Allopurinol (tablet)
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Sheng Shi, M.D. [2]; Sree Teja Yelamanchili, MBBS [3]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Allopurinol (tablet) is a xanthine oxidase inhibitor that is FDA approved for the treatment of primary or secondary gout (acute attacks, tophi, joint destruction, uric acid nephrolithiasis, and/or nephropathy), leukemia, lymphoma, solid tumor malignancies, and recurrent calcium oxalate calculi. Common adverse reactions include maculopapular eruption and pruritus.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Gout
- Dosing information (The dosage of allopurinol tablets to accomplish full control of gout and to lower serum uric acid to normal or near-normal levels varies with the severity of the disease.)
- The appropriate dosage may be administered in divided doses or as a single equivalent dose with the 300 mg tablet. Dosage requirements in excess of 300 mg should be administered in divided doses. The minimal effective dosage is 100 to 200 mg daily and the maximal recommended dosage is 800 mg daily.
- To reduce the possibility of flare-up of acute gouty attacks
- Recommended dosage: 100 mg PO qd and increase at weekly intervals by 100 mg until a serum uric acid level of 6 mg/dL or less is attained but without exceeding the maximal recommended dosage.
- Normal serum urate levels are usually achieved in 1 to 3 weeks. The upper limit of normal is about 7 mg/dL for men and postmenopausal women and 6 mg/dL for premenopausal women. Too much reliance should not be placed on a single serum uric acid determination since, for technical reasons, estimation of uric acid may be difficult. By selecting the appropriate dosage and, in certain patients, using uricosuric agents concurrently, it is possible to reduce serum uric acid to normal or, if desired, to as low as 2 to 3 mg/dL and keep it there indefinitely.
- While adjusting the dosage of allopurinol tablets in patients who are being treated with colchicine and/or anti-inflammatory agents, it is wise to continue the latter therapy until serum uric acid has been normalized and there has been freedom from acute gouty attacks for several months.
- In transferring a patient from a uricosuric agent to allopurinol tablets, the dose of the uricosuric agent should be gradually reduced over a period of several weeks and the dose of allopurinol tablets gradually increased to the required dose needed to maintain a normal serum uric acid level.
- It should also be noted that allopurinol tablets are generally better tolerated if taken following meals. A fluid intake sufficient to yield a daily urinary output of at least 2 liters and the maintenance of a neutral or, preferably, slightly alkaline urine are desirable.
- Since allopurinol tablets and its metabolites are primarily eliminated only by the kidney, accumulation of the drug can occur in renal failure, and the dose of allopurinol tablets should consequently be reduced. With a creatinine clearance of 10 to 20 mL/min, a daily dosage of 200 mg of allopurinol tablets is suitable. When the creatinine clearance is less than 10 mL/min, the daily dosage should not exceed 100 mg. With extreme renal impairment (creatinine clearance less than 3 mL/min) the interval between doses may also need to be lengthened.
- The correct size and frequency of dosage for maintaining the serum uric acid just within the normal range is best determined by using the serum uric acid level as an index.
- For the prevention of uric acid nephropathy during the vigorous therapy of neoplastic disease, treatment with 600 to 800 mg daily for 2 or 3 days is advisable together with a high fluid intake. Otherwise similar considerations to the above recommendations for treating patients with gout govern the regulation of dosage for maintenance purposes in secondary hyperuricemia.
- The dose of allopurinol tablets recommended for management of recurrent calcium oxalate stones in hyperuricosuric patients is 200 to 300 mg/day in divided doses or as the single equivalent.
- This dose may be adjusted up or down depending upon the resultant control of the hyperuricosuria based upon subsequent 24 hour urinary urate determinations.
- Clinical experience suggests that patients with recurrent calcium oxalate stones may also benefit from dietary changes such as the reduction of animal protein, sodium, refined sugars, oxalate-rich foods, and excessive calcium intake, as well as an increase in oral fluids and dietary fiber.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Allopurinol in adult patients.
