Mitral regurgitation surgery: Difference between revisions
(Removing all content from page) |
No edit summary |
||
| Line 1: | Line 1: | ||
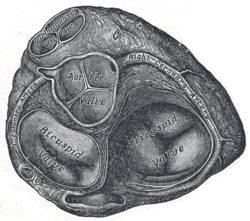
[[Image:250px-Gray495.png|right|frame|Base of ventricles exposed by removal of the atria. (Bicuspid (mitral) valve visible at bottom left.)]] | |||
{{Mitral valve regurgitation surgery}} | |||
'''For the WikiPatient page for this topic, click [[Mitral valve surgery (patient information)|here]]''' | |||
{{CMG}}; '''Associate Editor(s)-In-Chief:''' [[User:Mohammed Sbeih|Mohammed A. Sbeih, M.D.]] [mailto:msbeih@perfuse.org]; {{CZ}}; [[Varun Kumar]], M.B.B.S.; [[Lakshmi Gopalakrishnan]], M.B.B.S. | |||
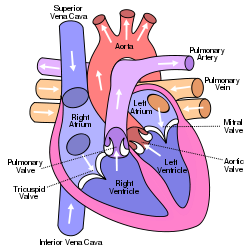
[[Image:250px-Diagram_of_the_human_heart_(cropped).svg.png|right|frame|Anterior (frontal) view of the opened heart. White arrows indicate normal blood flow. (Mitral valve labeled at center right.)]] | |||
==Overview== | |||
[[Mitral valve]] surgery is a surgery that can either repair or replace the mitral valve in the heart. | |||
Blood that flows between different chambers of the heart must flow through a valve. One such valve is called the mitral valve. It opens up enough so blood can flow from one chamber of the heart (left atria) to the next chamber (left ventricle). It then closes, keeping blood from flowing backwards. | |||
Regurgitation refers to leaking from a valve that doesn't close all the way. Diseases that weaken or damage the valve or the heart tissue around the valve cause mitral regurgitation. | |||
[[Mitral regurgitation]] is the most common type of heart [[valve insufficiency]]. After age 55, some degree of mitral regurgitation is found in almost 20% of men and women who have an echocardiogram. | |||
Mitral valve surgery is indicated when the [[mitral regurgitation]] is severe or when the patient is symptomatic. | |||
Decision between valve repair or valve replacement is made based on the type and severity of damage to [[mitral valve]]. | |||
In '''open surgery''', the surgeon makes a large cut in the sternum to reach the heart. | |||
'''Minimally invasive''' mitral valve surgery is done through much smaller surgical cuts than the large cuts needed for open surgery. | |||
==Anatomy and pathophysiology== | |||
'''Anatomy''' | |||
The '''mitral valve''' is typically 4–6 cm² in area. It has two [[cusps]], or leaflets, (the anteromedial leaflet and the posterolateral leaflet) that guard the opening. The opening is surrounded by a fibrous ring known as the mitral valve annulus <ref name="pmid1539731">{{cite journal| author=Shinoda H, Stern PH| title=Diurnal rhythms in Ca transfer into bone, Ca release from bone, and bone resorbing activity in serum of rats. | journal=Am J Physiol | year= 1992 | volume= 262 | issue= 2 Pt 2 | pages= R235-40 | pmid=1539731 | doi= | pmc= | url= }} </ref>. The anterior cusp protects approximately two-thirds of the valve (imagine a crescent moon within the circle, where the crescent represents the posterior cusp). Note that although the anterior leaflet takes up a larger part of the ring and rises higher, the posterior leaflet has a larger surface area. These valve leaflets are prevented from prolapsing into the left atrium by the action of tendons attached to the posterior surface of the valve, [[chordae tendineae]]. | |||
The inelastic [[chordae tendineae]] are attached at one end to the [[papillary muscles]] and the other to the valve cusps. Papillary muscles are fingerlike projections from the wall of the left ventricle. Chordae tendineae from each muscle are attached to both leaflets of the mitral valve. Thus, when the left ventricle contracts, the intraventricular pressure forces the valve to close, while the tendons keep the leaflets coapting together and prevent the valve from opening in the wrong direction (thus preventing blood to flow back to the left atrium). Each chord has a different thickness. The thinnest ones are attached to the free leaflet margin, whereas thickest ones are attached quite away from the free margin. This disposition has important effects on systolic stress distribution physiology <ref name="pmid10901521">{{cite journal| author=Nazari S, Carli F, Salvi S, Banfi C, Aluffi A, Mourad Z et al.| title=Patterns of systolic stress distribution on mitral valve anterior leaflet chordal apparatus. A structural mechanical theoretical analysis. | journal=J Cardiovasc Surg (Torino) | year= 2000 | volume= 41 | issue= 2 | pages= 193-202 | pmid=10901521 | doi= | pmc= | url= }} </ref>. | |||
'''Pathophysiology''' | |||
During left ventricular [[diastole]], after the pressure drops in the left ventricle due to relaxation of the ventricular [[myocardium]], the mitral valve opens, and blood travels from the left atrium to the left ventricle. About 70-80% of the blood that travels across the mitral valve occurs during the early filling phase of the left ventricle. This early filling phase is due to active relaxation of the ventricular myocardium, causing a pressure gradient that allows a rapid flow of blood from the left atrium, across the mitral valve. This early filling across the mitral valve is seen on doppler [[echocardiography]] of the mitral valve as the E wave. | |||
After the E wave, there is a period of slow filling of the ventricle. | |||
Left atrial contraction (during left ventricular diastole) causes added blood to flow across the mitral valve immediately before left ventricular systole. This late flow across the open mitral valve is seen on doppler echocardiography of the mitral valve as the A wave. The late filling of the LV contributes about 20% to the volume in the left ventricle prior to ventricular systole, and is known as the atrial kick. | |||
The mitral annulus changes in shape and size during the cardiac cycle. It is smaller at the end of atrial systole due to the contraction of the left atrium around it, like a sphincter. This reduction in annulus size at the end of atrial systole may be important for the proper coapting of the leaflets of the mitral valve when the left ventricle contracts and pumps blood <ref name="pmid12578332">{{cite journal| author=Pai RG, Varadarajan P, Tanimoto M| title=Effect of atrial fibrillation on the dynamics of mitral annular area. | journal=J Heart Valve Dis | year= 2003 | volume= 12 | issue= 1 | pages= 31-7 | pmid=12578332 | doi= | pmc= | url= }} </ref>. | |||
