Doripenem
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Gloria Picoy [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Doripenem is a beta-lactam that is FDA approved for the treatment of complicated intra-abdominal infections and complicated urinary tract infections, including pyelonephritis. Common adverse reactions include headache, nausea, diarrhea, rash and phlebitis.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Complicated Intra-Abdominal Infections
- Is indicated as a single agent for the treatment of complicated intra-abdominal infections caused by Escherichia coli, Klebsiella pneumoniae, Pseudomona aeruginosa, Bacteroides caccae, Bacteroides fragilis, Bacteroides thetaiotaomicron, Bacteroides uniformis, Bacteroides vulgatus, Streptococcus intermedius, Streptococcus constellatus and Peptostreptococcus micros.
- Dosage: 500 mg administered every 8 hours by intravenous infusion over one hour for 5-14 days
Complicated Urinary Tract Infections, Including Pyelonephritis
- Is indicated as a single agent for the treatment of complicated urinary tract infections, including pyelonephritis caused by Escherichia coli including cases with concurrent bacteremia, Klebsiella pneumoniae, Proteus mirabilis, Pseudomona aeruginosa, and Acinetobacter baumannii.
- Dosage: 500 mg administered every 8 hours by intravenous infusion over one hour for 10 days
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doripenem in adult patients.
Non–Guideline-Supported Use
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Safety and effectiveness not established in pediatric patients
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Doripenem in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Doripenem in pediatric patients.
Contraindications
Doripenem is contraindicated in patients with known serious hypersensitivity to doripenem or to other drugs in the same class or in patients who have demonstrated anaphylactic reactions to beta-lactams.
Warnings
Increased Mortality in Ventilator-Associated Bacterial Pneumonia
In a clinical trial of patients with ventilator-associated pneumonia comparing doripenem to imipenem, more subjects receiving doripenem died 23% (31/135) compared to those receiving imipenem 16.7% (22/132) based on 28-day all-cause mortality in the intent-to-treat (ITT) population. Clinical response rates were also lower in the doripenem arm. Doripenem is not approved for the treatment of ventilator associated pneumonia.
Hypersensitivity Reactions
Serious and occasionally fatal hypersensitivity (anaphylactic) and serious skin reactions have been reported in patients receiving beta-lactam antibiotics. These reactions are more likely to occur in individuals with a history of sensitivity to multiple allergens. Before therapy with doripenem is instituted, careful inquiry should be made to determine whether the patient has had a previous hypersensitivity reaction to other carbapenems, cephalosporins, penicillins or other allergens. If this product is to be given to a penicillin- or other beta-lactam-allergic patient, caution should be exercised because cross-reactivity among beta-lactam antibiotics has been clearly documented.
If an allergic reaction to doripenem occurs, discontinue the drug. Serious acute hypersensitivity (anaphylactic) reactions require emergency treatment, as clinically indicated.
Seizures
Seizures have been reported during treatment with doripenem. In clinical trials, doripenem-treated patients with pre-existing central nervous system (CNS) disorders (e.g. stroke or history of seizures), patients with compromised renal function and patients given doses greater than 500 mg every 8 hours appear to be at greater risk for developing seizures.
Interaction with Valproic Acid
Due to a drug interaction, patients with seizure disorders controlled with valproic acid or sodium valproate will be at an increased risk for breakthrough seizures when treated with doripenem concomitantly. Reduction in serum valproic acid concentrations to below the therapeutic concentration range (50 to 100 mcg/mL) was observed by 12 hours after the initiation of doripenem in healthy subjects co-administered both drugs. A similar drug interaction involving other carbapenem antibacterials and valproic acid has been described in published case reports. In some of these reports, increasing the dose of valproic acid or sodium valproate did not result in increased valproic acid serum concentrations. Alternative antibacterial therapies should be considered for patients receiving valproic acid or sodium valproate. If administration of doripenem is necessary, supplemental anti-convulsant therapy should be considered.
Clostridium difficile-Associated Diarrhea
Clostridium difficile-associated diarrhea (CDAD) has been reported with nearly all antibacterial agents and may range in severity from mild diarrhea to fatal colitis.
Treatment with antibacterial agents alters the normal flora of the colon and may permit overgrowth of C. difficile.