Non–Guideline-Supported Use
Leishmaniasis
- Dosing information
Malaria
- Dosing information
- 200 to 400 milligrams daily[3]
Schizophrenia
- Dosing information
- 300 milligrams (mg) PO qd or bid[4]
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Dosing in pediatric patients
- Children, 6 to 10 years of age, with secondary hyperuricemia associated with malignancies may be given 300 mg allopurinol tablets daily while those under 6 years are generally given 150 mg daily. The response is evaluated after approximately 48 hours of therapy and a dosage adjustment is made if necessary.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Allopurinol in pediatric patients.
Non–Guideline-Supported Use
Leishmaniasis
- Dosing information
- 10 to 36 milligrams/kilogram/day[5]
Contraindications
Patients who have developed a severe reaction to allopurinol tablets should not be restarted on the drug.
Warnings
- ALLOPURINOL TABLETS SHOULD BE DISCONTINUED AT THE FIRST APPEARANCE OF SKIN RASH OR OTHER SIGNS WHICH MAY INDICATE AN ALLERGIC REACTION.
- In some instances a skin rash may be followed by more severe hypersensitivity reactions such as exfoliative, urticarial, and purpuric lesions, as well as Stevens-Johnson syndrome (erythema multiforme exudativum), and/or generalized vasculitis, irreversible hepatotoxicity, and, on rare occasions, death.
- In patients receiving mercaptopurine or azathioprine, the concomitant administration of 300 to 600 mg of allopurinol tablets per day will require a reduction in dose to approximately one-third to one-fourth of the usual dose of mercaptopurine or azathioprine.
- Subsequent adjustment of doses of mercaptopurine or azathioprine should be made on the basis of therapeutic response and the appearance of toxic effects.
- A few cases of reversible clinical hepatotoxicity have been noted in patients taking allopurinol tablets, and in some patients, asymptomatic rises in serum alkaline phosphatase or serum transaminase have been observed.
- If anorexia, weight loss, or pruritus develop in patients on allopurinol tablets, evaluation of liver function should be part of their diagnostic workup.
- In patients with pre-existing liver disease, periodic liver function tests are recommended during the early stages of therapy.
- Due to the occasional occurrence of drowsiness, patients should be alerted to the need for due precaution when engaging in activities where alertness is mandatory.
- The occurrence of hypersensitivity reactions to allopurinol tablets may be increased in patients with decreased renal function receiving thiazides and allopurinol tablets concurrently.
- For this reason, in this clinical setting, such combinations should be administered with caution and patients should be observed closely.
General
- An increase in acute attacks of gout has been reported during the early stages of administration of allopurinol tablets, even when normal or subnormal serum uric acid levels have been attained.
- Accordingly, maintenance doses of colchicine generally should be given prophylactically when allopurinol tablets are begun.
- In addition, it is recommended that the patient start with a low dose of allopurinol tablets (100 mg daily) and increase at weekly intervals by 100 mg until a serum uric acid level of 6 mg/dL or less is attained but without exceeding the maximum recommended dose (800 mg per day).
- The use of colchicine or anti-inflammatory agents may be required to suppress gouty attacks in some cases.
- The attacks usually become shorter and less severe after several months of therapy.
- The mobilization of urates from tissue deposits which cause fluctuations in the serum uric acid levels may be a possible explanation for these episodes. *Even with adequate therapy with allopurinol tablets, it may require several months to deplete the uric acid pool sufficiently to achieve control of the acute attacks.
- A fluid intake sufficient to yield a daily urinary output of at least 2 liters and the maintenance of a neutral or, preferably, slightly alkaline urine are desirable to (1) avoid the theoretical possibility of formation of xanthine calculi under the influence of therapy with allopurinol tablets and (2) help prevent renal precipitation of urates in patients receiving concomitant uricosuric agents.
- Some patients with pre-existing renal disease or poor urate clearance have shown a rise in BUN during administration of allopurinol tablets. Although the mechanism responsible for this has not been established, patients with impaired renal function should be carefully observed during the early stages of administration of allopurinol tablets and the dosage decreased or the drug withdrawn if increased abnormalities in renal function appear and persist.
- Renal failure in association with administration of allopurinol tablets has been observed among patients with hyperuricemia secondary to neoplastic diseases. Concurrent conditions such as multiple myeloma and congestive myocardial disease were present among those patients whose renal dysfunction increased after allopurinol tablets were begun.
