Raxibacumab: Difference between revisions
No edit summary |
No edit summary |
||
| Line 241: | Line 241: | ||
* Intravenous | * Intravenous | ||
*The recommended dose of raxibacumab is weight-based, given as an intravenous infusion after dilution in a compatible solution to a final volume of 250 mL (adults and children 50 kg or heavier) or to a volume indicated based on the child’s weight (Table 2). Dilute raxibacumab using one of the following compatible solutions: | |||
:*0.9% Sodium Chloride Injection, USP | |||
:*0.45% Sodium Chloride Injection, USP | |||
*Keep vials in their cartons prior to preparation of an infusion solution to protect raxibacumab from light. Raxibacumab vials contain no preservative. | |||
: [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | : [[File:{{PAGENAME}}02.png|thumb|none|600px|This image is provided by the National Library of Medicine.]] | ||
*Preparation: Follow the steps below to prepare the raxibacumab intravenous infusion solution. | |||
:*Calculate the milligrams of raxibacumab injection by multiplying the recommended mg/kg dose in Table 2 by patient weight in kilograms. | |||
:*Calculate the required volume in milliliters of raxibacumab injection needed for the dose by dividing the calculated dose in milligrams (step 1) by the concentration, 50 mg/mL. Each single-use vial allows delivery of 34 mL raxibacumab. | |||
*Based on the total infusion volume selected in Table 2, prepare either a syringe or infusion bag as appropriate following the steps below. | |||
*Syringe Preparation | |||
:*Select an appropriate size syringe for the total volume of infusion to be administered, as described in Table 2. | |||
:*Using the selected syringe, withdraw the volume of raxibacumab as calculated in step 2. | |||
:*Withdraw an appropriate amount of compatible solution to prepare a total volume infusion syringe as specified in Table 2. | |||
:*Gently mix the solution. Do not shake. | |||
:*Discard any unused portion remaining in the raxibacumab vial(s). | |||
:*The prepared solution is stable for 8 hours stored at room temperature. | |||
*Infusion Bag Preparation | |||
:*Select appropriate size bag of compatible solution (see compatible solutions listed in Table 2), withdraw a volume of solution from the bag equal to the calculated volume in milliliters of raxibacumab in Table 2. Discard the solution that was withdrawn from the bag. | |||
:*Withdraw the required volume of raxibacumab injection from the raxibacumab vial(s). | |||
:*Transfer the required volume of raxibacumab injection to the selected infusion bag (step 3). Gently invert the bag to mix the solution. Do not shake. | |||
:*Discard any unused portion remaining in the raxibacumab vial(s). | |||
:*The prepared solution is stable for 8 hours stored at room temperature. | |||
*Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the solution if particulate matter is present or color is abnormal. [See Description (11).] | |||
*Administration: Administer the infusion solution as described in Table 2. The rate of infusion may be slowed or interrupted if the patient develops any signs of adverse reactions, including infusion-associated symptoms. | |||
|monitoring= | |monitoring= | ||
| Line 389: | Line 424: | ||
*Monkey study 2 and rabbit studies 3 and 4 evaluated treatment with raxibacumab alone at an earlier time point after exposure than rabbit study 1. Treatment with raxibacumab alone resulted in a statistically significant dose-dependent improvement in survival relative to placebo when administered at the time of initial manifestations of anthrax disease in the rabbit and monkey infection models (Table 5). Raxibacumab at 40 mg/kg IV single dose was superior to placebo in the rabbit and monkey studies in the all treated and the bacteremic animal analysis populations. All surviving animals developed toxin-neutralizing antibodies. | *Monkey study 2 and rabbit studies 3 and 4 evaluated treatment with raxibacumab alone at an earlier time point after exposure than rabbit study 1. Treatment with raxibacumab alone resulted in a statistically significant dose-dependent improvement in survival relative to placebo when administered at the time of initial manifestations of anthrax disease in the rabbit and monkey infection models (Table 5). Raxibacumab at 40 mg/kg IV single dose was superior to placebo in the rabbit and monkey studies in the all treated and the bacteremic animal analysis populations. All surviving animals developed toxin-neutralizing antibodies. | ||
: [[File:{{PAGENAME}}05.png|thumb|none| | : [[File:{{PAGENAME}}05.png|thumb|none|800px|This image is provided by the National Library of Medicine.]] | ||
*In other animal studies evaluating antibacterial drug alone and raxibacumab-[[antibacterial]] drug combination, the efficacy of an antibacterial drug alone ([[levofloxacin]] in rabbits and [[ciprofloxacin]] in monkeys) was very high (95-100%) when given at the initial manifestations of inhalational anthrax disease. The timing of treatment was similar to that reported for studies 2, 3, and 4 above. | *In other animal studies evaluating antibacterial drug alone and raxibacumab-[[antibacterial]] drug combination, the efficacy of an antibacterial drug alone ([[levofloxacin]] in rabbits and [[ciprofloxacin]] in monkeys) was very high (95-100%) when given at the initial manifestations of inhalational anthrax disease. The timing of treatment was similar to that reported for studies 2, 3, and 4 above. | ||
Revision as of 14:10, 20 February 2015
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Vignesh Ponnusamy, M.B.B.S. [2]
Disclaimer
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Raxibacumab is a monoclonal antibody that is FDA approved for the {{{indicationType}}} of inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs, and for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate. Common adverse reactions include rash, pain in extremity, pruritus, and somnolence.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
Inhalational Anthrax
- Raxibacumab is indicated for the treatment of adult with inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs. Raxibacumab is also indicated for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate.