The closing of the mitral valve and the tricuspid valve constitutes the first [[heart sound]] (S1). It is not actually the valve closure which produces a sound but rather the sudden cessation of blood flow caused by the closure of the mitral and tricuspid valves. The mitral valve opening is normally not heard except in [[mitral stenosis]] (narrowing of the valve) as the opening Snap. Flow of blood into the heart during rapid filling is not normally heard except in certain pathological states where it constitutes the third heart sound (S3). | |||
When the mitral valve doesn't close all the way, blood flows backward into the upper heart chamber (atrium). This leads to a decrease in blood flow to the rest of the body. As a result, the heart may try to pump harder. This may lead to [[congestive heart failure]]. | |||
Mitral regurgitation '''may begin suddenly''', most often after a [[heart attack]] due to papillary muscle rupture. When the regurgitation does not go away, it becomes chronic (long-term). | |||
'''Causes of chronic mitral regurgitation''' include: | |||
*Primary diseases of the valve leaflets such as [[mitral valve prolapse]]. MVP is a common cause. However, most patients with MVP do not develop severe mitral regurgitation. Older age, male gender, and auscultatory evidence of severe MR are prognostic clues that identify patients with mitral valve prolapse who are at a relatively high risk of complications. | |||
*[[Rheumatic heart disease]]. One out of three cases of chronic mitral regurgitation are caused by rheumatic heart disease, a complication of untreated strep throat that is becoming less common. | |||
*[[Coronary artery disease]] and heart attacks. | |||
*[[cardiomyopathy]]. | |||
*[[Endocarditis]]. | |||
*Heart tumors. | |||
*High blood pressure. | |||
*[[Marfan syndrome]]. | |||
*Swelling of the left lower heart chamber. | |||
*Untreated [[syphilis]] (rare). | |||
*Congenital (present from birth) mitral regurgitation is most often part of a more complex heart defect or syndrome. | |||
Chronic MR is usually well tolerated '''during pregnancy'''. The normal fall in systemic vascular resistance tends to reduce the degree of regurgitation. | |||
'''Severity of MR''' can be assessed by both clinical and echocardiographic criteria. careful history is important to establish an estimate of baseline exercise tolerance of the patient. | |||
The 2006 ACC/AHA guidelines included recommendations for echocardiographic monitoring in asymptomatic patients with chronic MR <ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref>. Echocardiography is performed to assess the left ventricular ejection fraction and end-systolic dimension. | |||
There are often no '''symptoms'''. When symptoms occur, they often develop gradually, and may include: | |||
* Cough. | |||
* Fatigue, exhaustion, and light-headedness. | |||
* [[Palpitations]] (related to [[atrial fibrillation]]). | |||
* Shortness of breath during activity and when lying down. | |||
* Urination, excessive at night. | |||
Chronic mitral regurgitation can be divided into '''three stages'''; compensated, transitional, and decompensated stage. The stage depends on the left ventricular (LV) chamber size and function. | |||
*In the compensated stage; the left ventricular (LV) end-diastolic dimension is less than 60 mm, and the end-systolic dimension is less than 40mm (Dimensions measured by echocardiography) | |||
*The transitional stage left ventricular (LV) dimensions is not precisely defined, but most studies indicates that surgery at this stage has a very good results. | |||
*The decompensated stage defined on the basis of decompensated ventricular function. At this stage; the patients are at risk for a poor results of valve replacement. | |||
Markers for '''decompensated ventricular function''' include: | |||
*Left ventricular end-diastolic dimension greater than 70 mm. | |||
*Left ventricular end-systolic dimension greater than 45 to 47 mm. | |||
*Left ventricular [[ejection fraction]] (LVEF) less than 50 to 55 percent. | |||
Knowing the stage of chronic mitral valve regurgitation enables the clinician to predict the LV function, so he or she can decide if the patient could get benefit from the surgical treatment. Usually, a corrective surgery for mitral valve regurgitation should be performed before the transition to the decompensated stage of the disease, because at this stage any treatment may provide symptomatic relief only, but ventricular enlargement and a low [[LVEF]] (Left ventricular ejection fraction) usually persist even with successful surgery. | |||
==Treatments for Mitral valve regurgitation== | |||
The choice of treatment depends on the symptoms present and the condition and function of the heart. Patients with high blood pressure or a weakened heart muscle may be given medications to reduce the strain on the heart and help improve the condition. | |||
[[Anticoagulant]] or [[antiplatelet]] medications (blood thinners) may be used to prevent clots from forming in patients with [[atrial fibrillation]]. | |||
[[Digitalis]] may be used to strengthen the heartbeat, along with [[diuretics]] (water pills) to remove excess fluid in the lungs. | |||
A low-sodium diet may be helpful. Most people have no symptoms; but if a person develops symptoms, activity may be restricted. | |||
Hospitalization may be required for diagnosis and treatment of severe symptoms. Surgical repair or replacement of the valve is recommended if heart function is poor, symptoms are severe, or the condition gets worse. Once the diagnosis of mitral regurgitation is made, the patient should have regular follow-ups with a specialist to determine whether he or she need surgery. | |||
In the past, patients with heart valve problems such as mitral regurgitation were given antibiotics before dental work or an invasive procedure, such as colonoscopy. The antibiotics were given to prevent an infection of the damaged heart valve. However, antibiotics are now used much less often before dental work and other procedures. | |||