- Renal failure is also frequently associated with gouty nephropathy and rarely with hypersensitivity reactions associated with allopurinol tablets.
- Albuminuria has been observed among patients who developed clinical gout following chronic glomerulonephritis and chronic pyelonephritis.
- Patients with decreased renal function require lower doses of allopurinol tablets than those with normal renal function. Lower than recommended doses should be used to initiate therapy in any patients with decreased renal function and they should be observed closely during the early stages of administration of allopurinol tablets.
- In patients with severely impaired renal function or decreased urate clearance, the half-life of oxipurinol in the plasma is greatly prolonged. Therefore, a dose of 100 mg per day or 300 mg twice a week, or perhaps less, may be sufficient to maintain adequate xanthine oxidase inhibition to reduce serum urate levels.
- Bone marrow depression has been reported in patients receiving allopurinol tablets, most of whom received concomitant drugs with the potential for causing this reaction. This has occurred as early as 6 weeks to as long as 6 years after the initiation of therapy of allopurinol tablets. Rarely, a patient may develop varying degrees of bone marrow depression, affecting one or more cell lines, while receiving allopurinol tablets alone.
Adverse Reactions
Clinical Trials Experience
Data upon which the following estimates of incidence of adverse reactions are made are derived from experiences reported in the literature, unpublished clinical trials and voluntary reports since marketing of allopurinol tablets began. Past experience suggested that the most frequent event following the initiation of allopurinol treatment was an increase in acute attacks of gout (average 6% in early studies). An analysis of current usage suggests that the incidence of acute gouty attacks has diminished to less than 1%. The explanation for this decrease has not been determined but may be due in part to initiating therapy more gradually .
The most frequent adverse reaction to allopurinol tablets is skin rash. Skin reactions can be severe and sometimes fatal. Therefore, treatment with allopurinol tablets should be discontinued immediately if a rash develops . Some patients with the most severe reaction also had fever, chills, arthralgias, cholestatic jaundice, eosinophilia and mild leukocytosis or leukopenia. Among 55 patients with gout treated with allopurinol tablets for 3 to 34 months (average greater than 1 year) and followed prospectively, Rundles observed that 3% of patients developed a type of drug reaction which was predominantly a pruritic maculopapular skin eruption, sometimes scaly or exfoliative. However, with current usage, skin reactions have been observed less frequently than 1%. The explanation for this decrease is not obvious. The incidence of skin rash may be increased in the presence of renal insufficiency. The frequency of skin rash among patients receiving ampicillin or amoxicillin concurrently with allopurinol tablets has been reported to be increased
Most Common Reactions
- Probably Causally Related:
Gastrointestinal: Diarrhea, nausea, alkaline phosphatase increase, SGOT/SGPT increase.
Metabolic and Nutritional: Acute attacks of gout.
Skin and Appendages: Rash, maculopapular rash.
- Early clinical studies and incidence rates from early clinical experience with allopurinol tablets suggested that these adverse reactions were found to occur at a rate of greater than 1%. The most frequent event observed was acute attacks of gout following the initiation of therapy. Analyses of current usage suggest that the incidence of these adverse reactions is now less than 1%. The explanation for this decrease has not been determined, but it may be due to following recommended usage .
Incidence Less Than 1% Probably Causally Related:
Body As a Whole: Ecchymosis, fever, headache.
Cardiovascular: Necrotizing angiitis, vasculitis.
Gastrointestinal: Hepatic necrosis, granulomatous hepatitis, hepatomegaly, hyperbilirubinemia, cholestatic jaundice, vomiting, intermittent abdominal pain, gastritis, dyspepsia.
Hemic and Lymphatic: Thrombocytopenia, eosinophilia, leukocytosis, leukopenia.
Musculoskeletal: Myopathy, arthralgias.
Nervous: Peripheral neuropathy, neuritis, paresthesia, somnolence.
Respiratory: Epistaxis.
Skin and Appendages: Erythema multiforme exudativum (Stevens-Johnson syndrome), toxic epidermal necrolysis (Lyell's syndrome), hypersensitivity vasculitis, purpura, vesicular bullous dermatitis, exfoliative dermatitis, eczematoid dermatitis, pruritus, urticaria, alopecia, onycholysis, lichen planus.