C. difficile produces toxins A and B which contribute to the development of CDAD. Hypertoxin producing strains of C. difficile cause increased morbidity and mortality, as these infections can be refractory to antimicrobial therapy and may require colectomy. CDAD must be considered in all patients who present with diarrhea following antibiotic use. Careful medical history is necessary since CDAD has been reported to occur over two months after the administration of antibacterial agents.
If CDAD is suspected or confirmed, ongoing antibiotic use not directed against C. difficile may need to be discontinued. Appropriate fluid and electrolyte management, protein supplementation, antibiotic treatment of C. difficile, and surgical evaluation should be instituted as clinically indicated.
Development of Drug-Resistant Bacteria
Prescribing doripenem in the absence of a proven or strongly suspected bacterial infection is unlikely to provide benefit to the patient and increases the risk of the development of drug-resistant bacteria.
Pneumonitis with Inhalational Use
When doripenem has been used investigationally via inhalation, pneumonitis has occurred. doripenem should not be administered by this route.
Adverse Reactions
Clinical Trials Experience
Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in clinical trials of a drug cannot be compared directly to rates from clinical trials of another drug and may not reflect rates observed in practice.
During clinical investigations, 1338 adult patients were treated with doripenem (1076 patients received doripenem 500 mg administered over 1 hour every 8 hours and 262 patients received doripenem 500 mg administered over 4 hours every 8 hours); in some patients parenteral therapy was followed by a switch to an oral antimicrobial. The median age of patients treated with doripenem was 54 years (range 18–90) in the comparative complicated urinary tract infections (cUTI) study, 46 years (range 18–94) in the pooled comparative complicated intra-abdominal infections (cIAI) studies, and 56 years (range 18-94) in the other Phase 3 trials. There was a female predominance (62%) in the comparative cUTI study and a male predominance (63% and 75%) in the comparative cIAI and other Phase 3 trials, respectively. The patients treated with doripenem were predominantly Caucasian (79%) in the five comparator-controlled Phase 3 studies.
The most common adverse drug reactions (≥ 5%) observed in the five doripenem comparator-controlled Phase 3 clinical trials were anemia, headache, nausea, diarrhea, rash, phlebitis, and elevated hepatic enzymes. During clinical trials, adverse events led to discontinuation of doripenem in 4.1% (55 of 1338) of patients compared to 4.3% (58 of 1325) of comparator-treated patients.
Adverse reactions due to doripenem 500 mg every 8 hours that occurred at a rate ≥ 1 % are listed in Table 4. Hypersensitivity reactions related to intravenous study drug occurred at a rate of less than 1%.

In a Phase 1 study of healthy subjects receiving doripenem doses greater than the approved dose of 500 mg every 8 hours for 10 to 14 days, the incidence of rash was higher than that observed in subjects who received 500 mg every 8 hours. The rash resolved within 10 days after doripenem administration was discontinued.
Postmarketing Experience
The following adverse reactions have been identified during post-approval use of doripenem. Because these reactions were reported voluntarily from a population of uncertain size, it is not possible to reliably estimate their frequency or establish a causal relationship to drug exposure.
- Anaphylaxis
- Leukopenia
- Neutropenia
- Seizure
- Thrombocytopenia
- Toxic epidermal necrolysis, Stevens-Johnson Syndrome
- Interstitial pneumonia
Drug Interactions
Valproic Acid
Co-administration of doripenem with valproic acid causes the serum concentrations of valproic acid to fall below the therapeutic range, increasing the risk for breakthrough seizures. Although the mechanism of this interaction is not fully understood, data from in vitro and animal studies suggest that doripenem may inhibit the hydrolysis of valproic acid's glucuronide metabolite (VPA-g) back to valproic acid, thus decreasing the plasma concentrations of valproic acid. This is consistent with case reports for other carbapenems, where serum concentrations of valproic acid were reduced upon co-administration with a carbapenem. If administration of doripenem is necessary, supplemental anti-convulsant therapy should be considered. The pharmacokinetics of doripenem were unaffected by the co-administration of valproic acid.
Probenecid
Probenecid interferes with the active tubular secretion of doripenem, resulting in increased plasma concentrations of doripenem. Coadministration of probenecid with doripenem is not recommended.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): B
Doripenem was not teratogenic and did not produce effects on ossification, developmental delays or fetal weight following intravenous administration during organogenesis at doses as high as 1 g/kg/day in rats and 50 mg/kg/day in rabbits (based on AUC, at least 2.4 and 0.8 times the exposure to humans dosed at 500 mg administered every 8 hours, respectively). There are no adequate and well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Doripenem in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Doripenem during labor and delivery.