==Indications for Mitral valve regurgitation surgery== | |||
Surgery is indicated in patients with '''symptomatic mitral valve regurgitation''', also it is indicated in patients with abnormalities in LV size or function (These include a [[left ventricular ejection fraction]] ([[LVEF]]) of less than 60% and a left ventricular end systolic dimension (LVESD) of greater than 45 mm), [[pulmonary hypertension]], or new onset [[atrial fibrillation]] even without symptoms <ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref>. The patient with severe LV dysfunction (an LVEF < 30% and/or a left ventricular end-systolic dimension greater than 55 mm poses a higher risk but may undergo surgery if chordal preservation is likely. [[ACC]]/[[AHA]] guidelines recommend that patients with chronic MR who become symptomatic are candidates for corrective mitral surgery <ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref>, even if the symptoms improve with medical therapy or the left ventricle appears to be compensated <ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref>. | |||
Surgery may be recommended in '''asymptomatic patients''' with preserved left ventricular function if the surgery performed in a center in which the likelihood of successful surgery is greater than 90 percent, otherwise; the patient can be safely treated with watchful waiting as long as the patient is carefully monitored <ref name="pmid16651470">{{cite journal| author=Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D et al.| title=Outcome of watchful waiting in asymptomatic severe mitral regurgitation. | journal=Circulation | year= 2006 | volume= 113 | issue= 18 | pages= 2238-44 | pmid=16651470 | doi=10.1161/CIRCULATIONAHA.105.599175 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=16651470 }} </ref>. The pstient should be seen every 6 to 12 months. Echocardiography should be obtained at these visits. The early surgery exposes the patient to [[perioperative morbidity]] and mortality as well as the long-term complications of a [[prosthetic valve]]. But it is important to have an objective measure of LV function in patients with asymptomatic MR, because there may be benefit from surgery prior to the onset of symptoms of the depression of the ventricular function in some cases. In patients with borderline values of ventricular size or function in whom access to such monitoring is limited; Surgery may be done earlier. | |||
<table border="1" cellpadding="5" cellspacing="0" align="left"> | |||
<caption>'''Indications for surgery for chronic mitral regurgitation'''<ref name="pmid9809971">{{cite journal |author= |title=ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease) |journal=[[Journal of the American College of Cardiology]] |volume=32 |issue=5 |pages=1486–588 |year=1998 |month=November |pmid=9809971 |doi= |url=http://linkinghub.elsevier.com/retrieve/pii/S0735109798004549 |accessdate=2011-03-16}}</ref> | |||
<tr> | |||
<th style="background:#efefef;">Symptoms</th> | |||
<th style="background:#efefef;">LV EF</th> | |||
<th style="background:#efefef;">LVESD</th> | |||
</tr> | |||
<tr><td>[[New York Heart Association Functional Classification|NYHA II - IV]]</td><td>> 60 percent</td><td>< 45 mm</td></tr> | |||
<tr><td>Asymptomatic or symptomatic</td><td>50 - 60 percent</td><td>≥ 45 mm</td></tr> | |||
<tr><td>Asymptomatic or symptomatic</td><td colspan=2>< 50 percent or ≥ 45 mm</td></tr> | |||
<tr><td colspan=3>[[Pulmonary artery]] systolic pressure ≥ 50 [[mmHg]]</td></tr> | |||
</table> | |||
The patient may also need valve surgery in the following conditions: | |||
*The changes in the mitral valve are causing major heart symptoms, such as [[angina]] (chest pain), shortness of breath, fainting spells ([[syncope]]), or heart failure. | |||
*Tests show that the changes in your mitral valve are beginning to seriously affect your heart function. | |||
*The heart valve has been damaged by [[endocarditis]] (infection of the heart valve). | |||
*The patient has received a new heart valve in the past, and it is not working well, or you have other problems such as blood clots, infection, or bleeding. | |||
'''[[Mitral valve repair]]''' is recommended in following: | |||
*Limited damage to certain areas of the mitral valve leaflets or [[chordae tendineae]]<ref name="pmid12830055">{{cite journal| author=Gillinov AM, Faber C, Houghtaling PL, Blackstone EH, Lam BK, Diaz R et al.| title=Repair versus replacement for degenerative mitral valve disease with coexisting ischemic heart disease. | journal=J Thorac Cardiovasc Surg | year= 2003 | volume= 125 | issue= 6 | pages= 1350-62 | pmid=12830055 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12830055 }} </ref>. | |||
*Limited calcification of the leaflets or annulus. | |||
*[[Mitral valve prolapse|Prolapse]] of less than one-third of either leaflet. | |||
*Pure annular dilatation. | |||
*Valvular perforations. | |||
*Incomplete [[papillary muscle rupture]]. | |||
'''[[Mitral valve replacement]]''' is recommended in following: | |||
*Extensive calcification or degeneration of a leaflet or annulus. | |||
*[[Mitral valve prolapse|Prolapse]] of more than one-third of the leaflet tissue. | |||
*Extensive chordal fusion, calcification, or [[papillary muscle rupture]]. | |||
*Extensive damage of mitral valve secondary to [[endocarditis]]. | |||
Based on above, '''ACC/AHA 2008 guidelines'''<ref name="pmid18848134">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=J Am Coll Cardiol | year= 2008 | volume= 52 | issue= 13 | pages= e1-142 | pmid=18848134 | doi=10.1016/j.jacc.2008.05.007 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18848134 }} </ref> recommend [[mitral valve repair]] rather than [[mitral valve replacement]] if the anatomy is appropriate, including patients with [[rheumatic]] mitral valve disease<ref name="pmid10612761">{{cite journal| author=Yau TM, El-Ghoneimi YA, Armstrong S, Ivanov J, David TE| title=Mitral valve repair and replacement for rheumatic disease. | journal=J Thorac Cardiovasc Surg | year= 2000 | volume= 119 | issue= 1 | pages= 53-60 | pmid=10612761 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=10612761 }} </ref> and mitral valve prolapse<ref name="pmid11568020">{{cite journal| author=Mohty D, Orszulak TA, Schaff HV, Avierinos JF, Tajik JA, Enriquez-Sarano M| title=Very long-term survival and durability of mitral valve repair for mitral valve prolapse. | journal=Circulation | year= 2001 | volume= 104 | issue= 12 Suppl 1 | pages= I1-I7 | pmid=11568020 | doi= | pmc= | url= }} </ref> (Grade 1C). The procedure should be performed at experienced surgical centers. | |||