- Administer raxibacumab as a single dose of 40 mg/kg intravenously over 2 hours and 15 minutes after dilution in 0.9% Sodium Chloride Injection, USP (normal saline) to a final volume of 250 mL. Administer 25 to 50 mg diphenhydramine within 1 hour prior to raxibacumab infusion to reduce the risk of infusion reactions. Diphenhydramine route of administration (oral or IV) should be based on the temporal proximity to the start of raxibacumab infusion.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Raxibacumab in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Raxibacumab in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
Inhalational Anthrax
- Raxibacumab is indicated for the treatment of pediatric patients with inhalational anthrax due to Bacillus anthracis in combination with appropriate antibacterial drugs. Raxibacumab is also indicated for prophylaxis of inhalational anthrax when alternative therapies are not available or are not appropriate.
- The recommended dose for pediatric patients is based on weight as shown in Table 1.
- Premedicate with diphenhydramine within 1 hour prior to raxibacumab infusion. Diphenhydramine route of administration (oral or IV) should be based on the temporal proximity to the start of raxibacumab infusion. Infuse raxibacumab over 2 hours and 15 minutes. No pediatric patients were studied during the development of raxibacumab. The dosing recommendations in Table 1 are derived from simulations designed to match the observed adult exposure to raxibacumab at a 40 mg/kg dose.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Raxibacumab in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Raxibacumab in pediatric patients.
Contraindications
- None.
Warnings
Precautions
- Infusion Reactions
- Infusion-related reactions were reported during administration of raxibacumab in clinical trials including reports of rash, urticaria, and pruritus. If these reactions occur, slow or interrupt raxibacumab infusion and administer appropriate treatment based on severity of the reaction.
- Premedicate with diphenhydramine within 1 hour prior to administering raxibacumab to reduce the risk of infusion reactions.
Adverse Reactions
Clinical Trials Experience
- Because clinical trials are conducted under widely varying conditions, adverse reaction rates observed in the clinical trials of a drug cannot be directly compared with rates in the clinical trials of another drug and may not reflect the rates observed in practice. The safety of raxibacumab has been studied only in healthy volunteers. It has not been studied in patients with inhalational anthrax.
- The safety of raxibacumab has been evaluated in 326 healthy subjects treated with a dose of 40 mg/kg in 3 clinical trials: a drug interaction trial with ciprofloxacin (Study 1), a repeat-dose trial of 20 subjects with the second raxibacumab dose administered ≥4 months after the first dose (Study 2), and a placebo-controlled trial evaluating single doses with a subset of subjects receiving 2 raxibacumab doses 14 days apart (Study 3). Raxibacumab was administered to 86 healthy subjects in Study 1. In Study 3, 240 healthy subjects received raxibacumab (217 received 1 dose and 23 received 2 doses) and 80 subjects received placebo.
- The overall safety of raxibacumab was evaluated as an integrated summary of these 3 clinical trials. Of 326 raxibacumab subjects, 283 received single doses, 23 received 2 doses 14 days apart, and 20 received 2 doses more than 4 months apart. The subjects were 18 to 88 years of age, 53% female, 74% white, 17% black/African American, 6% Asian, and 15% Hispanic.
