Prednicarbate
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-in-Chief: Ammu Susheela, M.D. [2]
WikiDoc MAKES NO GUARANTEE OF VALIDITY. WikiDoc is not a professional health care provider, nor is it a suitable replacement for a licensed healthcare provider. WikiDoc is intended to be an educational tool, not a tool for any form of healthcare delivery. The educational content on WikiDoc drug pages is based upon the FDA package insert, National Library of Medicine content and practice guidelines / consensus statements. WikiDoc does not promote the administration of any medication or device that is not consistent with its labeling. Please read our full disclaimer here.
Overview
Prednicarbate is an antiinflammatory that is FDA approved for the treatment of inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. Common adverse reactions include pruritis, edema, paresthesia, urticaria, burning, allergic contact dermatitis and rash.
Adult Indications and Dosage
FDA-Labeled Indications and Dosage (Adult)
- Prednicarbate emollient cream 0.1% is a medium-potency corticosteroid indicated for the relief of the inflammatory and pruritic manifestations of corticosteroid responsive dermatoses. Prednicarbate emollient cream 0.1% may be used with caution in pediatric patients 1 year of age or older. The safety and efficacy of drug use for longer than 3 weeks in this population have not been established. Since safety and efficacy of prednicarbate emollient cream 0.1% have not been established in pediatric patients below 1 year of age, its use in this age group is not recommended.
- Apply a thin film of prednicarbate emollient cream 0.1% to the affected skin areas twice daily. Rub in gently.
- Prednicarbate emollient cream 0.1 % may be used in pediatric patients 1 year of age or older. Safety and efficacy of prednicarbate emollient cream 0.1% in pediatric patients for more than 3 weeks of use have not been established. Use in pediatric patients under 1 year of age is not recommended.
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within 2 weeks, reassessment of the diagnosis may be necessary.
- Prednicarbate emollient cream 0.1% should not be used with occlusive dressings unless directed by the physician. Prednicarbate emollient cream 0.1% should not be applied in the diaper area if the child still requires diapers or plastic pants as these garments may constitute occlusive dressing.
Off-Label Use and Dosage (Adult)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prednicarbate in adult patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Prednicarbate in adult patients.
Pediatric Indications and Dosage
FDA-Labeled Indications and Dosage (Pediatric)
- Prednicarbate emollient cream 0.1% may be used with caution in pediatric patients 1 year of age or older, although the safety and efficacy of drug use longer than 3 weeks have not been established. The use of prednicarbate emollient cream 0.1% is supported by results of a three-week, uncontrolled study in 59 pediatric patients between the ages of 4 months and 12 years of age with atopic dermatitis. None of the 59 pediatric patients showed evidence of HPA-axis suppression. Safety and efficacy of prednicarbate emollient cream 0.1% in pediatric patients below 1 year of age have not been established, therefore use in this age group is not recommended. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA-axis suppression and Cushing's syndrome when they are treated with topical corticosteroids.
- They are therefore also at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. In an uncontrolled study in pediatric patients with atopic dermatitis, the incidence of adverse reactions possibly or probably associated with the use of prednicarbate emollient cream 0.1% was limited.
- Mild signs of atrophy developed in 5 patients (5/59, 8%) during the clinical trial, with 2 patients exhibiting more than one sign. Two patients (2/59, 3%) developed shininess, and two patients (2/59, 3%) developed thinness. Three patients (3/59, 5%) were observed with mild telangiectasia. It is unknown whether prior use of topical corticosterioids was a contributing factor in the development of telangiectasia in 2 of the patients.
- Adverse effects including striae have also been reported with inappropriate use of topical corticosteroids in infants and children. Pediatric patients applying topical corticosteroids to greater than 20% of body surface are at higher risk for HPA-axis suppression.
- HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain and intracranial hypertension have been reported in children receiving topical corti-costeroids. Manifestations of adrenal suppression in children include low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
- Prednicarbate emollient cream 0.1% should not be used in the treatment of diaper dermatitis.
Off-Label Use and Dosage (Pediatric)
Guideline-Supported Use
There is limited information regarding Off-Label Guideline-Supported Use of Prednicarbate in pediatric patients.
Non–Guideline-Supported Use
There is limited information regarding Off-Label Non–Guideline-Supported Use of Prednicarbate in pediatric patients.
Contraindications
- Prednicarbate emollient cream 0.1% is contraindicated in those patients with a history of hypersensitivity to any of the components in the preparations.
Warnings
General
- Systemic absorption of topical corticosteroids can produce reversible hypothalamic-pituitary-adrenal (HPA) axis suppression with the potential for glucocorticosteroid insufficiency after withdrawal of treatment.
- Manifestations of Cushing's syndrome, hyperglycemia, and glucosuria can also be produced in some patients by systemic absorption of topical corticosteroids while on treatment.