==Preoperative preparation== | |||
The patient may need to have some tests before the procedure. The Cardiologist usually conducts a physical examination and diagnose the condition within few days, he or she will assess the general health of the patient and will recommend the most appropriate treatment for the patient and if he or she needs surgery. Some of the '''tests that can be done before the procedure''' include: | |||
*[[Cardiac catheterization]]. | |||
*Chest X-ray. | |||
*Computed tomography (CT) scan. | |||
*[[Echocardiogram]] (Doppler echocardiogram). | |||
*[[Electrocardiogram]] (ECG). | |||
*[[Electrophysiology]] tests. | |||
*Exercise tests. | |||
*[[Holter monitor]]. | |||
*[[Magnetic resonance imaging]] (MRI). | |||
Many patients with chronic MR requiring surgery also have [[coronary artery disease]]<ref name="pmid11326232">{{cite journal| author=Lin SS, Lauer MS, Asher CR, Cosgrove DM, Blackstone E, Thomas JD et al.| title=Prediction of coronary artery disease in patients undergoing operations for mitral valve degeneration. | journal=J Thorac Cardiovasc Surg | year= 2001 | volume= 121 | issue= 5 | pages= 894-901 | pmid=11326232 | doi=10.1067/mtc.2001.112463 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11326232 }} </ref>. Usually coronary disease treated at the same operation if CABG (Coronary artery bypass grafting) is indicated. Studies showed that '''concurrent bypass surgery''' adds little morbidity to the valvular procedure and does not increase the mortality <ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref>. The 2006 [[ACC]]/[[AHA]] guidelines on the treatment of valvular heart disease included recommendations for coronary angiography prior to valve surgery in those who are suspected to have coronary artery disease and in those at risk for coronary disease <ref name="pmid18820172">{{cite journal| author=Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD et al.| title=2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. | journal=Circulation | year= 2008 | volume= 118 | issue= 15 | pages= e523-661 | pmid=18820172 | doi=10.1161/CIRCULATIONAHA.108.190748 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=18820172 }} </ref>. A noninvasive angiography using computed tomography (CT) or magnetic resonance imaging may be an alternative. | |||
'''Before the surgery''': | |||
*The surgeon needs to know if the patient is taking any drugs, supplements, or herbs before the procedure. | |||
*The patient may be able to store blood in the blood bank for transfusions during and after the surgery. The family members can also donate blood (autologous donation). | |||
*For the 2-week period before surgery, the patient should be asked to stop taking drugs that make it harder for the blood to clot. These might cause increased bleeding during the surgery. Some of these drugs are [[aspirin]], [[ibuprofen]] (Advil, Motrin), and [[naproxen]] (Aleve, Naprosyn). | |||
*The day before the surgery, the patient should shower and shampoo well and wash the whole body below the neck with a special soap. | |||
*The patient may also be asked to take an [[antibiotic]] to guard against infection. | |||
*The patient should be informed which drugs he or she should still take on the day of the surgery. | |||
*The patient should stop smoking. | |||
'''On the day of the surgery''': | |||
*An intravenous (IV) line will be placed into a blood vessel in the patient's arm or chest to give fluids and medicines. | |||
*The patient should be asked not to drink or eat anything after midnight the night before surgery. This includes chewing gum and using breath mints. The patient can rinse mouth with water if it feels dry without swallowing. | |||
*Make sure that the patient is taking the drugs that he or she needs to take with a small sip of water. | |||
*Hair near the incision site may be shaved immediately before the surgery. | |||
*The patient should be informed when to arrive to hospital on the day of the surgery. | |||
==The procedure== | |||
The Procedure can be done either by the traditional open heart surgery or by the [[Minimally invasive surgery]]. | |||
Before the surgery, the patient will receive '''[[general anesthesia]]'''. This will make the patient asleep and pain-free during the entire procedure. | |||
*In the'''traditional open heart surgery''': | |||
:*The surgeon will make a 10-inch-long cut in the middle of the chest (sternum). | |||
:*Next, the surgeon will separate the breastbone (sternum) to be able to see the heart. | |||
:*Most people are connected to a [[heart-lung bypass machine]] or bypass pump. The heart is stopped while the patient is connected to this machine. This machine does the work of the heart while the heart is stopped. | |||
:*A small cut is made in the left side of the heart so the surgeon can repair or replace the mitral valve. | |||
*In '''minimally invasive mitral valve surgery'''; there are several different ways to perform the procedure: | |||
:*The heart surgeon may make a 2-inch to 3-inch-long cut in the right part of your chest near the [[sternum]] (breastbone). Muscles in the area will be divided so the surgeon can reach the heart. A small cut is made in the left side of the heart so the surgeon can repair or replace the [[mitral valve]]. | |||
:*In '''[[Endoscopic surgery]]''', the surgeon makes one to four small holes in the chest, then he or she uses special instruments and a camera to do the surgery. | |||
:*For '''Robotically-assisted valve surgery''', the surgeon makes two to four tiny cuts (about a ½ to a ¾ inch) in the chest. The surgeon uses a special computer to control [[robotic]] arms during the surgery. The surgeon sees a three-dimensional view of the heart and mitral valve on the computer. This method is very precise. | |||