- Adverse Reactions Leading to Discontinuation of Raxibacumab Infusion
- Four subjects (1.2%) had their infusion of raxibacumab discontinued for adverse reactions: 2 subjects (neither of whom received diphenhydramine premedication) due to urticaria (mild), and 1 subject each discontinued for clonus (mild) and dyspnea (moderate).
- Most Frequently Reported Adverse Reactions
- The most frequently reported adverse reactions were rash, pain in extremity, pruritus, and somnolence.
- Rashes
- For all subjects exposed to raxibacumab in clinical trials, the rate of rash was 2.8% (9/326) compared with 1.3% (1/80) of placebo subjects. Mild to moderate infusion-related rashes were reported in 22.2% (6/27) of subjects who did not receive diphenhydramine premedication compared to 3.3% (2/61) of subjects who were premedicated with diphenhydramine in the ciprofloxacin/raxibacumab combination trial (Study 1). In the placebo-controlled raxibacumab study where all subjects received diphenhydramine (Study 3), the rate of rash was 2.5% in both placebo- and raxibacumab-treated subjects.
- Less Common Adverse Reactions
- Clinically significant adverse reactions that were reported in <1.5% of subjects exposed to raxibacumab and at rates higher than in placebo subjects are listed below:
Blood and lymphatic system
Anemia, leukopenia, lymphadenopathy
Cardiac disorders
Ear and labyrinth
General disorders and administration site
Fatigue, infusion site pain, peripheral edema
Investigations
Blood amylase increased, blood creatine phosphokinase increased, prothrombin time prolonged
Musculoskeletal and connective tissue
Nervous system
Syncope vasovagal
Psychiatric
Vascular
- Immunogenicity
- The development of anti-raxibacumab antibodies was evaluated in all subjects receiving single and double doses of raxibacumab in Studies 1, 2, and 3. Immunogenic responses against raxibacumab were not detected in any raxibacumab-treated human subjects following single or repeat doses of raxibacumab.
- The incidence of antibody formation is highly dependent on the sensitivity and specificity of the immunogenicity assay. Additionally, the observed incidence of any antibody positivity in an assay is highly dependent on several factors, including assay sensitivity and specificity, assay methodology, sample handling, timing of sample collection, concomitant medications, and underlying disease. For these reasons, comparison of the incidence of antibodies to raxibacumab with the incidence of antibodies to other products may be misleading.
Postmarketing Experience
There is limited information regarding Postmarketing Experience of Raxibacumab in the drug label.
Drug Interactions
- Ciprofloxacin
- Co-administration of 40 mg/kg raxibacumab IV with IV or oral ciprofloxacin in human subjects did not alter the PK of either ciprofloxacin or raxibacumab.
Use in Specific Populations
Pregnancy
- Pregnancy Category B
- A single embryonic-fetal development study was conducted in pregnant, healthy New Zealand White rabbits administered 2 intravenous doses of raxibacumab up to 120 mg/kg (3 times the human dose on a mg/kg basis) on gestation days 7 and 14. No evidence of harm to the pregnant dam or the fetuses due to raxibacumab was observed. Cmax values in rabbits after dosing with 120 mg/kg were 3,629 mcg/mL and 4,337 mcg/mL after the first and second dose of raxibacumab, respectively; these are more than 3 and 4 times the mean Cmax values in humans. Estimates of exposure (AUC) were not generated in the embryo-fetal rabbit study. No adequate and well-controlled studies in pregnant women were conducted. Because animal reproduction studies are not always predictive of human response, raxibacumab should be used during pregnancy only if clearly needed.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Raxibacumab in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Raxibacumab during labor and delivery.
Nursing Mothers
- Raxibacumab has not been evaluated in nursing women. Although human immunoglobulins are excreted in human milk, published data suggest that neonatal consumption of human milk does not result in substantial absorption of these maternal immunoglobulins into circulation. Inform a nursing woman that the effects of local gastrointestinal and systemic exposure to raxibacumab on a nursing infant are unknown.
Pediatric Use
- As in adults, the effectiveness of raxibacumab in pediatric patients is based solely on efficacy studies in animal models of inhalational anthrax. As exposure of healthy children to raxibacumab is not ethical, a population PK approach was used to derive dosing regimens that are predicted to provide pediatric patients with exposure comparable to the observed exposure in adults receiving 40 mg/kg. The dose for pediatric patients is based on weight.
