Progesterone (oral)
 | |
 | |
| Clinical data | |
|---|---|
| Synonyms | 4-pregnene-3,20-dione |
| Pregnancy category |
|
| Routes of administration | oral, implant |
| ATC code | |
| Pharmacokinetic data | |
| Bioavailability | prolonged absorption, half-life approx 25-50 hours |
| Protein binding | 96%-99% |
| Metabolism | hepatic to pregnanediols and pregnanolones |
| Elimination half-life | 34.8-55.13 hours |
| Excretion | renal |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| DrugBank | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C21H30O2 |
| Molar mass | 314.47 |
| Specific rotation | [α]D |
| Melting point | 126 °C (258.8 °F) |
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Overview
Progesterone is a C-21 steroid hormone involved in the female menstrual cycle, pregnancy (supports gestation) and embryogenesis of humans and other species. Progesterone belongs to a class of hormones called progestogens, and is the major naturally occurring human progestogen.
Progesterone should not be confused with progestins, which are synthetically produced progestogens.
Chemistry
Progesterone was independently discovered by four research groups.[1][2][3][4]
Willard Myron Allen who co-discovered Progesterone with his anatomy professor George Washington Corner at the University of Rochester Medical School in 1933. Allen first determined its melting point, molecular weight, and partial molecular structure. He also gave it the name PROGESTERONE derived from PROGEstational STERoidal ketONE.[5] An avid mathematician, Allen recognized that the molecular weight of progesterone is 100 x π = 314 dalt.
Like other steroids, progesterone consists of four interconnected cyclic hydrocarbons. Progesterone contains ketone and oxygenated functional groups, as well as two methyl branches. Like all steroid hormones, it is hydrophobic.
Synthesis

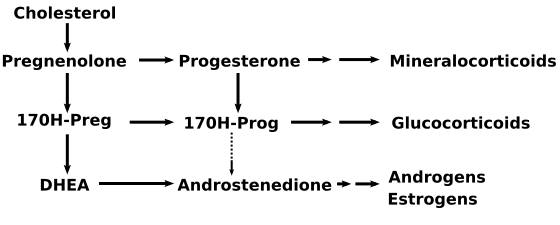
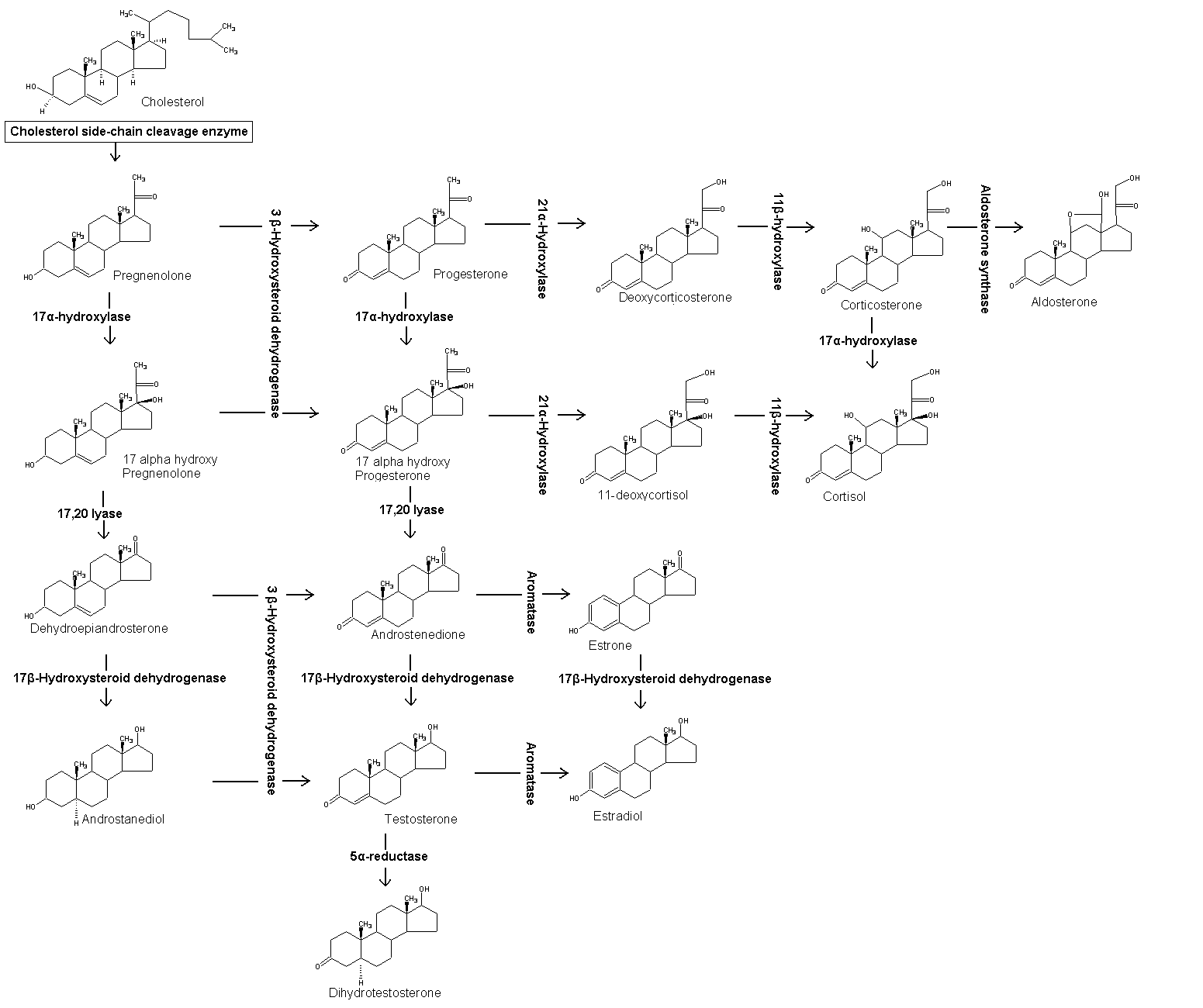
Progesterone, like all other steroid hormones, is synthesized from pregnenolone, a derivative of cholesterol. This conversion takes place in two steps. The 3-hydroxyl group is converted to a keto group and the double bond is moved to C-4, from C-5.
Progesterone is the precursor of the mineralocorticoid aldosterone, and after conversion to 17-hydroxyprogesterone (another natural progestogen) of cortisol and androstenedione. Androstenedione can be converted to testosterone, estrone and estradiol.
Sources