The patient may or may not need to be on a [[heart-lung machine]] for these types of surgery, but if not, the heart rate will be slowed by medicine or a mechanical device. | |||
If the surgeon can '''repair the mitral valve''', the patient may have: | |||
*Ring annuloplasty: The surgeon repairs the ring-like part around the valve by sewing a ring of metal, cloth, or tissue around the valve. | |||
*Valve repair: The surgeon trims, shapes, or rebuilds one or more of the three leaflets of the valve. The leaflets are flaps that open and close the valve. | |||
If the mitral valve is too damaged, the patient will need a new valve. This is called '''Replacement surgery'''. The surgeon will remove the mitral valve and sew a new one into place. There are two '''types of valves''': | |||
1. Mechanical which is made of man-made (synthetic) materials, such as a metal like titanium. These valves last the longest, but the patient will need to take blood-thinning medicine, such as [[warfarin]] (Coumadin) or [[aspirin]], for the rest of his or her life. | |||
2. [[Biological]] which made of human or animal tissue. These valves last 10 to 12 years, but the patient may not need to take blood thinners for life. | |||
Once the new or repaired valve is working, the surgeon will: | |||
*Close the heart and take you off the [[heart-lung machine]]. | |||
*Place [[catheters]] (tubes) around the heart to drain fluids that build up. | |||
*Close the sternum with stainless steel wires. It will take about 6 weeks for the bone to heal. The wires will stay inside the body. | |||
The patient may have a temporary [[pacemaker]] connected to the heart until his or her natural heart [[rhythm]] returns. | |||
The surgeon may also perform [[coronary artery bypass surgery]] at the same time, if needed. | |||
==Recovery== | |||
'''Recovery at hospital''' | |||
The patient may spend 4 to 7 days in the hospital after surgery (much less in Minimally invasive mitral valve surgery-3 to 5 days). Then patient will wake up in the [[intensive care unit]] (ICU) and recover there for 1 or 2 days. Two to three tubes will be in the patient's chest to [[drain]] fluid from around the heart. They are usually removed 1 to 3 days after surgery. | |||
The patient may have a [[catheter]] in the bladder to drain urine, and may also have intravenous lines to get fluids. Nurses will closely watch monitors that show information about the [[vital signs]] (pulse, temperature, and breathing). | |||
The patient will be moved to a regular hospital room from the ICU. The nurses and doctors will continue to monitor the heart and vital signs until the patient is stable enough to go home. The patient will receive pain medicine to control pain around your surgical cut. | |||
A nurse should help the patient to slowly resume some activity, and the patient should begin a physical therapy program to make the heart and body stronger. | |||
A temporary [[pacemaker]] may be placed in the patient's heart if the heart rate becomes too slow after surgery. | |||
'''Recovery at home''' | |||
The patient should be informed about the following: | |||
*Taking care for his or her healing incisions. | |||
*Recognizing signs of infection or other complications. | |||
*Coping with after-effects of surgery. | |||
*Followup appointments, medicines, and situations when he or she should call the doctor right away. | |||
*When he or she can go back to daily routine, such as working, driving, and physical activity. | |||
After-effects of heart surgery are normal. They may include muscle pain, chest pain, or swelling. | |||
Other after-effects may include loss of appetite, problems sleeping, constipation, and mood swings and [[depression]]. After-effects usually go away over time. | |||
Less recovery time is needed for off-pump heart surgery and [[minimally invasive]] heart surgery. | |||
'''Ongoing care''' | |||
Ongoing care after valve surgery may include periodic checkups with the doctor. During these visits, the patient may have blood tests, an [[EKG]] (electrocardiogram), [[echocardiography]], or a [[stress test]]. These tests will show how the patient's heart is working after the surgery. | |||
Routine tests should be done to make sure the patient is getting the right amount of the blood-thinning medicine in case of mechanical valve placement. | |||
The patient may be advised to change his or her lifestyle, this includes: quitting smoking, making changes to diet, being physically active, and reducing and managing stress. | |||
==Surgical outcome== | |||
The results of mitral valve repair are excellent in the centers that regularly perform this surgery. | |||
Techniques for minimally invasive heart valve surgery have improved greatly over the past 10 years. These techniques are safe for most patients, and they reduce recovery time and pain. | |||
'''Valve repair versus valve replacement''' | |||
Advantages of [[Mitral valve repair]] include: | |||
*Lower operative mortality rate <ref name="pmid9918527">{{cite journal| author=Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ et al.| title=Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications. | journal=Circulation | year= 1999 | volume= 99 | issue= 3 | pages= 400-5 | pmid=9918527 | doi= | pmc= | url= }} </ref><ref name="pmid3769948">{{cite journal| author=Krayenbuehl HP| title=Surgery for mitral regurgitation. Repair versus valve replacement. | journal=Eur Heart J | year= 1986 | volume= 7 | issue= 8 | pages= 638-43 | pmid=3769948 | doi= | pmc= | url= }} </ref> | |||
*Improves left ventricular [[EF]] and function <ref name="pmid7850937">{{cite journal| author=Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL| title=Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis. | journal=Circulation | year= 1995 | volume= 91 | issue= 4 | pages= 1022-8 | pmid=7850937 | doi= | pmc= | url= }} </ref>. | |||
*Preserves native heart valve and avoids the use of a prosthetic heart valve with its complications. | |||