- Safety or PK of raxibacumab have not been studied in the pediatric population.
Geriatic Use
- Clinical trials of raxibacumab did not include sufficient numbers of subjects aged 65 years and older to determine whether they respond differently from younger subjects. Of the total number of subjects in clinical trials of raxibacumab, 6.4% (21/326) were 65 years and older, while 1.5% (5/326) were 75 years and older. However, no alteration of dosing is needed for patients ≥65 years of age.
Gender
There is no FDA guidance on the use of Raxibacumab with respect to specific gender populations.
Race
There is no FDA guidance on the use of Raxibacumab with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Raxibacumab in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Raxibacumab in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Raxibacumab in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Raxibacumab in patients who are immunocompromised.
Administration and Monitoring
Administration
- Intravenous
- The recommended dose of raxibacumab is weight-based, given as an intravenous infusion after dilution in a compatible solution to a final volume of 250 mL (adults and children 50 kg or heavier) or to a volume indicated based on the child’s weight (Table 2). Dilute raxibacumab using one of the following compatible solutions:
- 0.9% Sodium Chloride Injection, USP
- 0.45% Sodium Chloride Injection, USP
- Keep vials in their cartons prior to preparation of an infusion solution to protect raxibacumab from light. Raxibacumab vials contain no preservative.
- Preparation: Follow the steps below to prepare the raxibacumab intravenous infusion solution.
- Calculate the milligrams of raxibacumab injection by multiplying the recommended mg/kg dose in Table 2 by patient weight in kilograms.
- Calculate the required volume in milliliters of raxibacumab injection needed for the dose by dividing the calculated dose in milligrams (step 1) by the concentration, 50 mg/mL. Each single-use vial allows delivery of 34 mL raxibacumab.
- Based on the total infusion volume selected in Table 2, prepare either a syringe or infusion bag as appropriate following the steps below.
- Syringe Preparation
- Select an appropriate size syringe for the total volume of infusion to be administered, as described in Table 2.
- Using the selected syringe, withdraw the volume of raxibacumab as calculated in step 2.
- Withdraw an appropriate amount of compatible solution to prepare a total volume infusion syringe as specified in Table 2.
- Gently mix the solution. Do not shake.
- Discard any unused portion remaining in the raxibacumab vial(s).
- The prepared solution is stable for 8 hours stored at room temperature.
- Infusion Bag Preparation
- Select appropriate size bag of compatible solution (see compatible solutions listed in Table 2), withdraw a volume of solution from the bag equal to the calculated volume in milliliters of raxibacumab in Table 2. Discard the solution that was withdrawn from the bag.
- Withdraw the required volume of raxibacumab injection from the raxibacumab vial(s).
- Transfer the required volume of raxibacumab injection to the selected infusion bag (step 3). Gently invert the bag to mix the solution. Do not shake.
- Discard any unused portion remaining in the raxibacumab vial(s).
- The prepared solution is stable for 8 hours stored at room temperature.
- Parenteral drug products should be inspected visually for particulate matter and discoloration prior to administration, whenever solution and container permit. Discard the solution if particulate matter is present or color is abnormal. [See Description (11).]
- Administration: Administer the infusion solution as described in Table 2. The rate of infusion may be slowed or interrupted if the patient develops any signs of adverse reactions, including infusion-associated symptoms.
Monitoring
There is limited information regarding Monitoring of Raxibacumab in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Raxibacumab in the drug label.
Overdosage
Chronic Overdose
There is limited information regarding Chronic Overdose of Raxibacumab in the drug label.
Pharmacology
Raxibacumab?
| |
| Therapeutic monoclonal antibody | |
| Source | u |
| Target | Protective antigen of anthrax toxin |
| Identifiers | |
| CAS number | |
| ATC code | J06 |
| PubChem | ? |
| Chemical data | |
| Formula | Template:OrganicBox atomTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox atomTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBoxTemplate:OrganicBox |
| Mol. mass | 142.93 kDa |
| Pharmacokinetic data | |
| Bioavailability | ? |
| Metabolism | ? |
| Half life | ? |
| Excretion | ? |
| Therapeutic considerations | |
| Pregnancy cat. |
? |
| Legal status | |
| Routes | ? |
Mechanism of Action
- Raxibacumab is a monoclonal antibody that binds free PA with an affinity equilibrium dissociation constant (Kd) of 2.78 ± 0.9 nM. Raxibacumab inhibits the binding of PA to its cellular receptors, preventing the intracellular entry of the anthrax lethal factor and edema factor, the enzymatic toxin components responsible for the pathogenic effects of anthrax toxin.