Progesterone is produced in the adrenal glands, the gonads (specifically after ovulation in the corpus luteum), the brain, and, during pregnancy, in the placenta.
In humans, increasing amounts of progesterone are produced during pregnancy:
- Initially, the source is the corpus luteum that has been "rescued" by the presence of human chorionic gonadotropins (hCG) from the conceptus.
- However, after the 8th week production of progesterone shifts over to the placenta. The placenta utilizes maternal cholesterol as the initial substrate, and most of the produced progesterone enters the maternal circulation, but some is picked up by the fetal circulation and is used as substrate for fetal corticosteroids. At term the placenta produces about 250 mg progesterone per day.
Levels
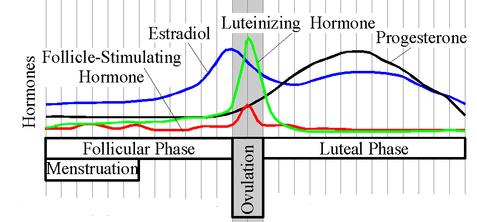
In women, progesterone levels are relatively low during the preovulatory phase of the menstrual cycle, rise after ovulation, and are elevated during the luteal phase. In women progesterone levels tend to be < 2 ng/ml prior to ovulation, and > 5 ng/ml after ovulation. If pregnancy occurs, progesterone levels are maintained at luteal levels initially. With the onset of the luteal-placental shift in progesterone support of the pregnancy levels start to rise further and may reach 100-200 ng/ml at term. Whether a decrease in progesterone levels is critical for the initiation of labor has been argued and may be species-specific. After delivery of the placenta and during lactation, progesterone levels are very low.
Progesterone levels are relatively low in children and postmenopausal women.[6] Adult males have levels similar to those in women during the follicular phase of the menstrual cycle.
Effects
Progesterone exerts its action primarily through the intracellular progesterone receptor though a distinct, membrane bound progesterone receptor which has recently been discovered. It has a number of physiological effects, often regulatory, especially of the effects of estrogen. Estrogen often induces a multiplication of progesterone receptors.
Reproductive system
Progesterone is sometimes called the "hormone of pregnancy"[7], and it has many roles relating to the development of the fetus:
- Progesterone converts the endometrium to its secretory stage to prepare the uterus for implantation. At the same time progesterone affects the vaginal epithelium and cervical mucus. If pregnancy does not occur, progesterone levels will decrease, leading, in the human, to menstruation. Normal menstrual bleeding is progesterone withdrawal bleeding.