Nursing Mothers
It is not known whether this drug is excreted in human milk. Because many drugs are excreted in human milk, caution should be exercised when doripenem is administered to a nursing woman.
Pediatric Use
Safety and effectiveness in pediatric patients have not been established.
Geriatic Use
Of the total number of subjects in clinical studies of doripenem, 28% were 65 and over, while 12% were 75 and over. Clinical cure rates in complicated intra-abdominal and complicated urinary tract infections were slightly lower in patients ≥ 65 years of age and also in the subgroup of patients ≥ 75 years of age versus patients < 65. These results were similar between doripenem and comparator treatment groups.
This drug is known to be excreted substantially by the kidney, and the risk of adverse reactions to this drug may be greater in patients with impaired renal function or pre-renal azotemia. Because elderly patients are more likely to have decreased renal function or pre-renal azotemia, care should be taken in dose selection, and it may be useful to monitor renal function.
Elderly subjects had greater doripenem plasma concentrations relative to non-elderly subjects; however, this increase in exposure was mainly attributed to age-related changes in renal function.
No overall differences in safety were observed between older and younger subjects, but greater sensitivity of some older individuals cannot be ruled out.
Gender
The effect of gender on the pharmacokinetics of doripenem was evaluated in healthy male (n=12) and female (n=12) subjects. Doripenem Cmax and AUC were similar between males and females. No dose adjustment is recommended based on gender.
Race
The effect of race on doripenem pharmacokinetics was examined using a population pharmacokinetic analysis of data from phase 1 and 2 studies. No significant difference in mean doripenem clearance was observed across race groups and therefore, no dosage adjustment is recommended based on race.
Renal Impairment
Dosage adjustment is required in patients with moderately or severely impaired renal function. In such patients, renal function should be monitored.
Hepatic Impairment
The pharmacokinetics of doripenem in patients with hepatic impairment have not been established. As doripenem does not appear to undergo hepatic metabolism, the pharmacokinetics of doripenem are not expected to be affected by hepatic impairment.
Females of Reproductive Potential and Males
Intravenous injection of doripenem had no adverse effects on general fertility of treated male and female rats or on postnatal development and reproductive performance of the offspring at doses as high as 1 g/kg/day (based on AUC, greater than 1.5 times the exposure to humans at the dose of 500 mg administered every 8 hours).
Immunocompromised Patients
There is no FDA guidance one the use of Doripenem in patients who are immunocompromised.
Administration and Monitoring
Administration
Intravenous
Monitoring
There is limited information regarding Doripenem Monitoring in the drug label.
IV Compatibility
There is limited information regarding the compatibility of Doripenem and IV administrations.
Overdosage
In the event of overdose, doripenem should be discontinued and general supportive treatment given.
Doripenem can be removed by hemodialysis. In subjects with end-stage renal disease administered doripenem 500 mg, the mean total recovery of doripenem and doripenem-M1 in the dialysate following a 4-hour hemodialysis session was 259 mg (52% of the dose). However, no information is available on the use of hemodialysis to treat overdosage.
Pharmacology
| |
Doripenem
| |
| Systematic (IUPAC) name | |
| (4R,5S,6S)-6-(1-hydroxyethyl)-4-methyl-7-oxo-3- [(3S,5S)-5-[(sulfamoylamino)methyl]pyrrolidin-3-yl] sulfanyl-1-azabicyclo[3.2.0]hept-2-ene-2-carboxylic acid | |
| Identifiers | |
| CAS number | |
| ATC code | J01 |
| PubChem | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 420.50426 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | Renal |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Licence data |
, |
| Pregnancy cat. |
B |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | IM, IV |
Mechanism of Action
Doripenem belongs to the carbapenem class of antimicrobials. Doripenem exerts its bactericidal activity by inhibiting bacterial cell wall biosynthesis. Doripenem inactivates multiple essential penicillin-binding proteins (PBPs) resulting in inhibition of cell wall synthesis with subsequent cell death. In E. coli and P. aeruginosa, doripenem binds to PBP 2, which is involved in the maintenance of cell shape, as well as to PBPs 3 and 4.