*Has good overall outcome with good survival rates <ref name="pmid9129898">{{cite journal| author=Lee EM, Shapiro LM, Wells FC| title=Superiority of mitral valve repair in surgery for degenerative mitral regurgitation. | journal=Eur Heart J | year= 1997 | volume= 18 | issue= 4 | pages= 655-63 | pmid=9129898 | doi= | pmc= | url= }} </ref><ref name="pmid11568020">{{cite journal| author=Mohty D, Orszulak TA, Schaff HV, Avierinos JF, Tajik JA, Enriquez-Sarano M| title=Very long-term survival and durability of mitral valve repair for mitral valve prolapse. | journal=Circulation | year= 2001 | volume= 104 | issue= 12 Suppl 1 | pages= I1-I7 | pmid=11568020 | doi= | pmc= | url= }} </ref><ref name="pmid12835220">{{cite journal| author=Thourani VH, Weintraub WS, Guyton RA, Jones EL, Williams WH, Elkabbani S et al.| title=Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement: effect of age and concomitant coronary artery bypass grafting. | journal=Circulation | year= 2003 | volume= 108 | issue= 3 | pages= 298-304 | pmid=12835220 | doi=10.1161/01.CIR.0000079169.15862.13 | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=12835220 }} </ref>. | |||
*Lower risk for [[endocarditis]]. | |||
*Avoids long term use of [[anticoagulants]]. | |||
'''Mechanical versus Biological valves''' | |||
Mechanical heart valves do not fail often. They last from 12 to 20 years. However, blood clots develop on them. If a blood clot forms, the patient may have a stroke. Bleeding can occur, but this is rare. | |||
[[Biological]] valves tend to fail over time <ref name="pmid8469251">{{cite journal| author=Hammermeister KE, Sethi GK, Henderson WG, Oprian C, Kim T, Rahimtoola S| title=A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or bioprosthesis. Veterans Affairs Cooperative Study on Valvular Heart Disease. | journal=N Engl J Med | year= 1993 | volume= 328 | issue= 18 | pages= 1289-96 | pmid=8469251 | doi=10.1056/NEJM199305063281801 | pmc= | url= }} </ref><ref name="pmid11028464">{{cite journal| author=Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH| title=Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial. | journal=J Am Coll Cardiol | year= 2000 | volume= 36 | issue= 4 | pages= 1152-8 | pmid=11028464 | doi= | pmc= | url=http://www.ncbi.nlm.nih.gov/entrez/eutils/elink.fcgi?dbfrom=pubmed&tool=sumsearch.org/cite&retmode=ref&cmd=prlinks&id=11028464 }} </ref>, but they have a lower risk of blood clots. | |||
==Possible complications== | |||
'''Risks for any surgery''' | |||
*Blood clots in the legs that may travel to the lungs. | |||
*Blood loss. | |||
*Breathing problems. | |||
*Infection, including in the lungs, kidneys, bladder, chest, or heart valves. | |||
*Reactions to medicines. | |||
'''Possible risks from having open-heart surgery''' | |||
*[[Heart attack]] or stroke. | |||
*Heart [[rhythm]] problems. | |||
*Infection in the cut, which is more likely to happen in people who are obese, have [[diabetes]], or have already had this surgery. | |||
*Memory loss and loss of mental clarity, or "fuzzy thinking." | |||
*[[Post-pericardiotomy syndrome]], which is a low-grade fever and chest pain. This could last for up to 6 months. | |||
'''Prosthetic heart valves are associated with a variety of complications''' | |||
*Structural deterioration, particularly with bioprosthetic valves. | |||
*Valve obstruction due to [[thrombosis]] or pannus formation. | |||
*Systemic [[embolization]]. | |||
*Bleeding. | |||
*[[Endocarditis]] and other infections. | |||
*Left ventricular systolic dysfunction, which may be preexisting. | |||
*[[Hemolytic anemia]]. | |||
==Videos== | |||
*'''Minimally invasive mitral valve surgery''' (Right thoracotomy approach video) | |||
<youtube v=EnJQh_W3r3A/> | |||
*'''Robotic mitral valve repair surgery animation-(1)''' | |||
<youtube v=VrIxRfWDOm8/> | |||
*'''Robotic mitral valve repair surgery animation-(2)''' | |||
<youtube v=GYAmSH2zwic/> | |||
==External links== | |||
http://en.wikipedia.org/wiki/Mitral_valve#cite_note-0 | |||
http://www.nlm.nih.gov/medlineplus/ency/article/000176.htm | |||
http://www.nhlbi.nih.gov/health/health-topics/topics/hs/before.html | |||
http://www.mayoclinic.org/mitral-valve-disease/ | |||
http://www.nlm.nih.gov/medlineplus/ency/article/007411.htm | |||
http://www.nhlbi.nih.gov/health/health-topics/topics/hs/during.html | |||
http://www.nhlbi.nih.gov/health/health-topics/topics/hs/after.html | |||
==References== | |||
<references/> | |||
[[Category:Cardiology]] | |||
[[Category:Surgery]] | |||
[[Category:Cardiac surgery]] | |||
[[Category:Surgical procedures]] | |||
[[Category:Overview complete]] | |||
[[Category:Template complete]] | |||
[[Category:For review]] | |||
{{WH}} | |||
{{WS}} | |||
Revision as of 19:43, 7 September 2011
For the WikiPatient page for this topic, click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]; Associate Editor(s)-In-Chief: Mohammed A. Sbeih, M.D. [2]; Cafer Zorkun, M.D., Ph.D. [3]; Varun Kumar, M.B.B.S.; Lakshmi Gopalakrishnan, M.B.B.S.
Overview
Mitral valve surgery is a surgery that can either repair or replace the mitral valve in the heart. Blood that flows between different chambers of the heart must flow through a valve. One such valve is called the mitral valve. It opens up enough so blood can flow from one chamber of the heart (left atria) to the next chamber (left ventricle). It then closes, keeping blood from flowing backwards. Regurgitation refers to leaking from a valve that doesn't close all the way. Diseases that weaken or damage the valve or the heart tissue around the valve cause mitral regurgitation. Mitral regurgitation is the most common type of heart valve insufficiency. After age 55, some degree of mitral regurgitation is found in almost 20% of men and women who have an echocardiogram. Mitral valve surgery is indicated when the mitral regurgitation is severe or when the patient is symptomatic. Decision between valve repair or valve replacement is made based on the type and severity of damage to mitral valve.
In open surgery, the surgeon makes a large cut in the sternum to reach the heart. Minimally invasive mitral valve surgery is done through much smaller surgical cuts than the large cuts needed for open surgery.