Special Senses: Taste loss/perversion.
Urogenital: Renal failure, uremia .
Incidence Less Than 1% Causal Relationship Unknown:
Body As a Whole: Malaise.
Cardiovascular: Pericarditis, peripheral vascular disease, thrombophlebitis, bradycardia, vasodilation.
Endocrine: Infertility (male), hypercalcemia, gynecomastia (male).
Gastrointestinal: Hemorrhagic pancreatitis, gastrointestinal bleeding, stomatitis, salivary gland swelling, hyperlipidemia, tongue edema, anorexia.
Hemic and Lymphatic: Aplastic anemia, agranulocytosis, eosinophilic fibrohistiocytic lesion of bone marrow, pancytopenia, prothrombin decrease, anemia, hemolytic anemia, reticulocytosis, lymphadenopathy, lymphocytosis.
Musculoskeletal: Myalgia.
Nervous: Optic neuritis, confusion, dizziness, vertigo, foot drop, decrease in libido, depression, amnesia, tinnitus, asthenia, insomnia.
Respiratory: Bronchospasm, asthma, pharyngitis, rhinitis.
Skin and Appendages: Furunculosis, facial edema, sweating, skin edema.
Special Senses: Cataracts, macular retinitis, iritis, conjunctivitis, amblyopia.
Urogenital: Nephritis, impotence, primary hematuria, albuminuria.
Postmarketing Experience
FDA Package Insert for Allopurinol contains no information regarding Postmarketing experience.
Drug Interactions
- In patients receiving mercaptopurine or azathioprine, the concomitant administration of 300 to 600 mg of allopurinol tablets per day will require a reduction in dose to approximately one third to one fourth of the usual dose of mercaptopurine or azathioprine. Subsequent adjustment of doses of mercaptopurine or azathioprine should be made on the basis of therapeutic response and the appearance of toxic effects .
- It has been reported that allopurinol tablets prolong the half-life of the anticoagulant, dicumarol. The clinical basis of this drug interaction has not been established but should be noted when allopurinol tablets are given to patients already on dicumarol therapy.
- Since the excretion of oxipurinol is similar to that of urate, uricosuric agents, which increase the excretion of urate, are also likely to increase the excretion of oxipurinol and thus lower the degree of inhibition of xanthine oxidase. The concomitant administration of uricosuric agents and allopurinol tablets has been associated with a decrease in the excretion of oxypurines (hypoxanthine and xanthine) and an increase in urinary uric acid excretion compared with that observed with allopurinol tablets alone. Although clinical evidence to date has not demonstrated renal precipitation of oxypurines in patients either on allopurinol tablets alone or in combination with uricosuric agents, the possibility should be kept in mind.
- The reports that the concomitant use of allopurinol tablets and thiazide diuretics may contribute to the enhancement of allopurinol toxicity in some patients have been reviewed in an attempt to establish a cause-and-effect relationship and a mechanism of causation. Review of these case reports indicates that the patients were mainly receiving thiazide diuretics for hypertension and that tests to rule out decreased renal function secondary to hypertensive nephropathy were not often performed. In those patients in whom renal insufficiency was documented, however, the recommendation to lower the dose of allopurinol tablets was not followed. Although a causal mechanism and a cause-and-effect relationship have not been established, current evidence suggests that renal function should be monitored in patients on thiazide diuretics and allopurinol tablets even in the absence of renal failure, and dosage levels should be even more conservatively adjusted in those patients on such combined therapy if diminished renal function is detected.
- An increase in the frequency of skin rash has been reported among patients receiving ampicillin or amoxicillin concurrently with allopurinol tablets compared to patients who are not receiving both drugs. The cause of the reported association has not been established.
- Enhanced bone marrow suppression by cyclophosphamide and other cytotoxic agents has been reported among patients with neoplastic disease, except leukemia, in the presence of allopurinol tablets. However, in a well-controlled study of patients with lymphoma on combination therapy, allopurinol tablets did not increase the marrow toxicity of patients treated with cyclophosphamide, doxorubicin, bleomycin, procarbazine, and/or mechlorethamine.
