Prednylidene: Difference between revisions
Jump to navigation
Jump to search
m (Protected "Prednylidene": Protecting pages from unwanted edits ([edit=sysop] (indefinite) [move=sysop] (indefinite))) |
No edit summary |
||
| Line 1: | Line 1: | ||
{{ | {{Drugbox | ||
| IUPAC_name = | | Verifiedfields = changed | ||
| image = Prednylidene. | | verifiedrevid = 464213580 | ||
| IUPAC_name = (11β)-11,17,21-trihydroxy-16-methylenepregna-1,4-diene-3,20-dione | |||
| image = Prednylidene.png | |||
<!--Clinical data--> | |||
| tradename = | |||
| pregnancy_AU = <!-- A / B1 / B2 / B3 / C / D / X --> | |||
| pregnancy_US = <!-- A / B / C / D / X --> | |||
| pregnancy_category = | |||
| legal_AU = <!-- Unscheduled / S2 / S3 / S4 / S8 --> | |||
| legal_UK = <!-- GSL / P / POM / CD --> | |||
| legal_US = <!-- OTC / Rx-only --> | |||
| legal_status = | |||
| routes_of_administration = | |||
<!--Pharmacokinetic data--> | |||
| bioavailability = | |||
| protein_bound = | |||
| metabolism = | |||
| elimination_half-life = | |||
| excretion = | |||
<!--Identifiers--> | |||
| CAS_number_Ref = {{cascite|changed|??}} | |||
| CAS_number = 599-33-7 | | CAS_number = 599-33-7 | ||
| ATC_prefix = H02 | | ATC_prefix = H02 | ||
| ATC_suffix = AB11 | | ATC_suffix = AB11 | ||
| PubChem = | | PubChem = 20055008 | ||
| DrugBank = | | DrugBank_Ref = {{drugbankcite|correct|drugbank}} | ||
| C=22 | H=28 | | | DrugBank = | ||
| ChemSpiderID_Ref = {{chemspidercite|correct|chemspider}} | |||
| ChemSpiderID = 16735979 | |||
| UNII_Ref = {{fdacite|correct|FDA}} | |||
| UNII = IF8PQP966U | |||
<!--Chemical data--> | |||
| C=22 | H=28 | O=5 | |||
| molecular_weight = 372.455 | | molecular_weight = 372.455 | ||
| | | smiles = OCC(=O)[C@@]2(O)C(=C)C[C@H]3[C@@H]4CC\C1=C\C(=O)\C=C/[C@]1(C)[C@H]4[C@@H](O)C[C@]23C | ||
| InChI = 1/C22H28O5/c1-12-8-16-15-5-4-13-9-14(24)6-7-20(13,2)19(15)17(25)10-21(16,3)22(12,27)18(26)11-23/h6-7,9,15-17,19,23,25,27H,1,4-5,8,10-11H2,2-3H3/t15-,16-,17-,19+,20-,21-,22-/m0/s1 | |||
| InChIKey = WSVOMANDJDYYEY-CWNVBEKCBU | |||
| StdInChI_Ref = {{stdinchicite|correct|chemspider}} | |||
| StdInChI = 1S/C22H28O5/c1-12-8-16-15-5-4-13-9-14(24)6-7-20(13,2)19(15)17(25)10-21(16,3)22(12,27)18(26)11-23/h6-7,9,15-17,19,23,25,27H,1,4-5,8,10-11H2,2-3H3/t15-,16-,17-,19+,20-,21-,22-/m0/s1 | |||
| | | StdInChIKey_Ref = {{stdinchicite|correct|chemspider}} | ||
| StdInChIKey = WSVOMANDJDYYEY-CWNVBEKCSA-N | |||
| | | synonyms = <small>(8''S'',9''S'',10''R'',11''S'',13''S'',14''S'',17''R'')-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-16-methylidene-6,7,8,9,11,12,14,15-octahydrocyclopenta[''a'']phenanthren-3-one</small> | ||
| | |||
| | |||
}} | }} | ||
{{ | '''Prednylidene''' is a [[glucocorticoid]]<ref name="pmid10423179">{{cite journal |author=Buttgereit F, Brand MD, Burmester GR |title=Equivalent doses and relative drug potencies for non-genomic glucocorticoid effects: a novel glucocorticoid hierarchy |journal=Biochem. Pharmacol. |volume=58 |issue=2 |pages=363–8 |date=July 1999 |pmid=10423179 |doi= 10.1016/S0006-2952(99)00090-8|url=http://linkinghub.elsevier.com/retrieve/pii/S0006-2952(99)00090-8}}</ref> for systemic use. | ||
{{ | |||
Substitution at position 16 also leads to more potent corticosteroids. The additional steric bulk introduced by such substituents adjacent to the dihydroxyacetone side chain also protects that moiety against metabolic degradation. | |||
==Synthesis== | |||
[[Curvularia]] | |||
[[File:Prednylidene synthesis.png|thumb|center|700px|Prednylidene synthesis:<ref>{{Cite doi|10.1016/S0040-4039(01)99336-0}}</ref>]] | |||
==References== | |||
{{Reflist|2}} | |||
{{Glucocorticoids}} | |||
{{Glucocorticoidics}} | |||
[[Category:Glucocorticoids]] | [[Category:Glucocorticoids]] | ||
{{ | |||
{{systemic-hormonal-drug-stub}} | |||
Revision as of 19:49, 15 April 2015
 | |
| Clinical data | |
|---|---|
| Synonyms | (8S,9S,10R,11S,13S,14S,17R)-11,17-dihydroxy-17-(2-hydroxyacetyl)-10,13-dimethyl-16-methylidene-6,7,8,9,11,12,14,15-octahydrocyclopenta[a]phenanthren-3-one |
| ATC code | |
| Identifiers | |
| |
| CAS Number | |
| PubChem CID | |
| ChemSpider | |
| UNII | |
| E number | {{#property:P628}} |
| ECHA InfoCard | {{#property:P2566}}Lua error in Module:EditAtWikidata at line 36: attempt to index field 'wikibase' (a nil value). |
| Chemical and physical data | |
| Formula | C22H28O5 |
| Molar mass | 372.455 |
| 3D model (JSmol) | |
| |
| |
| | |
Prednylidene is a glucocorticoid[1] for systemic use.
