aeruginosa in patients 18 years or older.
- The recommended dosage of ceftazidime and avibactam is 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered every 8 hours by intravenous (IV) infusion over 2 hours in patients 18 years of age and older in patients with normal renal function.
- Metronidazole should be given concurrently.
- Duration of treatment should range from 5 to 14 days.
- The recommended ceftazidime and avibactam dosage in patients with varying degrees of renal function is presented in TABLE 2. For patients with changing renal function, monitor CrCl at least daily and adjust the dosage of ceftazidime and avibactam accordingly.

Complicated Urinary Tract Infections (cUTI), including Pyelonephritis
Ceftazidime and avibactam is indicated for the treatment of complicated urinary tract infections (cUTI) including pyelonephritis caused by the following susceptible Gram-negative microorganisms: Escherichia coli, Klebsiella pneumoniae, Enterobacter cloacae, Citrobacter freundii complex, Proteus mirabilis, and Pseudomonas aeruginosa in patients 18 years or older.
- The recommended dosage of ceftazidime and avibactam is 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered every 8 hours by intravenous (IV) infusion over 2 hours in patients 18 years of age and older in patients with normal renal function.
- Duration of treatment should range from 7 to 14 days.
- The recommended ceftazidime and avibactam dosage in patients with varying degrees of renal function is presented in TABLE 2. For patients with changing renal function, monitor CrCl at least daily and adjust the dosage of ceftazidime and avibactam accordingly.

Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftazidime and avibactam in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceftazidime and avibactam in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
There is limited information regarding Ceftazidime and avibactam FDA-Labeled Indications and Dosage (Pediatric) in the drug label.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Ceftazidime and avibactam in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Ceftazidime and avibactam in pediatric patients.
Contraindications
Ceftazidime and avibactam is contraindicated in patients with known serious hypersensitivity to ceftazidime or avibactam, avibactam-containing products, or other members of the cephalosporin class.
Warnings
- Decreased Clinical Response in cIAI Patients with Baseline Creatinine Clearance of 30 to Less Than or Equal to 50 mL/min
- In a Phase 3 cIAI trial, clinical cure rates were lower in a subgroup of patients with baseline CrCl of 30 to less than or equal to 50 mL/min compared to those with CrCl greater than 50 mL/min (TABLE 4). The reduction in clinical cure rates was more marked in patients treated with ceftazidime and avibactam plus metronidazole compared to meropenem-treated patients. Within this subgroup, patients treated with ceftazidime and avibactam received a 33% lower daily dose than is currently recommended for patients with CrCl 30 to less than or equal to 50 mL/min.
- The decreased clinical response was not observed for patients with moderate renal impairment at baseline (CrCl of 30 to less than or equal to 50 mL/min) in the Phase 3 cUTI trials.
- Monitor CrCl at least daily in patients with changing renal function and adjust the dosage of ceftazidime and avibactam accordingly.

- Hypersensitivity Reactions
- Serious and occasionally fatal hypersensitivity (anaphylactic) reactions and serious skin reactions have been reported in patients receiving beta-lactam antibacterial drugs. Before therapy with ceftazidime and avibactam is instituted, careful inquiry about previous hypersensitivity reactions to other cephalosporins, penicillins, or carbapenems should be made.
- Exercise caution if this product is to be given to a penicillin or other beta-lactam-allergic patient because cross sensitivity among beta-lactam antibacterial drugs has been established. Discontinue the drug if an allergic reaction to ceftazidime and avibactam occurs.
- Clostridium difficile-associated Diarrhea
- Clostridium difficile-associated diarrhea (CDAD) has been reported for nearly all systemic antibacterial drugs, including ceftazidime and avibactam, and may range in severity from mild diarrhea to fatal colitis. Treatment with antibacterial drugs alters the normal flora of the colon and may permit overgrowth of C. difficile.
- C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy.
- CDAD must be considered in all patients who present with diarrhea following antibacterial use. Careful medical history is necessary because CDAD has been reported to occur more than 2 months after the administration of antibacterial drugs.
- If CDAD is suspected or confirmed, antibacterial drugs not directed against C. difficile may need to be discontinued. Manage fluid and electrolyte levels as appropriate, supplement protein intake, monitor antibacterial treatment of C. difficile, and institute surgical evaluation as clinically indicated.
- Central Nervous System Reactions
- Seizures, nonconvulsive status epilepticus (NCSE), encephalopathy, coma, asterixis, neuromuscular excitability, and myoclonia have been reported in patients treated with ceftazidime, particularly in the setting of renal impairment.
- Adjust dosing based on creatinine clearance
- Development of Drug-Resistant Bacteria
- Prescribing ceftazidime and avibactam in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared to rates in the clinical trials of another drug and may not reflect the rates observed in practice. Ceftazidime and avibactam was evaluated in five active-controlled clinical trials in patients with cIAI or cUTI, including pyelonephritis. These trials included two Phase 2 trials, one in cIAI and one in cUTI, as well as three Phase 3 trials, one in cIAI, one in cUTI (Trial 1), and one in cIAI or cUTI due to ceftazidime non-susceptible pathogens (Trial 2). Data from Trial 1 served as the primary dataset for ceftazidime and avibactam safety findings in cUTI as there was a single comparator. Trial 2 had an open-label design as well as multiple comparator regimens which prevented pooling, but provided supportive information. The five clinical trials included a total of 1373 adult patients treated with ceftazidime and avibactam and 1375 patients treated with comparators.
Complicated Intra-abdominal Infections
The Phase 3 cIAI trial included 529 adult patients treated with ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes every 8 hours plus 0.5 grams metronidazole administered intravenously over 60 minutes every 8 hours and 529 patients treated with meropenem. The median age of patients treated with ceftazidime and avibactam was 50 years (range 18 to 90 years) and 22.5% of patients were 65 years of age or older. Patients were predominantly male (62%) and Caucasian (76.6%).