Structure
- Raxibacumab is a human IgG1λ monoclonal antibody that binds the PA component of B. anthracis toxin. Raxibacumab has a molecular weight of approximately 146 kilodaltons. Raxibacumab is produced by recombinant DNA technology in a murine cell expression system.
- Raxibacumab is supplied as a sterile, liquid formulation in single-dose vials for intravenous infusion. Each vial contains 50 mg/mL raxibacumab in citric acid (0.13 mg/mL), glycine (18 mg/mL), polysorbate 80 [0.2 mg/mL (w/v)], sodium citrate (2.8 mg/mL), and sucrose (10 mg/mL), with a pH of 6.5. Each vial contains a minimum of 35.1 mL filled into a 50 mL vial (to allow delivery of 1,700 mg/34 mL). Raxibacumab is a clear to opalescent, colorless to pale yellow, liquid.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Raxibacumab in the drug label.
Pharmacokinetics
- The PK of raxibacumab are linear over the dose range of 1 to 40 mg/kg following single IV dosing in humans; raxibacumab was not tested at doses higher than 40 mg/kg in humans. Following single IV administration of raxibacumab 40 mg/kg in healthy, male and female human subjects, the mean Cmax and AUCinf were 1,020.3 ± 140.6 mcg/mL and 15,845.8 ± 4,333.5 mcg•day/mL, respectively. Mean raxibacumab steady-state volume of distribution was greater than plasma volume, suggesting some tissue distribution. Clearance values were much smaller than the glomerular filtration rate indicating that there is virtually no renal clearance of raxibacumab.
- Because the effectiveness of raxibacumab cannot be tested in humans, a comparison of raxibacumab exposures achieved in healthy human subjects to those observed in animal models of inhalational anthrax in therapeutic efficacy studies is necessary to support the dosage regimen of 40 mg/kg IV as a single dose for the treatment of inhalational anthrax in humans. Humans achieve similar or greater systemic exposure (Cmax and AUCinf) to raxibacumab following a single 40 mg/kg IV dose compared with New Zealand White rabbits and cynomolgus macaques receiving the same dosage regimen.
- Effects of Gender, Age, and Race
- Raxibacumab PK were evaluated via a population PK analysis using serum samples from 322 healthy subjects who received a single 40 mg/kg IV dose across 3 clinical trials. Based on this analysis, gender (female versus male), race (non-white versus white), or age (elderly versus young) had no meaningful effects on the PK parameters for raxibacumab.
- Raxibacumab PK have not been evaluated in children.
- Repeat Dosing
- Although raxibacumab is intended for single dose administration, the PK of raxibacumab following a second administration of 40 mg/kg IV given 14 days after the first 40 mg/kg IV dose was assessed in 23 healthy subjects (Study 3). The mean raxibacumab concentration at 28 days after the second dose was approximately twice the mean raxibacumab concentration at 14 days following the first dose. In the human trial assessing the immunogenicity of raxibacumab (Study 2), 20 healthy subjects who had initially received a single dose of raxibacumab 40 mg/kg IV received a second 40 mg/kg IV dose at ≥4 months following their first dose. No statistically significant differences in mean estimates of AUCinf, CL, or half-life of raxibacumab between the 2 doses administered ≥4 months apart were observed. The mean Cmax following the second dose was 15% lower than the Cmax following the first dose.
- Ciprofloxacin Interaction Trial
- In an open-label trial evaluating the effect of raxibacumab on ciprofloxacin PK in healthy adult male and female subjects (Study 1), the administration of 40 mg/kg raxibacumab IV following ciprofloxacin IV infusion or ciprofloxacin oral tablet ingestion did not alter the PK of ciprofloxacin administered orally and/or intravenously. Likewise, ciprofloxacin did not alter the PK of raxibacumab.
Nonclinical Toxicology
Carcinogenesis, Mutagenesis, Impairment of Fertility
- Carcinogenicity, genotoxicity, and fertility studies have not been conducted with raxibacumab.