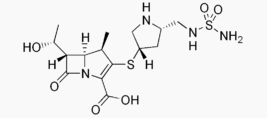
Structure
Its molecular weight is 438.52, and its chemical structure is:

Pharmacodynamics
Similar to other beta-lactam antimicrobial agents, the time that unbound plasma concentration of doripenem exceeds the MIC of the infecting organism has been shown to best correlate with efficacy in animal models of infection. However, the pharmacokinetic/pharmacodynamic relationship for doripenem has not been evaluated in patients.
In a randomized, positive- and placebo-controlled crossover QT study, 60 healthy subjects were administered doripenem 500 mg IV every 8 hours × 4 doses and doripenem 1 g IV every 8 hours × 4 doses, placebo, and a single oral dose of positive control. At both the 500 mg and 1 g doripenem doses, no significant effect on QTc interval was detected at peak plasma concentration or at any other time.
Pharmacokinetics
Plasma Concentrations
Mean plasma concentrations of doripenem following a single 1-hour intravenous infusion of a 500 mg dose of doripenem to 24 healthy subjects are shown below in Figure 1. The mean (SD) plasma Cmax and AUC0–∞ values were 23.0 (6.6) µg/mL and 36.3 (8.8) µg•hr/mL, respectively.

The pharmacokinetics of doripenem (Cmax and AUC) are linear over a dose range of 500 mg to 1 g when intravenously infused over 1 hour. There is no accumulation of doripenem following multiple intravenous infusions of either 500 mg or 1 g administered every 8 hours for 7 to 10 days in subjects with normal renal function.
Distribution
The average binding of doripenem to plasma proteins is approximately 8.1% and is independent of plasma drug concentrations. The median (range) volume of distribution at steady state in healthy subjects is 16.8 L (8.09–55.5 L), similar to extracellular fluid volume (18.2 L).
Doripenem penetrates into several body fluids and tissues, including those at the site of infection for the approved indications. Doripenem concentrations in peritoneal and retroperitoneal fluid either match or exceed those required to inhibit most susceptible bacteria; however, the clinical relevance of this finding has not been established. Concentrations achieved in selected tissues and fluids following administration of doripenem are shown in Table 5:

Metabolism
Metabolism of doripenem to a microbiologically inactive ring-opened metabolite (doripenem-M1) occurs primarily via dehydropeptidase-I. The mean (SD) plasma doripenem-M1-to-doripenem AUC ratio following single 500 mg and 1 g doses in healthy subjects is 18% (7.2%).
In pooled human liver microsomes, no in vitro metabolism of doripenem could be detected, indicating that doripenem is not a substrate for hepatic CYP450 enzymes.
Excretion
Doripenem is primarily eliminated unchanged by the kidneys. The mean plasma terminal elimination half-life of doripenem in healthy non-elderly adults is approximately 1 hour and mean (SD) plasma clearance is 15.9 (5.3) L/hour. Mean (SD) renal clearance is 10.3 (3.5) L/hour. The magnitude of this value, coupled with the significant decrease in the elimination of doripenem with concomitant probenecid administration, suggests that doripenem undergoes both glomerular filtration and active tubular secretion. In healthy adults given a single 500 mg dose of doripenem, a mean of 71% and 15% of the dose was recovered in urine as unchanged drug and the ring-opened metabolite, respectively, within 48 hours. Following the administration of a single 500 mg dose of radiolabeled doripenem to healthy adults, less than 1% of the total radioactivity was recovered in feces after one week.
Nonclinical Toxicology
Mechanism(s) of Resistance
Bacterial resistance mechanisms that affect doripenem include drug inactivation by carbapenem-hydrolyzing enzymes, mutant or acquired PBPs, decreased outer membrane permeability and active efflux. Doripenem is stable to hydrolysis by most beta-lactamases, including penicillinases and cephalosporinases produced by Gram-positive and Gram-negative bacteria, with the exception of carbapenem hydrolyzing beta-lactamases. Although cross-resistance may occur, some isolates resistant to other carbapenems may be susceptible to doripenem.
Carcinogenesis and Mutagenesis
Because of the short duration of treatment and intermittent clinical use, long-term carcinogenicity studies have not been conducted with doripenem.
Doripenem did not show evidence of mutagenic activity in standard tests that included bacterial reverse mutation assay, chromosomal aberration assay with Chinese hamster lung fibroblast cells, and mouse bone marrow micronucleus assay.