Treatment discontinuation due to an adverse reaction occurred in 2.6% (14/529) of patients receiving ceftazidime and avibactam plus metronidazole and 1.3% (7/529) of patients receiving meropenem. There was no specific adverse reaction leading to discontinuation.
Adverse reactions occurring at 5% or greater in patients receiving ceftazidime and avibactam plus metronidazole were diarrhea, nausea and vomiting.
TABLE 5 lists adverse reactions occurring in 1% or more of patients receiving ceftazidime and avibactam plus metronidazole and with incidences greater than the comparator in the Phase 3 cIAI clinical trial.

Increased Mortality:
In the Phase 3 cIAI trial, death occurred in 2.5% (13/529) of patients who received ceftazidime and avibactam plus metronidazole and in 1.5% (8/529) of patients who received meropenem. Among a subgroup of patients with baseline CrCl 30 to less than or equal to 50 mL/min, death occurred in 19.5% (8/41) of patients who received ceftazidime and avibactam plus metronidazole and in 7.0% (3/43) of patients who received meropenem. Within this subgroup, patients treated with ceftazidime and avibactam received a 33% lower daily dose than is currently recommended for patients with CrCl 30 to less than or equal to 50 mL/min.
In patients with normal renal function or mild renal impairment (baseline CrCl greater than 50 mL/min), death occurred in 1.0% (5/485) of patients who received ceftazidime and avibactam plus metronidazole and in 1.0% (5/484) of patients who received meropenem. The causes of death varied and contributing factors included progression of underlying infection, baseline pathogens isolated that were unlikely to respond to the study drug, and delayed surgical intervention.
Complicated Urinary Tract Infections, Including Pyelonephritis
Trial 1 included 511 adult patients treated with ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered intravenously over 120 minutes every 8 hours and 509 patients treated with doripenem; in some patients parenteral therapy was followed by a switch to an oral antimicrobial agent. Median age of patients treated with ceftazidime and avibactam was 54 years (range 18 to 89 years). Patients were predominantly female (68.3%) and Caucasian (82.4%). Patients with CrCl less than 30 mL/min were excluded.
There were no deaths in Trial 1. Treatment discontinuation due to adverse reactions occurred in 1.4% (7/511) of patients receiving ceftazidime and avibactam and 1.2% (6/509) of patients receiving doripenem. There was no specific adverse reaction leading to discontinuation.
The most common adverse reactions occurring in 3% of cUTI patients treated with ceftazidime and avibactam were nausea and diarrhea.
TABLE 6 lists adverse reactions occurring in 1% or more of patients receiving ceftazidime and avibactam and with incidences greater than the comparator in Trial 1.

Other Adverse Reactions of Ceftazidime and Avibactam and Ceftazidime Alone
The following selected adverse reactions were reported in ceftazidime and avibactam-treated patients at a rate of less than 1% in the Phase 3 trials.
- Blood and lymphatic disorders - Thrombocytopenia
- General disorders and administration site conditions - Injection site phlebitis
- Infections and infestations - Candidiasis
- Investigations - Increased aspartate aminotransferase, increased alanine aminotransferase, increased gamma-glutamyltransferase
- Metabolism and nutrition disorders - Hypokalemia
- Nervous system disorders - Dysgeusia
- Renal and urinary disorders - Acute renal failure, renal impairment, nephrolithiasis
- Skin and subcutaneous tissue disorders - Rash, rash maculo-papular, urticaria, pruritus
- Psychiatric disorders - Anxiety
Additionally, adverse reactions reported with ceftazidime alone that were not reported in ceftazidime and avibactam-treated patients in the Phase 3 trials are listed below:
- Blood and lymphatic disorders - Agranulocytosis, hemolytic anemia, leukopenia, lymphocytosis, neutropenia, thrombocytosis, eosinophilia
- General disorders and administration site conditions - Infusion site inflammation, injection site hematoma, injection site thrombosis
- Hepatobiliary disorders – Jaundice
- Investigations - Increased blood lactate dehydrogenase, prolonged prothrombin time
- Nervous system disorders - Paresthesia
- Renal and urinary disorders - Tubulointerstitial nephritis
- Reproductive and breast disorders - Vaginal inflammation
- Skin and subcutaneous tissue disorders - Angioedema, erythema multiforme, Stevens-Johnson syndrome, Toxic epidermal necrolysis
Laboratory Changes
Seroconversion from a negative to a positive direct Coombs' test result at any time up to the last visit occurred in 31/240 (12.9%) of patients receiving ceftazidime and avibactam plus metronidazole with initial negative Coombs' test and at least one follow up test and in 7/235 (3.0%) of patients receiving meropenem in the Phase 3 cIAI trial. Seroconversion from a negative to a positive direct Coombs' test result at any time up to the last visit occurred in 7/216 (3.2%) of patients receiving ceftazidime and avibactam with initial negative Coombs' test and at least one follow up test and 2/214 (0.9%) of patients receiving doripenem in the Phase 3 cUTI trial. No adverse reactions representing hemolytic anemia were reported in any treatment group.
Postmarketing Experience
There is limited information regarding Ceftazidime and avibactam Postmarketing Experience in the drug label.
Drug Interactions
- Probenecid
- In vitro, avibactam is a substrate of OAT1 and OAT3 transporters which might contribute to the active uptake from the blood compartment, and thereby its excretion.
- As a potent OAT inhibitor, probenecid inhibits OAT uptake of avibactam by 56% to 70% in vitro and, therefore, has the potential to decrease the elimination of avibactam when co-administered.
- Because a clinical interaction study of ceftazidime and avibactam or avibactam alone with probenecid has not been conducted, co-administration of ceftazidime and avibactam with probenecid is not recommended.
- Drug/Laboratory Test Interactions
- The administration of ceftazidime may result in a false-positive reaction for glucose in the urine with certain methods.