Animal Toxicology
- Healthy cynomolgus macaques administered 3 intravenous doses or 3 subcutaneous doses of 40 mg/kg raxibacumab once every 12 days, or a single intramuscular dose (40 mg/kg) of raxibacumab, showed no adverse effects, including no effects up to 120 days post-dosing.
- Studies with raxibacumab in rabbit, cynomolgus macaque, and human donor tissues showed no cross reactivity with brain.
- Anthrax-infected rabbits and monkeys administered an intravenous injection of raxibacumab (40 mg/kg) at time of PA toxemia reproducibly showed greater severity of central nervous system (CNS) lesions (bacteria, inflammation, hemorrhage, and necrosis) in non-surviving animals compared to dead placebo control animals, with no difference in mean time to death from spore challenge. The raxibacumab monoclonal antibody appears unable to penetrate the CNS until compromise of the blood-brain barrier (BBB) during the later stages of anthrax infection. The most severe brain lesions in rabbits were associated with bacteria and raxibacumab tissue binding in a similar pattern as endogenous IgG antibody that leaked across the compromised BBB. No dose/exposure-response relationship for brain histopathology was identified. Surviving rabbits and monkeys at the end of the 28-day studies showed no microscopic evidence of CNS lesions. CNS toxicity was not observed in healthy monkeys administered raxibacumab (40 mg/kg) or in GLP combination treatment studies with antibacterials in rabbits (levofloxacin) or in monkeys (ciprofloxacin) at any time.
Clinical Studies
- Because it is not feasible or ethical to conduct controlled clinical trials in humans with inhalational anthrax, the effectiveness of raxibacumab for therapeutic treatment of inhalational anthrax is based on efficacy studies in rabbits and monkeys. Raxibacumab effectiveness has not been studied in humans. Because the animal efficacy studies are conducted under widely varying conditions, the survival rates observed in the animal studies cannot be directly compared between studies and may not reflect the rates observed in clinical practice.
- The efficacy of raxibacumab for treatment of inhalational anthrax was studied in a monkey model (study 2) and a rabbit model (studies 3 and 4) of inhalational anthrax disease. These 3 studies tested raxibacumab efficacy compared to placebo. Another study in a rabbit model (study 1) evaluated the efficacy of raxibacumab in combination with an antibacterial drug relative to the antibacterial drug alone. Studies were randomized and blinded.
- The animals were challenged with aerosolized B. anthracis spores (Ames strain) at 200xLD50 to achieve 100% mortality if untreated. In rabbit study 1, treatment was delayed until 84 hours after spore challenge. In monkey study 2, study treatment commenced at the time of a positive serum electrochemiluminescence (ECL) assay for B. anthracis PA. The mean time between spore challenge and initiation of study treatment was 42 hours. In rabbit studies 3 and 4, sustained elevation of body temperature above baseline for 2 hours or a positive result on serum ECL assay for PA served as the trigger for initiation of study treatment. The mean time between spore challenge and initiation of study treatment was 28 hours post-exposure. Efficacy in all therapeutic studies in animals was determined based on survival at the end of the study. Most study animals (88% to 100%) were bacteremic and had a positive ECL assay for PA prior to treatment in all 4 studies.
Treatment of Inhalational Anthrax in Combination With Antibacterial Drug
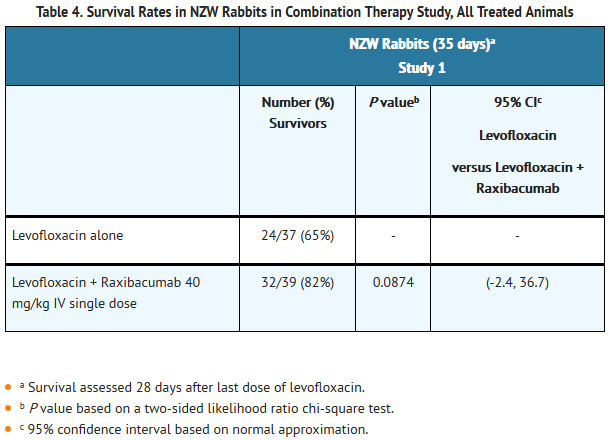
- The efficacy of raxibacumab administered with levofloxacin as treatment of animals with systemic anthrax disease (84 hours after spore challenge) was evaluated in New Zealand White rabbits (study 1). The dose of levofloxacin was chosen to yield a comparable exposure to that achieved by the recommended doses in humans. Levofloxacin and raxibacumab PK in this study were unaffected by product co-administration. Forty-two percent of challenged animals survived to treatment. Treatment with antibacterial drug plus raxibacumab resulted in 82% survival compared to 65% survival in rabbits treated with antibacterial drug alone, P = 0.0874 (Table 4).