- Tolbutamide's conversion to inactive metabolites has been shown to be catalyzed by xanthine oxidase from rat liver. The clinical significance, if any, of these observations is unknown.
- Chlorpropamide's plasma half-life may be prolonged by allopurinol tablets, since allopurinol and chlorpropamide may compete for excretion in the renal tubule. The risk of hypoglycemia secondary to this mechanism may be increased if allopurinol tablets and chlorpropamide are given concomitantly in the presence of renal insufficiency.
- Rare reports indicate that cyclosporine levels may be increased during concomitant treatment with allopurinol tablets. Monitoring of cyclosporine levels and possible adjustment of cyclosporine dosage should be considered when these drugs are coadministered.
Drug/Laboratory Test Interactions
Allopurinol tablets are not known to alter the accuracy of laboratory tests.
Use in Specific Populations
Pregnancy
Pregnancy Category (FDA): C Teratogenic Effects: Pregnancy Category C. Reproductive studies have been performed in rats and rabbits at doses up to twenty times the usual human dose (5 mg/kg per day), and it was concluded that there was no impaired fertility or harm to the fetus due to allopurinol. There is a published report of a study in pregnant mice given 50 or 100 mg/kg allopurinol intraperitoneally on gestation days 10 or 13. There were increased numbers of dead fetuses in dams given 100 mg/kg allopurinol but not in those given 50 mg/kg. There were increased numbers of external malformations in fetuses at both doses of allopurinol on gestation day 10 and increased numbers of skeletal malformations in fetuses at both doses on gestation day 13. It cannot be determined whether this represented a fetal effect or an effect secondary to maternal toxicity. There are, however, no adequate or well-controlled studies in pregnant women. Because animal reproduction studies are not always predictive of human response, this drug should be used during pregnancy only if clearly needed.
Experience with allopurinol tablets during human pregnancy has been limited partly because women of reproductive age rarely require treatment with allopurinol tablets. There are two unpublished reports and one published paper of women giving birth to normal offspring after receiving allopurinol tablets during pregnancy.
Pregnancy Category (AUS):
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Allopurinol (tablet) in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Allopurinol (tablet) during labor and delivery.
Nursing Mothers
Allopurinol and oxipurinol have been found in the milk of a mother who was receiving allopurinol tablets. Since the effect of allopurinol on the nursing infant is unknown, caution should be exercised when allopurinol tablets are administered to a nursing woman.
Pediatric Use
Allopurinol tablets are rarely indicated for use in children with the exception of those with hyperuricemia secondary to malignancy or to certain rare inborn errors of purine metabolism.
Geriatic Use
There is no FDA guidance on the use of Allopurinol (tablet) in geriatric settings.
Gender
There is no FDA guidance on the use of Allopurinol (tablet) with respect to specific gender populations.
Race
There is no FDA guidance on the use of Allopurinol (tablet) with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Allopurinol (tablet) in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Allopurinol (tablet) in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Allopurinol (tablet) in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Allopurinol (tablet) in patients who are immunocompromised.
Administration and Monitoring
Administration
Oral
Monitoring
FDA Package Insert for Allopurinol contains no information regarding Drug Monitoring.
IV Compatibility
There is limited information about the IV Compatibility.
Overdosage
Massive overdosing or acute poisoning by allopurinol tablets has not been reported.
In mice, the 50% lethal dose (LD50) is 160 mg/kg given intraperitoneally (IP) with deaths delayed up to 5 days and 700 mg/kg orally (PO) (approximately 140 times the usual human dose) with deaths delayed up to 3 days. In rats, the acute LD50 is 750 mg/kg IP and 6000 mg/kg PO (approximately 1200 times the human dose).
In the management of overdosage there is no specific antidote for allopurinol tablets. There has been no clinical experience in the management of a patient who has taken massive amounts of allopurinol tablets.
Both allopurinol and oxipurinol are dialyzable; however, the usefulness of hemodialysis or peritoneal dialysis in the management of an overdose of allopurinol tablets is unknown.