- It is recommended that glucose tests based on enzymatic glucose oxidase reactions be used.
Use in Specific Populations
Pregnancy
- Risk Summary
- There are no adequate and well-controlled studies of ceftazidime and avibactam, ceftazidime, or avibactam in pregnant women.
- Neither ceftazidime nor avibactam were teratogenic in rats at doses 40 and 9 times the recommended human clinical dose. In the rabbit, at twice the exposure as seen at the human clinical dose, there were no effects on embryofetal development with avibactam.
- The background risk of major birth defects and miscarriage for the indicated population is unknown.
- The background risk of major birth defects is 2-4% and of miscarriage is 15-20% of clinically recognized pregnancies within the general population.
- Because animal reproduction studies are not always predictive of human response, this drug should be used in pregnancy only if clearly needed.
- Data
- Animal Data
- Ceftazidime
- Reproduction studies have been performed in mice and rats at doses up to 40 times the human dose and showed no evidence of harm to the fetus due to ceftazidime.
- Avibactam
- Avibactam was not teratogenic in rats or rabbits.
- In the rat, intravenous studies with 0, 250, 500 and 1000 mg/kg/day avibactam during gestation days 6-17 showed no embryofetal toxicity at doses up to 1000 mg/kg/day, approximately 9 times the human dose based on exposure (AUC).
- In a rat pre- and post-natal study at up to 825 mg/kg/day intravenously (11 times the human exposure based on AUC), there were no effects on pup growth and viability.
- A dose-related increase in the incidence of renal pelvic and ureter dilatation was observed in female weaning pups that was not associated with pathological changes to renal parenchyma or renal function, with renal pelvic dilatation persisting after female weaning pups became adults.
- Rabbits administered intravenous avibactam on gestation days 6-19 at 0, 100, 300 and 1000 mg/kg/day showed no effects on embryofetal development at a dose of 100 mg/kg, twice the human exposure (AUC).
- At higher doses, increased post-implantation loss, lower mean fetal weights, delayed ossification of several bones and other anomalies were observed.
- Ceftazidime
- Animal Data
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Ceftazidime and avibactam in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Ceftazidime and avibactam during labor and delivery.
Nursing Mothers
- Risk Summary
- Ceftazidime is excreted in human milk in low concentrations. It is not known whether avibactam is excreted into human milk, although avibactam was shown to be excreted in the milk of rats.
- No information is available on the effects of ceftazidime and avibactam on the breast-fed child or on milk production.
- The developmental and health benefits of breastfeeding should be considered along with the mother's clinical need for ceftazidime and avibactam and any potential adverse effects on the breastfed child from ceftazidime and avibactam or from the underlying maternal conditions.
- Data
- In a rat pre- and post-natal study at doses up to 825 mg/kg/day intravenously (11 times the human exposure based on AUC), the exposure to avibactam was minimal in the pups in comparison to the dams.
- Exposure to avibactam was observed in both pups and milk on PND 7.
Pediatric Use
Safety and effectiveness in patients less than 18 years of age have not been established.
Geriatic Use
Of the 1373 patients treated with ceftazidime and avibactam in the Phase 2 and Phase 3 clinical trials 385 (28%) were 65 years of age and older, including 173 (15.3 %) patients 75 years of age and older.
In the pooled Phase 2 and Phase 3 cIAI ceftazidime and avibactam clinical trials, 20% (126/630) of patients treated with ceftazidime and avibactam were 65 years of age and older, including 49 (7.8%) patients 75 years of age and older. The incidence of adverse reactions in both treatment groups was higher in older patients (≥ 65 years of age) and similar in both treatment groups; clinical cure rates for patients 65 years of age or older were 73.0% (73/100) in the ceftazidime and avibactam plus metronidazole arm and 78.6% (77/98) in the meropenem arm.
In the Phase 3 cUTI trial, 30.7% (157/511) of patients treated with ceftazidime and avibactam were 65 years of age or older, including 78 (15.3%) patients 75 years of age or older. The incidence of adverse reactions in both treatment groups was lower in older patients (≥ 65 years of age) and similar between treatment groups. Among patients 65 years of age or older in the Phase 3 cUTI trial, 66.1% (82/124) of patients treated with ceftazidime and avibactam had symptomatic resolution at Day 5 compared with 56.6% (77/136) of patients treated with doripenem. The combined response (microbiological cure and symptomatic response) observed at the test-of-cure (TOC) visit for patients 65 years of age or older were 58.1% (72/124) in the ceftazidime and avibactam arm and 58.8% (80/136) in the doripenem arm.
Ceftazidime and avibactam are known to be substantially excreted by the kidney; therefore, the risk of adverse reactions to ceftazidime and avibactam may be greater in patients with decreased renal function. Because elderly patients are more likely to have decreased renal function, care should be taken in dose selection and it may be useful to monitor renal function. Healthy elderly subjects had 17% greater exposure relative to healthy young subjects when administered the same single dose of avibactam, which may have been related to decreased renal function in the elderly subjects. Dosage adjustment for elderly patients should be based on renal function.
Gender
There is no FDA guidance on the use of Ceftazidime and avibactam with respect to specific gender populations.
Race
There is no FDA guidance on the use of Ceftazidime and avibactam with respect to specific racial populations.
Renal Impairment
Dosage adjustment is required in patients with moderately or severely impaired renal function (CrCl 50 mL/min or less). For patients with changing renal function, CrCl should be monitored at least daily, particularly early in treatment, and dosage of ceftazidime and avibactam adjusted accordingly. Both ceftazidime and avibactam are hemodialyzable; thus, ceftazidime and avibactam should be administered after hemodialysis on hemodialysis days.
Hepatic Impairment
There is no FDA guidance on the use of Ceftazidime and avibactam in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Ceftazidime and avibactam in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Ceftazidime and avibactam in patients who are immunocompromised.