Post-exposure Prophylaxis/Early Treatment of Inhalational Anthrax
- Monkey study 2 and rabbit studies 3 and 4 evaluated treatment with raxibacumab alone at an earlier time point after exposure than rabbit study 1. Treatment with raxibacumab alone resulted in a statistically significant dose-dependent improvement in survival relative to placebo when administered at the time of initial manifestations of anthrax disease in the rabbit and monkey infection models (Table 5). Raxibacumab at 40 mg/kg IV single dose was superior to placebo in the rabbit and monkey studies in the all treated and the bacteremic animal analysis populations. All surviving animals developed toxin-neutralizing antibodies.
- In other animal studies evaluating antibacterial drug alone and raxibacumab-antibacterial drug combination, the efficacy of an antibacterial drug alone (levofloxacin in rabbits and ciprofloxacin in monkeys) was very high (95-100%) when given at the initial manifestations of inhalational anthrax disease. The timing of treatment was similar to that reported for studies 2, 3, and 4 above.
- In another study, rabbits were exposed to 100xLD50 B. anthracis spores and administered raxibacumab at a single dose of 40 mg/kg at the time of exposure, 12 hours, 24 hours, or 36 hours after exposure. Survival was 12/12 (100%) in animals treated at time of exposure or 12 hours, but decreased to 6/12 (50%) and 5/12 (42%) at 24 hours and 36 hours, respectively.
How Supplied
- Raxibacumab is supplied in single-use vials containing 1,700 mg/34 mL (50 mg/mL) raxibacumab injection and is available in the following packaging configuration:
- Single Unit Carton: Contains one (1) single-use vial of raxibacumab 1,700 mg/34 mL (deliverable) (NDC 49401-103-01).
- Raxibacumab must be refrigerated at 2° to 8°C (36° to 46°F). DO NOT FREEZE. Protect the vial from exposure to light, prior to use. Brief exposure to light, as with normal use, is acceptable. Store vial in original carton until time of use.
Storage
There is limited information regarding Raxibacumab Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Raxibacumab |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Raxibacumab |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
- Efficacy Based on Animal Models: Inform patients that the efficacy of raxibacumab is based solely on efficacy studies demonstrating a survival benefit in animals and that the effectiveness of raxibacumab has not been tested in humans with anthrax. The safety of raxibacumab has been tested in healthy adults, but no safety data are available in children or pregnant women. Limited data are available in geriatric patients.
- Pregnancy and Nursing Mothers: Inform patients that raxibacumab has not been studied in pregnant women or nursing mothers so the effects of raxibacumab on pregnant women or nursing infants are not known. Instruct patients to tell their healthcare professional if they are pregnant, become pregnant, or are thinking about becoming pregnant. Instruct patients to tell their healthcare professional if they plan to breastfeed their infant.
- Infusion Reactions: Infusion-related reactions were reported during administration of raxibacumab in clinical trials, including reports of rash, urticaria, and pruritus.
- Prophylactic administration of diphenhydramine is recommended within 1 hour prior to administering raxibacumab. Diphenhydramine route of administration (oral or IV) should be based on the temporal proximity to the start of raxibacumab infusion.
Precautions with Alcohol
- Alcohol-Raxibacumab interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- RAXIBACUMAB®[1]
Look-Alike Drug Names
There is limited information regarding Raxibacumab Look-Alike Drug Names in the drug label.
Drug Shortage Status
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Page Name=Raxibacumab |Pill Name=No image.jpg |Drug Name= |Pill Ingred=|+sep=; |Pill Imprint= |Pill Dosage= |Pill Color=|+sep=; |Pill Shape= |Pill Size (mm)= |Pill Scoring= |Pill Image= |Drug Author= |NDC=
}}
{{#subobject:
|Label Page=Raxibacumab |Label Name=Raxibacumab07.png
}}
{{#subobject:
|Label Page=Raxibacumab |Label Name=Raxibacumab08.png
}}
{{#subobject:
|Label Page=Raxibacumab |Label Name=Raxibacumab09.png
}}