Anatomy and pathophysiology
Anatomy
The mitral valve is typically 4–6 cm² in area. It has two cusps, or leaflets, (the anteromedial leaflet and the posterolateral leaflet) that guard the opening. The opening is surrounded by a fibrous ring known as the mitral valve annulus [1]. The anterior cusp protects approximately two-thirds of the valve (imagine a crescent moon within the circle, where the crescent represents the posterior cusp). Note that although the anterior leaflet takes up a larger part of the ring and rises higher, the posterior leaflet has a larger surface area. These valve leaflets are prevented from prolapsing into the left atrium by the action of tendons attached to the posterior surface of the valve, chordae tendineae.
The inelastic chordae tendineae are attached at one end to the papillary muscles and the other to the valve cusps. Papillary muscles are fingerlike projections from the wall of the left ventricle. Chordae tendineae from each muscle are attached to both leaflets of the mitral valve. Thus, when the left ventricle contracts, the intraventricular pressure forces the valve to close, while the tendons keep the leaflets coapting together and prevent the valve from opening in the wrong direction (thus preventing blood to flow back to the left atrium). Each chord has a different thickness. The thinnest ones are attached to the free leaflet margin, whereas thickest ones are attached quite away from the free margin. This disposition has important effects on systolic stress distribution physiology [2].
Pathophysiology
During left ventricular diastole, after the pressure drops in the left ventricle due to relaxation of the ventricular myocardium, the mitral valve opens, and blood travels from the left atrium to the left ventricle. About 70-80% of the blood that travels across the mitral valve occurs during the early filling phase of the left ventricle. This early filling phase is due to active relaxation of the ventricular myocardium, causing a pressure gradient that allows a rapid flow of blood from the left atrium, across the mitral valve. This early filling across the mitral valve is seen on doppler echocardiography of the mitral valve as the E wave. After the E wave, there is a period of slow filling of the ventricle.
Left atrial contraction (during left ventricular diastole) causes added blood to flow across the mitral valve immediately before left ventricular systole. This late flow across the open mitral valve is seen on doppler echocardiography of the mitral valve as the A wave. The late filling of the LV contributes about 20% to the volume in the left ventricle prior to ventricular systole, and is known as the atrial kick.
The mitral annulus changes in shape and size during the cardiac cycle. It is smaller at the end of atrial systole due to the contraction of the left atrium around it, like a sphincter. This reduction in annulus size at the end of atrial systole may be important for the proper coapting of the leaflets of the mitral valve when the left ventricle contracts and pumps blood [3].
The closing of the mitral valve and the tricuspid valve constitutes the first heart sound (S1). It is not actually the valve closure which produces a sound but rather the sudden cessation of blood flow caused by the closure of the mitral and tricuspid valves. The mitral valve opening is normally not heard except in mitral stenosis (narrowing of the valve) as the opening Snap. Flow of blood into the heart during rapid filling is not normally heard except in certain pathological states where it constitutes the third heart sound (S3).
When the mitral valve doesn't close all the way, blood flows backward into the upper heart chamber (atrium). This leads to a decrease in blood flow to the rest of the body. As a result, the heart may try to pump harder. This may lead to congestive heart failure.
Mitral regurgitation may begin suddenly, most often after a heart attack due to papillary muscle rupture. When the regurgitation does not go away, it becomes chronic (long-term).
Causes of chronic mitral regurgitation include:
- Primary diseases of the valve leaflets such as mitral valve prolapse. MVP is a common cause. However, most patients with MVP do not develop severe mitral regurgitation. Older age, male gender, and auscultatory evidence of severe MR are prognostic clues that identify patients with mitral valve prolapse who are at a relatively high risk of complications.
- Rheumatic heart disease. One out of three cases of chronic mitral regurgitation are caused by rheumatic heart disease, a complication of untreated strep throat that is becoming less common.
- Coronary artery disease and heart attacks.
- cardiomyopathy.
- Endocarditis.
- Heart tumors.
- High blood pressure.
- Marfan syndrome.
- Swelling of the left lower heart chamber.
- Untreated syphilis (rare).
- Congenital (present from birth) mitral regurgitation is most often part of a more complex heart defect or syndrome.
Chronic MR is usually well tolerated during pregnancy. The normal fall in systemic vascular resistance tends to reduce the degree of regurgitation.
Severity of MR can be assessed by both clinical and echocardiographic criteria. careful history is important to establish an estimate of baseline exercise tolerance of the patient. The 2006 ACC/AHA guidelines included recommendations for echocardiographic monitoring in asymptomatic patients with chronic MR [4]. Echocardiography is performed to assess the left ventricular ejection fraction and end-systolic dimension.
There are often no symptoms. When symptoms occur, they often develop gradually, and may include:
- Cough.
- Fatigue, exhaustion, and light-headedness.
- Palpitations (related to atrial fibrillation).
- Shortness of breath during activity and when lying down.
- Urination, excessive at night.
Chronic mitral regurgitation can be divided into three stages; compensated, transitional, and decompensated stage. The stage depends on the left ventricular (LV) chamber size and function.
- In the compensated stage; the left ventricular (LV) end-diastolic dimension is less than 60 mm, and the end-systolic dimension is less than 40mm (Dimensions measured by echocardiography)
- The transitional stage left ventricular (LV) dimensions is not precisely defined, but most studies indicates that surgery at this stage has a very good results.
- The decompensated stage defined on the basis of decompensated ventricular function. At this stage; the patients are at risk for a poor results of valve replacement.
Markers for decompensated ventricular function include:
- Left ventricular end-diastolic dimension greater than 70 mm.
- Left ventricular end-systolic dimension greater than 45 to 47 mm.
- Left ventricular ejection fraction (LVEF) less than 50 to 55 percent.
Knowing the stage of chronic mitral valve regurgitation enables the clinician to predict the LV function, so he or she can decide if the patient could get benefit from the surgical treatment. Usually, a corrective surgery for mitral valve regurgitation should be performed before the transition to the decompensated stage of the disease, because at this stage any treatment may provide symptomatic relief only, but ventricular enlargement and a low LVEF (Left ventricular ejection fraction) usually persist even with successful surgery.