Administration and Monitoring
Administration
Ceftazidime and avibactam is supplied as a dry powder, which must be constituted and subsequently diluted, using aseptic technique prior to intravenous infusion.
- Constitute the powder in the ceftazidime and avibactam vial with 10 mL of sterile water for injection, USP; 0.9% of sodium chloride injection, USP (normal saline); 5% of dextrose injection, USP; all combinations of dextrose injection and sodium chloride injection, USP, containing up to 2.5% dextrose, USP, and 0.45% sodium chloride, USP; or lactated Ringer's injection, USP
- Mix gently. The constituted ceftazidime and avibactam solution will have an approximate ceftazidime concentration of 0.167 grams/mL and an approximate avibactam concentration of 0.042 grams/mL. The final volume is approximately 12 mL. The constituted solution is not for direct injection. The constituted solution must be diluted before intravenous infusion.
- Prepare the required dose for intravenous infusion by withdrawing the appropriate volume determined from Table 3 from the constituted vial.
This image is provided by the National Library of Medicine - Before infusion, dilute the withdrawn volume of the constituted ceftazidime and avibactam solution further with the same diluent used for constitution of the powder (except sterile water for injection), to achieve a total volume between 50 mL (ceftazidime 0.04 grams/mL and avibactam 0.01 grams/mL) to 250 mL (ceftazidime 0.008 grams/mL and avibactam 0.002 grams/mL) in an infusion bag. If sterile water for injection was used for constitution, use any of the other appropriate constitution diluents for dilution.
- Mix gently and ensure that the contents are dissolved completely. Visually inspect the diluted ceftazidime and avibactam solution (for administration) for particulate matter and discoloration prior to administration (the color of the ceftazidime and avibactam infusion solution for administration ranges from clear to light yellow).
- Use the diluted ceftazidime and avibactam solution in the infusion bags within 12 hours when stored at room temperature.
- The diluted ceftazidime and avibactam solution in the infusion bags may be stored under refrigeration at 2 to 8°C (36 to 46°F) up to 24 hours following dilution and used within 12 hours of subsequent storage at room temperature.
Drug Compatibility:
The ceftazidime and avibactam solution for administration at the range of diluted concentrations of ceftazidime 0.008 g/mL and avibactam 0.002 g/mL to ceftazidime 0.04 g/mL and avibactam 0.01 g/mL is compatible with the more commonly used intravenous infusion fluids in infusion bags (including Baxter® Mini-Bag Plus™) such as 0.9% sodium chloride injection, USP; 5% dextrose injection, USP; all combinations of dextrose injection and sodium chloride injection, USP, containing up to 2.5% dextrose, USP, and 0.45% sodium chloride, USP; lactated ringer's injection, USP; and Baxter® Mini-Bag Plus™ containing 0.9% sodium chloride injection or 5% dextrose injection. Compatibility of ceftazidime and avibactam solution for administration with other drugs has not been established.
Storage of Constituted Solutions:
Upon constitution with appropriate diluent, the constituted ceftazidime and avibactam solution may be held for no longer than 30 minutes prior to transfer and dilution in a suitable infusion bag.
Following dilution of the constituted solutions with the appropriate diluents, ceftazidime and avibactam solutions in the infusion bags are stable for 12 hours when stored at room temperature.
Following dilution of the constituted solutions with the appropriate diluents, ceftazidime and avibactam solutions in the infusion bags may also be refrigerated at 2 to 8°C (36 to 46°F) for up to 24 hours; and then should be used within 12 hours of subsequent storage at room temperature.
Monitoring
There is limited information regarding Ceftazidime and avibactam Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Ceftazidime and avibactam and IV administrations.
Overdosage
In the event of overdose, discontinue ceftazidime and avibactam and institute general supportive treatment.
Ceftazidime and avibactam can be removed by hemodialysis. In subjects with end-stage renal disease (ESRD) administered 1 gram ceftazidime, the mean total recovery in dialysate following a 4-hour hemodialysis session was 55% of the administered dose. In subjects with ESRD administered 100 mg avibactam, the mean total recovery in dialysate following a 4-hour hemodialysis session started 1 hour after dosing was approximately 55% of the dose.
No clinical information is available on the use of hemodialysis to treat ceftazidime and avibactam overdosage.
Pharmacology


Mechanism of Action
The ceftazidime component of ceftazidime and avibactam is a cephalosporin antibacterial drug with in vitro activity against certain gram-negative and gram-positive bacteria. The bactericidal action of ceftazidime is mediated through binding to essential penicillin-binding proteins (PBPs). The avibactam component of ceftazidime and avibactam is a non-beta-lactam beta-lactamase inhibitor that inactivates some beta-lactamases and protects ceftazidime from degradation by certain beta-lactamases. Avibactam does not decrease the activity of ceftazidime against ceftazidime-susceptible organisms.
Ceftazidime and avibactam demonstrated in vitro activity against Enterobacteriaceae in the presence of some beta-lactamases and extended-spectrum beta-lactamases (ESBLs) of the following groups: TEM, SHV, CTX-M, Klebsiella pneumoniae carbapenemase (KPCs), AmpC, and certain oxacillinases (OXA). Ceftazidime and avibactam also demonstrated in vitro activity against P. aeruginosa in the presence of some AmpC beta-lactamases, and certain strains lacking outer membrane porin (OprD). Ceftazidime and avibactam is not active against bacteria that produce metallo-beta lactamases and may not have activity against gram-negative bacteria that overexpress efflux pumps or have porin mutations.
Structure
Ceftazidime and avibactam is an antibacterial combination product consisting of the semisynthetic cephalosporin ceftazidime pentahydrate and the beta-lactamase inhibitor avibactam sodium for intravenous administration.