Substitution at position 16 also leads to more potent corticosteroids. The additional steric bulk introduced by such substituents adjacent to the dihydroxyacetone side chain also protects that moiety against metabolic degradation.
Synthesis
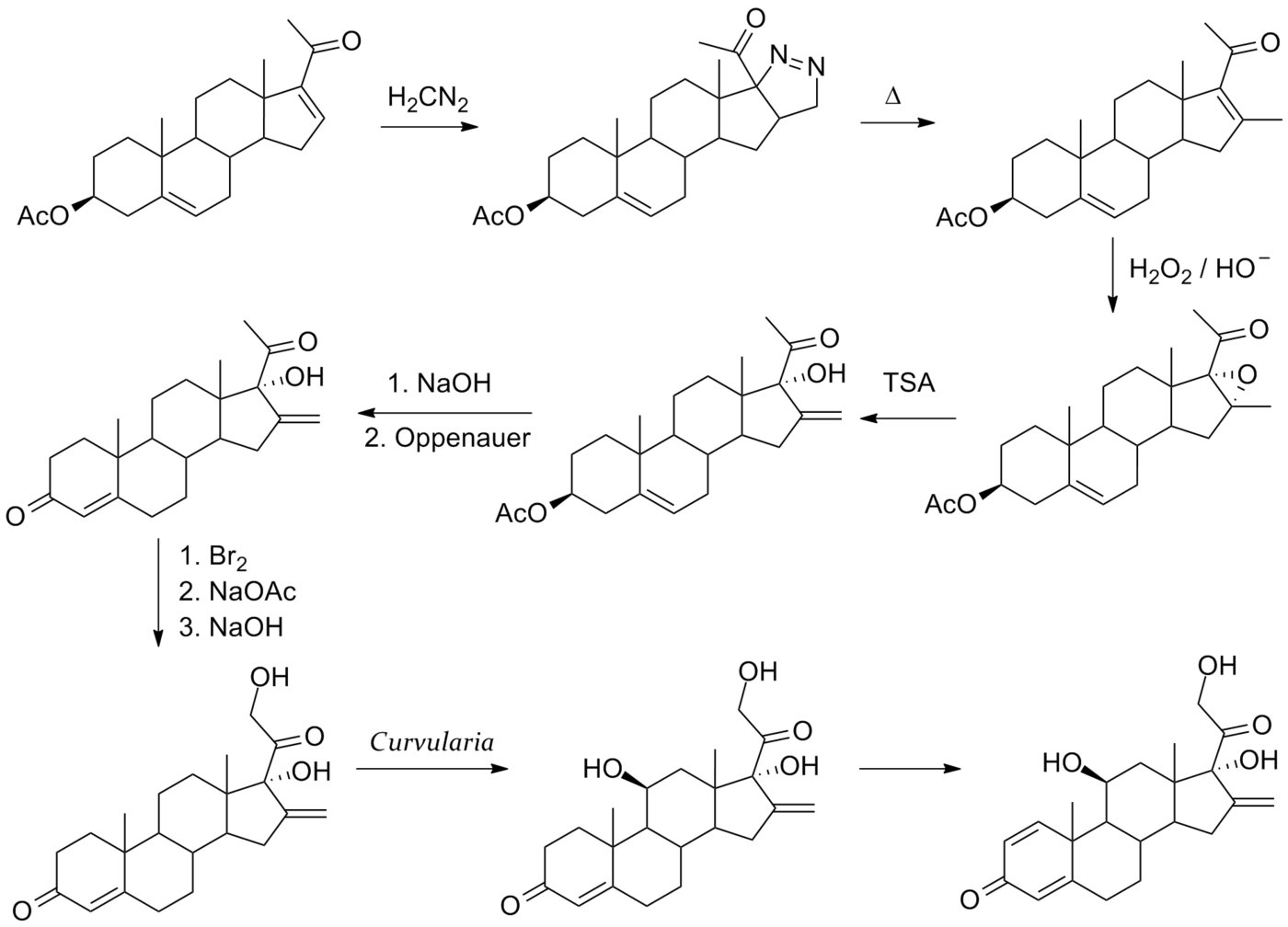
References
- ↑ Buttgereit F, Brand MD, Burmester GR (July 1999). "Equivalent doses and relative drug potencies for non-genomic glucocorticoid effects: a novel glucocorticoid hierarchy". Biochem. Pharmacol. 58 (2): 363–8. doi:10.1016/S0006-2952(99)00090-8. PMID 10423179.
- ↑ Template:Cite doi
Categories:
- Pages with script errors
- CS1 maint: Multiple names: authors list
- Articles with changed CASNo identifier
- E number from Wikidata
- ECHA InfoCard ID from Wikidata
- Chemical articles with unknown parameter in Infobox drug
- Articles without EBI source
- Chemical pages without DrugBank identifier
- Articles without KEGG source
- Drugs with no legal status
- Drugboxes which contain changes to verified fields
- Glucocorticoids