Pharmacology
| |
| |
Allopurinol (tablet)
| |
| Systematic (IUPAC) name | |
| 1H-pyrazolo[3,4-d]pyrimidin-4(2H)-one | |
| Identifiers | |
| CAS number | |
| ATC code | M04 |
| PubChem | |
| DrugBank | |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 136.112 g/mol |
| SMILES | & |
| Pharmacokinetic data | |
| Bioavailability | 78±20% |
| Protein binding | Negligible |
| Metabolism | hepatic (80% oxypurinol, 10% allopurinol ribosides) |
| Half life | 2 h (oxypurinol 18-30 h) |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
C(USA) |
| Legal status |
[[Prescription drug|Template:Unicode-only]](US) |
| Routes | tablet (100, 300 mg) |
Mechanism of Action
Allopurinol is a purine analog; it is a structural isomer of hypoxanthine (a naturally occurring purine in the body) and is an inhibitor of the enzyme xanthine oxidase.[6] Xanthine oxidase is responsible for the successive oxidation of hypoxanthine and xanthine, resulting in the production of uric acid, the product of human purine metabolism.[6] In addition to blocking uric acid production, inhibition of xanthine oxidase causes an increase in hypoxanthine and xanthine. While xanthine cannot be converted to purine ribotides, hypoxanthine can be salvaged to the purine ribotides adenosine and guanosine monophosphates. Increased levels of these ribotides may cause feedback inhibition of amidophosphoribosyl transferase, the first and rate-limiting enzyme of purine biosynthesis. Allopurinol, therefore, decreases uric acid formation and may also inhibit purine synthesis.
Structure
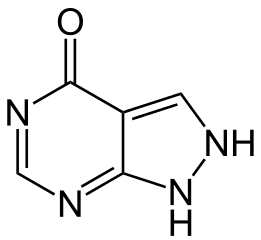
Allopurinol has the following structural formula:

Allopurinol is known chemically as 1, 5-dihydro-4H-pyrazolo [3, 4-d] pyrimidin-4-one. It is a xanthine oxidase inhibitor which is administered orally. Its solubility in water at 37°C is 80.0 mg/dL and is greater in an alkaline solution. Each scored white to off-white tablet contains 100 mg allopurinol and the inactive ingredients lactose monohydrate, corn starch, sodium starch glycolate, povidone and stearic acid. Each scored peach tablet contains 300 mg allopurinol and the inactive ingredients lactose monohydrate, corn starch, sodium starch glycolate , FD & C yellow no. 6, povidone and stearic acid.
Pharmacodynamics
There is limited information regarding Allopurinol (tablet) Pharmacodynamics in the drug label.
Pharmacokinetics
There is limited information regarding Allopurinol (tablet) Pharmacokinetics in the drug label.
Nonclinical Toxicology
There is limited information regarding Allopurinol (tablet) Nonclinical Toxicology in the drug label.
Clinical Studies
FDA Package Insert for Allopurinol contains no information regarding Clinical Studies.
How Supplied
100 mg white to off-white colored, round, beveled edged uncoated tablets with "RG10" embossed on one side and breakline on the other side.
The tablets are supplied in the following package sizes:
Bottles of 100 tablets NDC 63304-539-01
Bottles of 1000 tablets NDC 63304-539-10
300 mg peach colored, round, beveled edged uncoated tablets with "RG11" embossed on one side and breakline on the other side.
The tablets are supplied in the following package sizes:
Bottles of 100 tablets NDC 63304-540-01
Bottles of 500 tablets NDC 63304-540-05
Storage
Store at 20° - 25°C (68° - 77°F)
Images
Drug Images
{{#ask: Page Name::Allopurinol (tablet) |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel




{{#ask: Label Page::Allopurinol (tablet) |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Patients should be informed of the following: (1) They should be cautioned to discontinue allopurinol tablets and to consult their physician immediately at the first sign of a skin rash, painful urination, blood in the urine, irritation of the eyes, or swelling of the lips or mouth. (2) They should be reminded to continue drug therapy prescribed for gouty attacks since optimal benefit of allopurinol tablets may be delayed for 2 to 6 weeks. (3) They should be encouraged to increase fluid intake during therapy to prevent renal stones. (4) If a single dose of allopurinol tablets is occasionally forgotten, there is no need to double the dose at the next scheduled time. (5) There may be certain risks associated with the concomitant use of allopurinol tablets and dicumarol, sulfinpyrazone, mercaptopurine, azathioprine, ampicillin, amoxicillin, and thiazide diuretics, and they should follow the instructions of their physician. (6) Due to the occasional occurrence of drowsiness, patients should take precautions when engaging in activities where alertness is mandatory. (7) Patients may wish to take allopurinol tablets after meals to minimize gastric irritation.