Ceftazidime is a semisynthetic, beta-lactam antibacterial drug. It is the pentahydrate of (6R,7R,Z)-7-(2-(2-aminothiazol-4-yl)-2-(2-carboxypropan-2-yloxyimino)acetamido)-8-oxo-3-(pyridinium-1-ylmethyl)-5-thia-1-aza-bicyclo[4.2.0]oct-2-ene-2-carboxylate. Its molecular weight is 636.6. The empirical formula is C22 H32 N6 O12 S2.

Avibactam:
Avibactam sodium chemical name is sodium [(2S,5R)-2-carbamoyl-7-oxo-1,6-diazabicyclo[3.2.1]octan-6-yl] sulfate. Its molecular weight is 287.23. The empirical formula is C7 H10 N3 O6 SNa.

Ceftazidime and avibactam 2.5 grams (ceftazidime and avibactam) for injection is a white to yellow sterile powder for constitution consisting of ceftazidime pentahydrate and avibactam sodium packaged in glass vials. The formulation also contains sodium carbonate.
Each ceftazidime and avibactam 2.5 grams single-dose vial contains ceftazidime 2 grams (equivalent to 2.635 grams sterile ceftazidime pentahydrate/sodium carbonate) and avibactam 0.5 grams (equivalent to 0.551 grams sterile avibactam sodium). The sodium carbonate content of the mixture is 239.6 mg/vial. The total sodium content of the mixture is approximately 146 mg (6.4 mEq)/vial.
Pharmacodynamics
As with other beta-lactam antimicrobial drugs, the time that unbound plasma concentrations of ceftazidime exceeds the ceftazidime and avibactam minimum inhibitory concentration (MIC) against the infecting organism has been shown to best correlate with efficacy in a neutropenic murine thigh infection model with Enterobacteriaceae and Pseudomonas aeruginosa. The time above a threshold concentration has been determined to be the parameter that best predicts the efficacy of avibactam in in vitro and in vivo nonclinical models.
Cardiac Electrophysiology
In a thorough QT study, a supratherapeutic dose of ceftazidime (3 grams) was investigated for QT effects in combination with a supratherapeutic dose of avibactam (2 grams) given as a 30-minute single infusion. No significant effect on QTcF interval was detected at peak plasma concentration or at any other time. The largest 90% upper bound for the placebo corrected mean change from baseline was 5.9 ms. There were no QTcF intervals greater than 450 ms, nor were there any QTcF interval changes from baseline greater than 30 ms.
Pharmacokinetics
The mean pharmacokinetic parameters for ceftazidime and avibactam in healthy adult male subjects with normal renal function after single and multiple 2-hour intravenous infusions of ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered every 8 hours are summarized in TABLE 7.
Pharmacokinetic parameters of ceftazidime and avibactam were similar for single and multiple dose administration of ceftazidime and avibactam and were similar to those determined when ceftazidime or avibactam were administered alone.

The Cmax and AUC of ceftazidime increase in proportion to dose. Avibactam demonstrated approximately linear pharmacokinetics across the dose range studied (50 mg to 2000 mg) for single intravenous administration. No appreciable accumulation of ceftazidime or avibactam was observed following multiple intravenous infusions of ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) administered every 8 hours for up to 11 days in healthy adults with normal renal function.
Distribution
Less than 10% of ceftazidime was protein bound. The degree of protein binding was independent of concentration. The binding of avibactam to human plasma proteins was low (5.7% to 8.2%) and was similar across the range of concentrations tested in vitro (0.5 to 50 mg/L).
The steady-state volumes of distribution of ceftazidime and avibactam were 17 L and 22.2 L, respectively, in healthy adults following multiple doses of ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) infused every 8 hours over 2 hours for 11 days.
Ceftazidime is mostly (80% to 90% of the dose) eliminated as unchanged drug. No metabolism of avibactam was observed in human liver preparations (microsomes and hepatocytes). Unchanged avibactam was the major drug-related component in human plasma and urine after a single intravenous dose of 0.5 grams 14C-labelled avibactam.
Excretion
Both ceftazidime and avibactam are excreted mainly by the kidneys.
Approximately 80% to 90% of an intravenous dose of ceftazidime is excreted unchanged by the kidneys over a 24-hour period. After the intravenous administration of single 0.5-grams or 1-gram doses, approximately 50% of the dose appeared in the urine in the first 2 hours. An additional 20% was excreted between 2 and 4 hours after dosing, and approximately another 12% of the dose appeared in the urine between 4 and 8 hours later. The elimination of ceftazidime by the kidneys resulted in high therapeutic concentrations in the urine. The mean renal clearance of ceftazidime was approximately 100 mL/min. The calculated plasma clearance of approximately 115 mL/min indicated nearly complete elimination of ceftazidime by the renal route.
Following administration of a single 0.5-grams intravenous dose of radiolabelled avibactam, an average of 97% of administered radioactivity was recovered from the urine, with over 95% recovered within 12 hours of dosing. An average of 0.20% of administered total radioactivity was recovered in feces within 96 hours of dosing. An average of 85% of administered avibactam was recovered from the urine as unchanged drug within 96 hours, with over 50% recovered within 2 hours of the start of the infusion. Renal clearance was 158 mL/min, which is greater than the glomerular filtration, suggesting that active tubular secretion contributes to the excretion of avibactam in addition to glomerular filtration.
Specific Populations
Patients with Renal Impairment:
Ceftazidime is eliminated almost solely by the kidneys; its serum half-life is significantly prolonged in patients with impaired renal function.
The clearance of avibactam was significantly decreased in subjects with mild (CrCl greater than 50 to 80 mL/min, n = 6), moderate (CrCl 30 to less than or equal to 50 mL/min, n = 6), and severe (CrCl 30 mL/min or less, not requiring hemodialysis; n = 6) renal impairment compared to healthy subjects with normal renal function (CrCl greater than 80 mL/min, n = 6) following administration of a single 100-mg intravenous dose of avibactam. The slower clearance resulted in increases in systemic exposure (AUC) of avibactam of 2.6-fold, 3.8-fold, and 7-fold in subjects with mild, moderate, and severe renal impairment, respectively, compared to subjects with normal renal function.