- Patients applying a topical steroid to a large surface area or under occlusion should be evaluated periodically for evidence of HPA-axis suppression. This may be done by using the ACTH stimulation, A.M. plasma cortisol, and urinary free cortisol tests.
- Prednicarbate emollient cream 0.1% did not produce significant HPA-axis suppression when used at a dose of 30g/day for a week in 10 adult patients with extensive psoriasis or atopic dermatitis. Prednicarbate emollient cream 0.1% did not produce HPA-axis suppression in any of 59 pediatric patients with extensive atopic dermatitis when applied BID for 3 weeks to > 20% of the body surface.
- If HPA-axis suppression is noted, an attempt should be made to withdraw the drug, to reduce the frequency of the application, or to substitute a less potent corticosteroid. Recovery of HPA-axis function is generally prompt upon discontinuation of topical corticosteroids. Infrequently, signs and symptoms of glucocorticosteroid insufficiency may occur, requiring supplemental systemic corticosteroids. For information on systemic supplementation, see prescribing information for those products.
- Pediatric patients may be more susceptible to systemic toxicity from equivalent doses due to their larger skin surface to body mass ratios.
- If irritation develops, prednicarbate emollient cream 0.1% should be discontinued and appropriate therapy instituted. Allergic contact dermatitis with corticosteroids is usually diagnosed by observing a failure to heal rather than noting a clinical exacerbation, as observed with most topical products not containing corticosteroids. Such an observation should be corroborated with appropriate diagnostic patch testing.
- If concomitant skin infections are present or develop, an appropriate antifungal or antibacterial agent should be used.
- If a favorable response does not occur promptly, use of prednicarbate emollient cream 0.1% should be discontinued until the infection has been adequately controlled.
Adverse Reactions
Clinical Trials Experience
There is limited information regarding Clinical Trial Experience of Prednicarbate in the drug label.
Postmarketing Experience
- In controlled adult clinical studies, the incidence of adverse reactions probably or possibly associated with the use of prednicarbate emollient cream 0.1% was approximately 4%. Reported reactions included mild signs of skin atrophy in 1% of treated patients, as well as the following reactions which were reported in less than 1% of patients: pruritis, edema, paresthesia, urticaria, burning, allergic contact dermatitis and rash.
- In an uncontrolled study in pediatric patients with atopic dermatitis, the incidence of adverse reactions possibly or probably associated with the use of prednicarbate emollient cream 0.1 % was limited. Mild signs of atrophy developed in 5 patients (5/59, 8%) during the clinical trial, with 2 patients exhibiting more than one sign. Two patients (2/59, 3%) developed shininess, and 2 patients (2/59, 3%) developed thinness. Three patients (3/59, 5 %) were observed with mild telangiectasia. It is unknown whether prior use of topical corticosteroids was a contributing factor in the development of telangiectasia in 2 of the patients.
- The following additional local adverse reactions have been reported infrequently with topical corticosteroids, but may occur more frequently with the use of occlusive dressings. These reactions are listed in an approximate decreasing order of occurrence: folliculitis, acneiform eruptions, hypopigmentation, perioral dermatitis, secondary infection, striae and miliaria.
Drug Interactions
There is limited information regarding Prednicarbate Drug Interactions in the drug label.
Use in Specific Populations
Pregnancy
- Corticosteroids have been shown to be teratogenic in laboratory animals when administered systemically at relatively low dosage levels. Some corticosteroids have been shown to be teratogenic after dermal application in laboratory animals.
- Prednicarbate has been shown to be teratogenic and embryotoxic in Wistar rats and Himalayan rabbits when given subcutaneously during gestation at doses 1900 times and 45 times the recommended topical human dose, assuming a percutaneous absorption of approximately 3%. In the rats, slightly retarded fetal development and an incidence of thickened and wavy ribs higher than the spontaneous rate were noted.
- In rabbits, increased liver weights and slight increase in the fetal intrauterine death rate were observed. The fetuses that were delivered exhibited reduced placental weight, increased frequency of cleft palate, ossification disorders in the sternum, omphalocele, and anomalous posture of the forelimbs.
- There are no adequate and well-controlled studies in pregnant women on teratogenic effects of prednicarbate. Prednicarbate emollient cream 0.1% should be used during pregnancy only if the potential benefit justifies the potential risk to the fetus.
- Australian Drug Evaluation Committee (ADEC) Pregnancy Category
There is no Australian Drug Evaluation Committee (ADEC) guidance on usage of Prednicarbate in women who are pregnant.
Labor and Delivery
There is no FDA guidance on use of Prednicarbate during labor and delivery.