- During implantation and gestation, progesterone appears to decrease the maternal immune response to allow for the acceptance of the pregnancy.
- Progesterone decreases contractility of the uterine smooth muscle. [7]
- In addition progesterone inhibits lactation during pregnancy. The fall in progesterone levels following delivery is one of the triggers for milk production.
- A drop in progesterone levels is possibly one step that facilitates the onset of labor.
The fetus metabolizes placental progesterone in the production of adrenal mineralo- and glucosteroids.
Nervous system
Progesterone, like pregnenolone and dehydroepiandrosterone, belongs to the group of neurosteroids that are found in high concentrations in certain areas in the brain and are synthesized there.
Neurosteroids affect synaptic functioning, are neuroprotective, and affect myelinization.[8] They are investigated for their potential to improve memory and cognitive ability.
Progesterone as a neuroprotectant affects regulation of apoptotic genes.
Its effect as a neurosteroid works predominantly through the GSK-3 beta pathway, as an inhibitor. (Other GSK-3 beta inhibitors include bipolar mood stabilizers, lithium and valproic acid.)
Other systems
- It raises epidermal growth factor-1 levels, a factor often used to induce proliferation, and used to sustain cultures, of stem cells.
- It increases core temperature (thermogenic function) during ovulation.[9]
- It reduces spasm and relaxes smooth muscle. Bronchi are widened and mucus regulated. (Progesterone receptors are widely present in submucosal tissue.)
- It acts as an antiinflammatory agent and regulates the immune response.
- It reduces gall-bladder activity.[10]
- It normalizes blood clotting and vascular tone, zinc and copper levels, cell oxygen levels, and use of fat stores for energy.
- It assists in thyroid function, in bone building by osteoblasts, in bone, teeth, gums, joint, tendon, ligament and skin resilience and in some cases healing by regulating various types of collagen, and in nerve function and healing by regulating myelin.
- It appears to prevent endometrial cancer (involving the uterine lining) by regulating the effects of estrogen.
Medical applications
The use of progesterone and its analogues have many medical applications -- both to address acute situations, and to address the long-term decline of natural progesterone levels. Because of the poor bioavailability of progesterone when taken orally, many synthetic progestins have been designed. However, the roles of progesterone may not be fulfilled by the synthetic progestins which in some cases were designed solely to mimic progesterone's uterine effects.
Bioavailability
Progesterone is poorly absorbed by oral ingestion unless micronised and in oil, or with fatty foods; it does not dissolve in water. Products such as Prometrium, Utrogestan, Minagest and Microgest are therefore capsules containing micronised progesterone in oil - in all three mentioned the oil is peanut oil, which may cause serious allergic reactions in some people, but compounding pharmacies, which have the facilities and licenses to make their own products, can use alternatives. Vaginal and rectal application is also effective, with products such as CRINONE and PROCHIEVE bioadhesive progesterone vaginal gels (the only progesterone products FDA-approved for use in infertility and during pregnancy) and Cycff, which is progesterone in cocoa butter in the form of pessaries. Progesterone can be given by injection, but because it has a short half-life they need to be daily. Implants, for a longer period, are also available. Marketing of progesterone phamaceutical products, country to country, varies considerably, with many countries having no oral progesterone products marketed, but they can usually be specially imported by pharmacies through international wholesalers.
"Natural progesterone" products derived from yams, do not require a prescription. Wild yams contain a plant steroid called diosgenin, which the human body cannot metabolize into progesterone. Diosgenin can only be chemically processed into progesterone in labs.
Specific uses
- Progesterone is used to control anovulatory bleeding. It is also used to prepare uterine lining in infertility therapy and to support early pregnancy. Patients with recurrent pregnancy loss due to inadequate progesterone production may receive progesterone.
- Progesterone is being investigated as potentially beneficial in treating multiple sclerosis, since the characteristic deterioration of nerve myelin insulation halts during pregnancy, when progesterone levels are raised; deterioration commences again when the levels drop.