A single 100-mg dose of avibactam was administered to subjects with ESRD (n = 6) either 1 hour before or after hemodialysis. The avibactam AUC following the post-hemodialysis infusion was 19.5-fold the AUC of healthy subjects with normal renal function. Avibactam was extensively removed by hemodialysis, with an extraction coefficient of 0.77 and a mean hemodialysis clearance of 9.0 L/h. Approximately 55% of the avibactam dose was removed during a 4-hour hemodialysis session.
Dosage adjustment of ceftazidime and avibactam is recommended in patients with moderate and severe renal impairment and end-stage renal disease. Population PK models for ceftazidime and avibactam were used to conduct simulations for patients with impaired renal function. Simulations demonstrated that the recommended dose adjustments provide comparable exposures of ceftazidime and avibactam in patients with moderate and severe renal impairment and end-stage renal disease to those in patients with normal renal function or mild renal impairment. Because the exposure of both ceftazidime and avibactam is highly dependent on renal function, monitor CrCl at least daily and adjust the dosage of ceftazidime and avibactam accordingly for patients with changing renal function.
Patients with Hepatic Impairment:
The presence of hepatic dysfunction had no effect on the pharmacokinetics of ceftazidime in individuals administered 2 grams intravenously every 8 hours for 5 days.
The pharmacokinetics of avibactam in patients with hepatic impairment have not been established. Avibactam does not appear to undergo significant hepatic metabolism, therefore the systemic clearance of avibactam is not expected to be significantly affected by hepatic impairment.
Dose adjustments are not currently considered necessary for ceftazidime and avibactam in patients with impaired hepatic function.
Geriatric Patients:
Following single-dose administration of 0.5 grams avibactam as a 30-minute infusion the mean AUC for avibactam was 17% higher in healthy elderly subjects (65 years of age and older, n = 16) than in healthy young adult subjects (18 to 45 years of age, n = 17). There was no statistically significant age effect for avibactam Cmax.
No dose adjustment is recommended based on age. Dosage adjustment for ceftazidime and avibactam in elderly patients should be based on renal function.
Gender:
Following single-dose administration of 0.5 grams avibactam as a 30-minute infusion, healthy male subjects (n = 17) had 18% lower avibactam Cmax values than healthy female subjects (n = 16). There was no gender effect for avibactam AUC parameters.
No dose adjustment is recommended based on gender.
Drug Interactions
Avibactam at clinically relevant concentrations does not inhibit the cytochrome P450 isoforms CYP1A2, CYP2A6, CYP2B6, CYP2C8, CYP2C9, CYP2C19, CYP2D6, CYP2E1 and CYP3A4/5 in vitro in human liver microsomes. Avibactam showed no potential for in vitro induction of CYP1A2, 2B6, 2C9 and 3A4 isoenzymes in human hepatocytes. Against CYP2E1, avibactam showed a slight induction potential at very high concentrations that exceed any clinically relevant exposure. Ceftazidime was evaluated independently in human hepatocytes and showed no induction potential on the activity or mRNA expression of CYP1A1/2, CYP2B6, and CYP3A4/5.
Neither ceftazidime nor avibactam was found to be an inhibitor of the following hepatic and renal transporters in vitro at clinically relevant concentrations: MDR1, BCRP, OAT1, OAT3, OATP1B1, OATP1B3, BSEP, MRP4, OCT1 and OCT2. Avibactam was not a substrate of MDR1, BCRP, MRP4, or OCT2, but was a substrate of human OAT1 and OAT3 kidney transporters based on results generated in human embryonic kidney cells expressing these transporters. Probenecid inhibits 56% to 70% of the uptake of avibactam by OAT1 and OAT3 in vitro. Ceftazidime does not inhibit avibactam transport mediated by OAT1 and OAT3. The clinical impact of potent OAT inhibitors on the pharmacokinetics of avibactam is not known. Co-administration of ceftazidime and avibactam with probenecid is not recommended. Administration of ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) to healthy male subjects (n = 28) as a 2-hour infusion following a 1-hour infusion of metronidazole every 8 hours for 3 days, did not affect the Cmax and AUC values for avibactam or ceftazidime compared to administration of ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) alone. Administration of 0.5 grams metronidazole to healthy male subjects as a 1-hour infusion before a 2-hour infusion of ceftazidime and avibactam 2.5 grams (ceftazidime 2 grams and avibactam 0.5 grams) every 8 hours for 3 days did not affect the Cmax and AUC of metronidazole compared to administration of 0.5 grams metronidazole alone.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
Ceftazidime and avibactam were each evaluated for mutagenic potential in several in vitro and in vivo assays. Ceftazidime was negative for mutagenicity in a mouse micronucleus test and an Ames test. Avibactam was negative for genotoxicity in the Ames assay, unscheduled DNA synthesis, chromosomal aberration assay, and a rat micronucleus study.
Avibactam had no adverse effects on fertility of male and female rats given up to 1 g/kg/day (approximately 20 fold higher than the recommended clinical dose on a body surface area basis). There was a dose-related increase in the percentage of pre- and post-implantation loss relative to controls, resulting in a lower mean litter size at doses 0.5 g/kg and greater with intravenous administration to female rats beginning 2 weeks prior to mating.
Clinical Studies
Complicated Intra-abdominal Infections
A total of 1058 adults hospitalized with cIAI were randomized and received trial medications in a multinational, multi-center, double-blind trial comparing ceftazidime and avibactam 2.5 g (ceftazidime 2 grams and avibactam 0.5 grams) intravenously every 8 hours plus metronidazole (0.5 grams intravenously every 8 hours) to meropenem (1 gram intravenously every 8 hours) for 5 to 14 days of therapy. Complicated intra-abdominal infections included appendicitis, cholecystitis, diverticulitis, gastric/duodenal perforation, perforation of the intestine, and other causes of intra-abdominal abscesses and peritonitis.