Nursing Mothers
- Systemically administered corticosteroids appear in human milk and could suppress growth, interfere with endogenous corticosteroid production, or cause other untoward effects. It is not known whether topical administration of corticosteroids could result in sufficient systemic absorption to produce detectable quantities in human milk. Because many drugs are excreted in human milk, caution should be exercised when prednicarbate emollient cream 0.1% is administered to a nursing woman.
Pediatric Use
- Prednicarbate emollient cream 0.1% may be used with caution in pediatric patients 1 year of age or older, although the safety and efficacy of drug use longer than 3 weeks have not been established. The use of prednicarbate emollient cream 0.1% is supported by results of a three-week, uncontrolled study in 59 pediatric patients between the ages of 4 months and 12 years of age with atopic dermatitis. None of the 59 pediatric patients showed evidence of HPA-axis suppression. Safety and efficacy of prednicarbate emollient cream 0.1% in pediatric patients below 1 year of age have not been established, therefore use in this age group is not recommended. Because of a higher ratio of skin surface area to body mass, pediatric patients are at a greater risk than adults of HPA-axis suppression and Cushing's syndrome when they are treated with topical corticosteroids. They are therefore also at greater risk of adrenal insufficiency during and/or after withdrawal of treatment. In an uncontrolled study in pediatric patients with atopic dermatitis, the incidence of adverse reactions possibly or probably associated with the use of prednicarbate emollient cream 0.1% was limited.
- Mild signs of atrophy developed in 5 patients (5/59, 8%) during the clinical trial, with 2 patients exhibiting more than one sign. Two patients (2/59, 3%) developed shininess, and two patients (2/59, 3%) developed thinness. Three patients (3/59, 5%) were observed with mild telangiectasia. It is unknown whether prior use of topical corticosterioids was a contributing factor in the development of telangiectasia in 2 of the patients. Adverse effects including striae have also been reported with inappropriate use of topical corticosteroids in infants and children. Pediatric patients applying topical corticosteroids to greater than 20% of body surface are at higher risk for HPA-axis suppression.
- HPA axis suppression, Cushing's syndrome, linear growth retardation, delayed weight gain and intracranial hypertension have been reported in children receiving topical corti-costeroids. Manifestations of adrenal suppression in children include low plasma cortisol levels, and absence of response to ACTH stimulation. Manifestations of intracranial hypertension include bulging fontanelles, headaches, and bilateral papilledema.
- Prednicarbate emollient cream 0.1% should not be used in the treatment of diaper dermatitis.
Geriatic Use
There is no FDA guidance on the use of Prednicarbate with respect to geriatric patients.
Gender
There is no FDA guidance on the use of Prednicarbate with respect to specific gender populations.
Race
There is no FDA guidance on the use of Prednicarbate with respect to specific racial populations.
Renal Impairment
There is no FDA guidance on the use of Prednicarbate in patients with renal impairment.
Hepatic Impairment
There is no FDA guidance on the use of Prednicarbate in patients with hepatic impairment.
Females of Reproductive Potential and Males
There is no FDA guidance on the use of Prednicarbate in women of reproductive potentials and males.
Immunocompromised Patients
There is no FDA guidance one the use of Prednicarbate in patients who are immunocompromised.
Administration and Monitoring
Administration
Monitoring
There is limited information regarding Monitoring of Prednicarbate in the drug label.
IV Compatibility
There is limited information regarding IV Compatibility of Prednicarbate in the drug label.
Overdosage
- Topically applied corticosteroids can be absorbed in sufficient amounts to produce systemic effects.
Pharmacology
Mechanism of Action
- In common with other topical corticosteroids, prednicarbate has anti-inflammatory, antipruritic, and vasoconstrictive properties. In general, the mechanism of the anti-inflammatory activity of topical steroids is unclear.
- However, corticosteroids are thought to act by the induction of phospholipase A2 inhibitory proteins, collectively called lipocortins. It is postulated that these proteins control the biosynthesis of potent mediators of inflammation such as prostaglandins and leukotrienes by inhibiting the release of their common precursor arachidonic acid. Arachidonic acid is released from membrane phospholipids by phospholipase A2.