- Progesterone is used in hormone therapy for transsexual women, and some intersex women - especially when synthetic progestins have been ineffective or caused side-effects - since normal breast tissue cannot develop except in the presence of both progestogen and estrogen. Mammary glandular tissue is otherwise fibrotic, the breast shape conical and the areola immature. Progesterone can correct those even after years of inadequate hormonal treatment. Research usually cited against such value was conducted using Provera, a synthetic progestin. Progesterone also has a role in skin elasticity and bone strength, in respiration, in nerve tissue and in female sexuality, and the presence of progesterone receptors in certain muscle and fat tissue may hint at a role in sexually-dimorphic proportions of those.
- Progesterone receptor antagonists, or selective progesterone receptor modulators (SPRM)s, such as RU-486 (Mifepristone), can be used to prevent conception or induce medical abortions.
Note that methods of hormonal contraception do not contain progesterone but a progestin.
Progesterone may affect male behavior: 'Progesterone receptors mediate male aggression toward infants' PNAS 2003 100: 2951-2956; 10.1073/pnas.0130100100
Lawn chemicals and no-till agricultual practices may disturb both estrogen and progesterone metabolism. [refs required]
Aging
Since most progesterone in males is created during testicular production of testosterone, and most in females by the ovaries, the shutting down (whether by natural or chemical means), or removal, of those inevitably causes a considerable reduction in progesterone levels. Previous concentration upon the role of progestagens (progesterone and molecules with similar effects) in female reproduction, when progesterone was simply considered a "female hormone", obscured the significance of progesterone elsewhere in both sexes.
The tendency for progesterone to have a regulatory effect, the presence of progesterone receptors in many types of body tissue, and the pattern of deterioration (or tumor formation) in many of those increasing in later years when progesterone levels have dropped, is prompting widespread research into the potential value of maintaining progesterone levels in both males and females.
Brain Damage
Under investigation is the potential for progesterone therapy given within 24 hours of a brain injury to minimize brain damage. Evetually, this could become a standard treatment for the many people suffering traumatic brain injuries annually. This research has been pioneered by Dr. Donald G. Stein at Emory University. Human trials are underway and have shown similar results to the studies he performed years ago where female rats subjected to induced brain injuries recovered with much less permanent damage than males with much lower progesterone levels.
See also
References
- ↑ Allen WM (1935). "The isolation of crystalline progestin". Science. 82 (2118): 89–93. doi:10.1126/science.82.2118.89. PMID 17747122.
- ↑ Butenandt A, Westphal U (1934). "Zur Isolierung und Charakterisierung des Corpusluteum-Hormons". Berichte Deutsche chemische Gesellschaft. 67: 1440–1442.
- ↑ Hartmann M, Wettstein A (1934). "Ein krystallisiertes Hormon aus Corpus luteum". Helvetica Chimica Acta. 17: 878–882.
- ↑ Slotta KH, Ruschig H, Fels E (1934). "Reindarstellung der Hormone aus dem Corpusluteum". Berichte Deutsche chemische Gesellschaft. 67: 1270–1273.
- ↑ Allen WM (1970). "Progesterone: how did the name originate?". South. Med. J. 63 (10): 1151–5. PMID 4922128.
- ↑ http://cclnprod.cc.nih.gov/dlm/testguide.nsf/Index/CB26894E1EB28DEF85256BA5005B000E?OpenDocument
- ↑ 7.0 7.1 http://www.vivo.colostate.edu/hbooks/pathphys/reprod/placenta/endocrine.html
- ↑ Schumacher M, Guennoun R, Robert F, et al. Local synthesis and dual actions of progesterone in the nervous system: neuroprotection and myelination. Growth Horm IGF Res. 2004 Jun;14 Suppl A:S18-33. PMID 15135772
- ↑ Essentials of Human Physiology by Thomas M. Nosek. Section 5/5ch9/s5ch9_13.
- ↑ Hould F, Fried G, Fazekas A, Tremblay S, Mersereau W (1988). "Progesterone receptors regulate gallbladder motility". J Surg Res. 45 (6): 505–12. PMID 3184927.
Additional images
Template:Sex hormones Template:SIB
de:Progesteron it:Progesterone he:פרוגסטרון lt:Progesteronas nl:Progesteron no:Progesteron fi:Keltarauhashormoni sv:Progesteron
- Pages with script errors
- CS1 maint: Multiple names: authors list
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without ChemSpiderID
- Articles without KEGG source
- Articles without InChI source
- Articles without UNII source
- Drugs with no legal status
- Articles containing unverified chemical infoboxes
- Obstetrics
- Gynecology
- Drugs
- Endocrinology
- Progestagens
- Neurosteroids