The microbiologically modified intent-to treat (mMITT) population, which included all patients who had at least one baseline intra-abdominal pathogen, consisted of 823 patients; the median age was 51 years and 62.8% were male. The majority of patients (64.9%) were from Eastern Europe; 7.5% were from the United States. Less than 1.0% of patients were of Pacific Island or African descent. The most common primary cIAI diagnosis was appendiceal perforation or peri-appendiceal abscess, occurring in 44.7% of patients. Bacteremia at baseline was present in 4.3% of patients.
Clinical cure was defined as complete resolution or significant improvement in signs and symptoms of the index infection at the test-of-cure (TOC) visit which occurred 28 to 35 days after randomization. TABLE 10 presents the clinical cure in the mMITT population and in the microbiologically evaluable (ME) population, which included all protocol-adherent mMITT patients. Ceftazidime and avibactam plus metronidazole was non-inferior to meropenem with regard to the primary endpoint (clinical cure rate at the TOC visit in the mMITT population). Clinical cure rates at the TOC visit by pathogen in the mMITT population are presented in TABLE 11.

Of the 823 patients in the mMITT population, 14 (1.7%) had baseline E. coli bacteremia; 7/10 (70.0%) of patients in the ceftazidime and avibactam arm and 3/4 (75.0%) of patients in the meropenem arm had a clinical cure.

At baseline, 111 patients in the mMITT population had Gram-negative isolates that were not susceptible to ceftazidime, including 61 patients with E. coli and 26 patients with K. pneumoniae isolates. Cure rates were 39/47 (83.0%) in patients who received ceftazidime and avibactam and 55/64 (85.9%) of patients who received meropenem.
In a subset of Gram-negative pathogens from both arms of the Phase 3 cIAI trial that met phenotypic screening criteria for the presence of a beta-lactamase, genotypic testing identified certain ESBL groups (e.g., TEM-1, SHV-12, CTX-M-15, OXA-48) and AmpC that were expected to be inhibited by avibactam in isolates from 105 (12.8%) of the 823 patients in the mMITT population. Clinical cure rates in this subset were similar to the overall results.
Complicated Urinary Tract Infections, Including Pyelonephritis
The efficacy of ceftazidime and avibactam in patients with cUTI was evaluated in two randomized, actively controlled clinical trials (Trial 1 and Trial 2) as described below.
Trial 1:
A total of 1020 adults hospitalized with cUTI were randomized and received trial medications in a multinational, multi-center, double-blind trial comparing ceftazidime and avibactam 2.5 g (ceftazidime 2 grams and avibactam 0.5 grams) intravenously every 8 hours to doripenem 0.5 grams intravenously every 8 hours for 10 to 14 days of total therapy. A switch to an oral antimicrobial agent was allowed after 5 days of intravenous dosing. Complicated urinary tract infections included acute pyelonephritis and complicated lower urinary tract infections.
The mMITT population, which included all patients who had at least one uropathogen isolated at baseline (greater or equal to 105 CFU/mL), consisted of 810 patients; the median age was 55 years and 69.8% were female. The majority of patients (75.4%) were from Eastern Europe; less than 1% of patients were from the United States. The majority of patients were White (83%) or Asian (7.8%); other racial subgroups were each represented at less than 1%. The most common diagnosis was acute pyelonephritis, occurring in 72% of patients. Bacteremia was present at baseline in 8.8% of patients.
Clinical efficacy was determined by comparing the response rate of ceftazidime and avibactam to doripenem at both primary endpoints; symptom response rates at Day 5 and combined microbiological cure and symptom response rates at the TOC visit (21 to 25 days after randomization). A symptom response was based on the resolution of patient-reported cUTI symptoms, defined as frequency/urgency/dysuria/suprapubic pain, as well as an improvement in flank pain for individuals with acute pyelonephritis. Microbiological cure was defined as a reduction of all baseline uropathogens to less than 104 CFU/mL in the urine.
Ceftazidime and avibactam was noninferior to doripenem with regard to both primary endpoints as presented in TABLE 12.
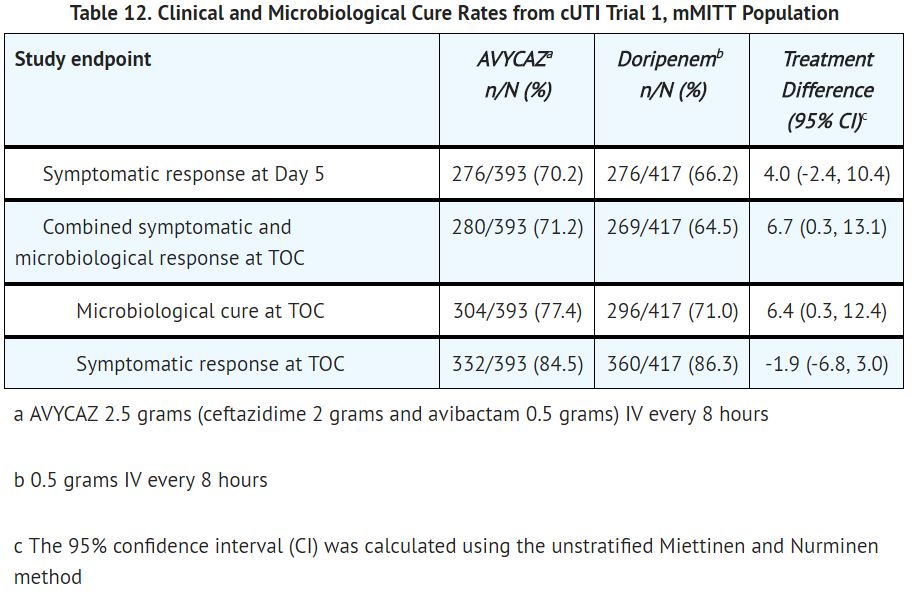
Microbiological cure rates by pathogen are presented in TABLE 13. Microbiological cure in individuals with bacteremia at baseline was achieved in 31/38 (81.6%) patients in the ceftazidime and avibactam arm and 24/33 (72.7%) patients in the doripenem arm at the TOC visit in the mMITT population. The most common pathogen isolated from blood was Escherichia coli, for which 31/32 (96.9%) patients in the ceftazidime and avibactam arm were microbiological cures, compared with 28/28 (100%) patients in the doripenem arm.