Clinical Studies
Complicated Intra-Abdominal Infections
A total of 946 adults with complicated intra-abdominal infections were randomized and received study medications in two identical multinational, multi-center, double-blind studies comparing doripenem (500 mg administered over 1 hour every 8 hours) to meropenem (1 g administered over 3–5 minutes every 8 hours). Both regimens allowed the option to switch to oral amoxicillin/clavulanate (875 mg/125 mg administered twice daily) after a minimum of 3 days of intravenous therapy for a total of 5–14 days of intravenous and oral treatment. Patients with complicated appendicitis, or other complicated intra-abdominal infections, including bowel perforation, cholecystitis, intra-abdominal or solid organ abscess and generalized peritonitis were enrolled.
Doripenem was non-inferior to meropenem with regard to clinical cure rates in microbiologically evaluable (ME) patients, i.e., in patients with susceptible pathogens isolated at baseline and no major protocol deviations at test of cure (TOC) visit, 25–45 days after completing therapy. Doripenem was also non-inferior to meropenem in microbiological modified intent-to-treat (mMITT) patients, i.e., patients with baseline pathogens isolated regardless of susceptibility. Clinical cure rates at TOC are displayed by patient populations in Table 8. Microbiological cure rates at TOC by pathogen in ME patients are presented in Table 9.

Complicated Urinary Tract Infections, Including Pyelonephritis
A total of 1171 adults with complicated urinary tract infections, including pyelonephritis (49 percent of microbiologically evaluable patients) were randomized and received study medications in two multi-center, multinational studies. Complicated pyelonephritis, i.e., pyelonephritis associated with predisposing anatomical or functional abnormality, comprised 17% of patients with pyelonephritis. One study was double-blind and compared doripenem (500 mg administered over 1 hour every 8 hours) to IV levofloxacin (250 mg administered every 24 hours). The second study was a non-comparative study but of otherwise similar design. Both studies permitted the option of switching to oral levofloxacin (250 mg administered every 24 hours) after a minimum of 3 days of IV therapy for a total of 10 days of treatment. Patients with confirmed concurrent bacteremia were allowed to receive 500 mg of IV levofloxacin (either IV or oral as appropriate) for a total of 10 to 14 days of treatment.
doripenem was non-inferior to levofloxacin with regard to the microbiological eradication rates in microbiologically evaluable (ME) patients, i.e., patients with baseline uropathogens isolated, no major protocol deviations and urine cultures at test of cure (TOC) visit 5-11 days after completing therapy. Doripenem was also non-inferior to levofloxacin in microbiological modified intent-to-treat (mMITT) patients, i.e., patients with pretreatment urine cultures. Overall microbiological eradication rates at TOC and the 95% CIs for the comparative study are displayed in Table 10. Microbiological eradication rates at TOC by pathogen in ME patients are presented in Table 11.

How Supplied
- Doripenem 500 mg/vial
- Doripenem 250 mg/vial
Storage
Doripenem should be stored at 25°C (77°F)
Images
Drug Images
{{#ask: Page Name::Doripenem |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel


{{#ask: Label Page::Doripenem |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Patients should be advised that allergic reactions, including serious allergic reactions, could occur and that serious reactions require immediate treatment. They should report any previous hypersensitivity reactions to doripenem, other carbapenems, beta-lactams or other allergens.
- Patients should be counseled that anti-bacterial drugs including doripenem should only be used to treat bacterial infections. They do not treat viral infections (e.g., the common cold). When doripenem is prescribed to treat a bacterial infection, patients should be told that although it is common to feel better early in the course of therapy, the medication should be taken exactly as directed. Skipping doses or not completing the full course of therapy may (1) decrease the effectiveness of the immediate treatment and (2) increase the likelihood that bacteria will develop resistance and will not be treatable by doripenem or other antibacterial drugs in the future.
- Patients should be counseled to inform their physician
- if they have central nervous system disorders such as stroke or history of seizures. Seizures have been reported during treatment with doripenem and with closely related antibiotics
- if they are taking valproic acid or sodium valproate. Valproic acid concentrations in the blood will drop below the therapeutic range upon co-administration with doripenem. If treatment with doripenem is necessary and continued, alternative or supplemental anti-convulsant medication to prevent and/or treat seizures may be needed.
- Keep out of the reach of children.
Precautions with Alcohol
Alcohol-Doripenem interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Doribax [1]
Look-Alike Drug Names
There is limited information regarding Doripenem Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Doripenem |Label Name=Doripenem 250 mg.png
}}
{{#subobject:
|Label Page=Doripenem |Label Name=Doripenem 500 mg.png
}}