Structure
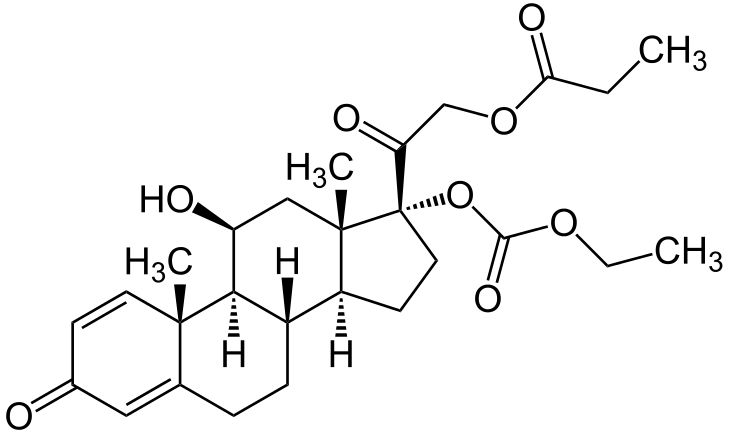
- Prednicarbate emollient cream 0.1% contains prednicarbate, a synthetic corticosteroid for topical dermatologic use. The chemical name of prednicarbate is 11β, 17, 21-trihydroxypregna-1,4-diene-3,20-dione 17-(ethyl carbonate) 21-propionate. Prednicarbate has the empirical formula C27H36O8 and a molecular weight of 488.58. Topical corticosteroids constitute a class of primarily synthetic steroids used topically as anti-inflammatory and antipruritic agents.
- The CAS Registry Number is 73771-04-7. The chemical structure is:
- Prednicarbate is a practically odorless white to yellow-white powder insoluble to practically insoluble in water and freely soluble in ethanol.
- Each gram of prednicarbate emollient cream 0.1% contains 1.0 mg of prednicarbate in a base consisting of white petrolatum USP, purified water USP, isopropyl myristate NF, lanolin alcohols NF, mineral oil USP, cetostearyl alcohol NF, aluminum stearate, edetate disodium USP, lactic acid USP, and magnesium stearate DAB 9.
Pharmacodynamics
There is limited information regarding Pharmacodynamics of Prednicarbate in the drug label.
Pharmacokinetics
- The extent of percutaneous absorption of topical corticosteroids is determined by many factors, including the vehicle and the integrity of the epidermal barrier. Use of occlusive dressings with hydrocortisone for up to 24 hours have not been shown to increase penetration; however, occlusion of hydrocortisone for 96 hours does markedly enhance penetration.
- Topical corticosteroids can be absorbed from normal intact skin. Inflammation and/or other disease processes in the skin increase percutaneous absorption.
- Studies performed with prednicarbate emollient cream 0.1 % indicate that the drug product is in the medium range of potency compared with other topical corticosteroids.
Nonclinical Toxicology
There is limited information regarding Nonclinical Toxicology of Prednicarbate in the drug label.
Clinical Studies
There is limited information regarding Clinical Studies of Prednicarbate in the drug label.
How Supplied
Storage
There is limited information regarding Prednicarbate Storage in the drug label.
Images
Drug Images
{{#ask: Page Name::Prednicarbate |?Pill Name |?Drug Name |?Pill Ingred |?Pill Imprint |?Pill Dosage |?Pill Color |?Pill Shape |?Pill Size (mm) |?Pill Scoring |?NDC |?Drug Author |format=template |template=DrugPageImages |mainlabel=- |sort=Pill Name }}
Package and Label Display Panel
{{#ask: Label Page::Prednicarbate |?Label Name |format=template |template=DrugLabelImages |mainlabel=- |sort=Label Page }}
Patient Counseling Information
Information for Patients
- Patients using topical corticosteroids should receive the following information and instructions:
- This medication is to be used as directed by the physician. It is for external use only. Avoid contact with the eyes.
- This medication should not be used for any disorder other than that for which it was prescribed.
- The treated skin area should not be bandaged, otherwise covered or wrapped so as to be occlusive, unless directed by the physician.
- Patients should report to their physician any signs of local adverse reactions.
- Parents of pediatric patients should be advised not to use this medication in the treatment of diaper dermatitis. This medication should not be applied in the diaper area as diapers or plastic pants may constitute occlusive dressing.
- This medication should not be used on the face, underarms, or groin areas.
- Contact between prednicarbate emollient cream 0.1% and latex containing products (eg. condoms, diaphragm etc.) should be avoided since paraffin in contact with latex can cause damage and reduce the effectiveness of any latex containing products. If latex products come into contact with prednicarbate emollient cream 0.1%, patients should be advised to discard the latex products. Patients should be advised that this medication is to be used externally only, not intravaginally.
- As with other corticosteroids, therapy should be discontinued when control is achieved. If no improvement is seen within two weeks, contact the physician.
Precautions with Alcohol
- Alcohol-Prednicarbate interaction has not been established. Talk to your doctor about the effects of taking alcohol with this medication.
Brand Names
- PREDNICARBATE ®[1]
Look-Alike Drug Names
There is limited information regarding Prednicarbate Look-Alike Drug Names in the drug label.
Price
References
The contents of this FDA label are provided by the National Library of Medicine.
{{#subobject:
|Label Page=Prednicarbate |Label Name=PRED 02.jpg
}}
{{#subobject:
|Label Page=Prednicarbate |Label Name=DailyMed - PREDNICARBATE- prednicarbate cream .png
}}