At baseline, 159 patients in the mMITT population had Gram-negative isolates that were not susceptible to ceftazidime, including 75 patients in the ceftazidime and avibactam arm and 84 in the doripenem arm. Microbiological and clinical cure rates at TOC were 47/75 (62.7%) and 67/75 (89.3%), respectively, in patients who received ceftazidime and avibactam, compared to 51/84 (60.7%) and 75/84 (89.3%) in patients who received doripenem.
In a subset of Gram-negative pathogens from both arms of the Phase 3 cUTI trial that met phenotypic screening criteria for the presence of a beta-lactamase, genotypic testing identified certain ESBL groups (e.g., TEM-1, SHV-12, CTX-M-15, CTX-M-27, OXA-48) and AmpC that were expected to be inhibited by avibactam in isolates from 176 (21.7%) of the 810 patients in the mMITT population. Microbiological and clinical cure rates in this subset were similar to the overall trial results.
Trial 2: In a multinational, multi-center, open-label study of adults hospitalized with ceftazidime non-susceptible (CAZ-NS) Gram-negative infections, 305 subjects with cUTI were randomized to receive ceftazidime and avibactam 2.5 g (ceftazidime 2 grams and avibactam 0.5 grams) intravenously every 8 hours or the best available intravenous therapy (BAT) for 5 to 21 days of treatment. There was no optional switch to oral therapy. The majority (96.1%) of patients in the BAT arm received monotherapy with a carbapenem antibacterial drug. Complicated urinary tract infections included acute pyelonephritis and complicated lower urinary tract infections.
The mMITT population consisted of 281 cUTI patients with at least one baseline CAZ-NS uropathogen (defined as MIC greater or equal to 8 mg/L for Enterobacteriaceae and greater or equal to 16 mg/L for P. aeruginosa). The median age was 65 years and 54.8% were male. The majority of cUTI patients (82.2%) were from Eastern Europe; 2.8% were from the United States. The majority of patients (95%) were White. The most common diagnosis was cUTI without pyelonephritis, occurring in 54.8% of patients. Bacteremia at baseline was present in 3.6% of patients.
Clinical efficacy was based on evaluation of both the clinical cure (defined as resolution or significant improvement of baseline cUTI signs and symptoms) and microbiological cure (all baseline uropathogens were reduced to less than 104 CFU/mL) rates at the follow-up visit (21 to 25 calendar days from randomization) in the mMITT population.
The clinical and microbiological response rates at the follow-up visit in the mMITT population are presented in TABLE 14. The microbiological response rates at the follow-up visit by baseline CAZ-NS uropathogen in the mMITT population are presented in TABLE 15.


Among Gram-negative uropathogens from both arms of Trial 2, genotypic testing identified certain ESBL groups (e.g., TEM-1, SHV-12, CTX-M-15, CTX-M-27, KPC-2, KPC-3, OXA-48) and AmpC beta-lactamases expected to be inhibited by avibactam in isolates from 273/281 (97.2%) patients in the mMITT population. Clinical and microbiological cure rates in this subset were similar to the overall results.
How Supplied
Ceftazidime and avibactam 2.5 grams (ceftazidime and avibactam) for injection is supplied in single-dose, clear glass vial containing: ceftazidime 2 grams (equivalent to 2.635 grams of ceftazidime pentahydrate/sodium carbonate) and avibactam 0.5 grams (equivalent to 0.551 grams of avibactam sodium). Vials are supplied as individual vial (NDC# 0456-2700-01) and in cartons containing 10 vials (NDC# 0456-2700-10)
Storage
Ceftazidime and avibactam vials should be stored at 25°C (77°F); excursions permitted between 15°C and 30°C (59°F and 86°F). Protect from light. Store in carton until time of use.
Images
Drug Images
{{#ask: Page Name::Ceftazidime and avibactam |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel



{{#ask: Label Page::Ceftazidime and avibactam |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Serious Allergic Reactions
- Advise patients, their families, or caregivers that allergic reactions, including serious allergic reactions, could occur that require immediate treatment.
- Ask them about any previous hypersensitivity reactions to ceftazidime and avibactam, other beta-lactams (including cephalosporins), or other allergens.
- Potentially Serious Diarrhea
- Advise patients, their families, or caregivers that diarrhea is a common problem caused by antibacterial drugs.
- Sometimes, frequent watery or bloody diarrhea may occur and may be a sign of a more serious intestinal infection. If severe watery or bloody diarrhea develops, tell them to contact his or her healthcare provider.
- Nervous System Reactions
- Advise patients, their families, or caregivers that neurological adverse reactions can occur with ceftazidime and avibactam use.
- Instruct patients their families, or caregivers to inform a healthcare provider at once of any neurological signs and symptoms, including encephalopathy (disturbance of consciousness including confusion, hallucinations, stupor, and coma), myoclonus, and seizures, for immediate treatment, dosage adjustment, or discontinuation of ceftazidime and avibactam.
- Antibacterial Resistance
- Counsel patients, their families, or caregivers that antibacterial drugs including ceftazidime and avibactam should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold).
- When ceftazidime and avibactam is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed.
- Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by ceftazidime and avibactam or other antibacterial drugs in the future.
Precautions with Alcohol
Alcohol-Ceftazidime and avibactam interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
AVYCAZ
Look-Alike Drug Names
There is limited information regarding Ceftazidime and avibactam Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