Precautions with Alcohol
Alcohol-Allopurinol interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- Zyloprim[7]
Look-Alike Drug Names
There is limited information about the look-like drug names.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
- ↑ Martinez S, Gonzalez M, Vernaza ME (1997). "Treatment of cutaneous leishmaniasis with allopurinol and stibogluconate". Clin Infect Dis. 24 (2): 165–9. PMID 9114142.
- ↑ Shapiro TA, Were JB, Danso K, Nelson DJ, Desjardins RE, Pamplin CL (1991). "Pharmacokinetics and metabolism of allopurinol riboside". Clin Pharmacol Ther. 49 (5): 506–14. PMID 2029827.
- ↑ Sarma PS, Mandal AK, Khamis HJ (1998). "Allopurinol as an additive to quinine in the treatment of acute complicated falciparum malaria". Am J Trop Med Hyg. 58 (4): 454–7. PMID 9574791.
- ↑ Buie LW, Oertel MD, Cala SO (2006). "Allopurinol as adjuvant therapy in poorly responsive or treatment refractory schizophrenia". Ann Pharmacother. 40 (12): 2200–4. doi:10.1345/aph.1H222. PMID 17119103.
- ↑ Velez I, Agudelo S, Hendrickx E, Puerta J, Grogl M, Modabber F; et al. (1997). "Inefficacy of allopurinol as monotherapy for Colombian cutaneous leishmaniasis. A randomized, controlled trial". Ann Intern Med. 126 (3): 232–6. PMID 9027276.
- ↑ 6.0 6.1
- ↑ "ALLOPURINOL- allopurinol tablet".
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_03780181.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=M;71 |Pill Dosage=300 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=2 |Pill Image= |Drug Author=Mylan Pharmaceuticals Inc. |NDC=03780181
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_05915543.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=DAN;DAN;5543 |Pill Dosage=100 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=2 |Pill Image= |Drug Author=Watson Laboratories, Inc. |NDC=05915543
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_05915544.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=DAN;DAN;5544 |Pill Dosage=300 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=2 |Pill Image= |Drug Author=Watson Laboratories, Inc. |NDC=05915544
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_06032115.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=2083;V |Pill Dosage=300 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=2 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032115
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_06032116.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=2084;V |Pill Dosage=100 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=2 |Pill Image= |Drug Author=Qualitest Pharmaceuticals |NDC=06032116
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_510790206.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=M;71 |Pill Dosage=300 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=2 |Pill Image= |Drug Author=Mylan Institutional Inc. |NDC=510790206
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_534890156.jpg |Drug Name=Allopurinol |Pill Ingred=Allopurinol[Allopurinol]|+sep=; |Pill Imprint=MP;71 |Pill Dosage=100 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=2 |Pill Image= |Drug Author=Mutual Pharmaceutical |NDC=534890156
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_675440371.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=2084;V |Pill Dosage=300 mg |Pill Color=Orange|+sep=; |Pill Shape=Round |Pill Size (mm)=11 |Pill Scoring=2 |Pill Image= |Drug Author=Aphena Pharma Solutions - Tennessee, Inc. |NDC=675440371
}}
{{#subobject:
|Page Name=Allopurinol (tablet) |Pill Name=Allopurinol_NDC_675440988.jpg |Drug Name=Allopurinol |Pill Ingred=ALLOPURINOL[ALLOPURINOL]|+sep=; |Pill Imprint=2083;V |Pill Dosage=100 mg |Pill Color=White|+sep=; |Pill Shape=Round |Pill Size (mm)=10 |Pill Scoring=2 |Pill Image= |Drug Author=Aphena Pharma Solutions - Tennessee, Inc. |NDC=675440988
}}