Treatments for Mitral valve regurgitation
The choice of treatment depends on the symptoms present and the condition and function of the heart. Patients with high blood pressure or a weakened heart muscle may be given medications to reduce the strain on the heart and help improve the condition.
Anticoagulant or antiplatelet medications (blood thinners) may be used to prevent clots from forming in patients with atrial fibrillation.
Digitalis may be used to strengthen the heartbeat, along with diuretics (water pills) to remove excess fluid in the lungs.
A low-sodium diet may be helpful. Most people have no symptoms; but if a person develops symptoms, activity may be restricted.
Hospitalization may be required for diagnosis and treatment of severe symptoms. Surgical repair or replacement of the valve is recommended if heart function is poor, symptoms are severe, or the condition gets worse. Once the diagnosis of mitral regurgitation is made, the patient should have regular follow-ups with a specialist to determine whether he or she need surgery.
In the past, patients with heart valve problems such as mitral regurgitation were given antibiotics before dental work or an invasive procedure, such as colonoscopy. The antibiotics were given to prevent an infection of the damaged heart valve. However, antibiotics are now used much less often before dental work and other procedures.
Indications for Mitral valve regurgitation surgery
Surgery is indicated in patients with symptomatic mitral valve regurgitation, also it is indicated in patients with abnormalities in LV size or function (These include a left ventricular ejection fraction (LVEF) of less than 60% and a left ventricular end systolic dimension (LVESD) of greater than 45 mm), pulmonary hypertension, or new onset atrial fibrillation even without symptoms [4]. The patient with severe LV dysfunction (an LVEF < 30% and/or a left ventricular end-systolic dimension greater than 55 mm poses a higher risk but may undergo surgery if chordal preservation is likely. ACC/AHA guidelines recommend that patients with chronic MR who become symptomatic are candidates for corrective mitral surgery [4], even if the symptoms improve with medical therapy or the left ventricle appears to be compensated [4].
Surgery may be recommended in asymptomatic patients with preserved left ventricular function if the surgery performed in a center in which the likelihood of successful surgery is greater than 90 percent, otherwise; the patient can be safely treated with watchful waiting as long as the patient is carefully monitored [5]. The pstient should be seen every 6 to 12 months. Echocardiography should be obtained at these visits. The early surgery exposes the patient to perioperative morbidity and mortality as well as the long-term complications of a prosthetic valve. But it is important to have an objective measure of LV function in patients with asymptomatic MR, because there may be benefit from surgery prior to the onset of symptoms of the depression of the ventricular function in some cases. In patients with borderline values of ventricular size or function in whom access to such monitoring is limited; Surgery may be done earlier.
| Symptoms | LV EF | LVESD |
|---|---|---|
| NYHA II - IV | > 60 percent | < 45 mm |
| Asymptomatic or symptomatic | 50 - 60 percent | ≥ 45 mm |
| Asymptomatic or symptomatic | < 50 percent or ≥ 45 mm | |
| Pulmonary artery systolic pressure ≥ 50 mmHg | ||
The patient may also need valve surgery in the following conditions:
- The changes in the mitral valve are causing major heart symptoms, such as angina (chest pain), shortness of breath, fainting spells (syncope), or heart failure.
- Tests show that the changes in your mitral valve are beginning to seriously affect your heart function.
- The heart valve has been damaged by endocarditis (infection of the heart valve).
- The patient has received a new heart valve in the past, and it is not working well, or you have other problems such as blood clots, infection, or bleeding.
Mitral valve repair is recommended in following:
- Limited damage to certain areas of the mitral valve leaflets or chordae tendineae[7].
- Limited calcification of the leaflets or annulus.
- Prolapse of less than one-third of either leaflet.
- Pure annular dilatation.
- Valvular perforations.
- Incomplete papillary muscle rupture.
Mitral valve replacement is recommended in following:
- Extensive calcification or degeneration of a leaflet or annulus.
- Prolapse of more than one-third of the leaflet tissue.
- Extensive chordal fusion, calcification, or papillary muscle rupture.
- Extensive damage of mitral valve secondary to endocarditis.
Based on above, ACC/AHA 2008 guidelines[8] recommend mitral valve repair rather than mitral valve replacement if the anatomy is appropriate, including patients with rheumatic mitral valve disease[9] and mitral valve prolapse[10] (Grade 1C). The procedure should be performed at experienced surgical centers.
Preoperative preparation
The patient may need to have some tests before the procedure. The Cardiologist usually conducts a physical examination and diagnose the condition within few days, he or she will assess the general health of the patient and will recommend the most appropriate treatment for the patient and if he or she needs surgery. Some of the tests that can be done before the procedure include:
- Cardiac catheterization.
- Chest X-ray.
- Computed tomography (CT) scan.
- Echocardiogram (Doppler echocardiogram).
- Electrocardiogram (ECG).
- Electrophysiology tests.
- Exercise tests.
- Holter monitor.
- Magnetic resonance imaging (MRI).
Many patients with chronic MR requiring surgery also have coronary artery disease[11]. Usually coronary disease treated at the same operation if CABG (Coronary artery bypass grafting) is indicated. Studies showed that concurrent bypass surgery adds little morbidity to the valvular procedure and does not increase the mortality [4]. The 2006 ACC/AHA guidelines on the treatment of valvular heart disease included recommendations for coronary angiography prior to valve surgery in those who are suspected to have coronary artery disease and in those at risk for coronary disease [4]. A noninvasive angiography using computed tomography (CT) or magnetic resonance imaging may be an alternative.
Before the surgery:
- The surgeon needs to know if the patient is taking any drugs, supplements, or herbs before the procedure.
- The patient may be able to store blood in the blood bank for transfusions during and after the surgery. The family members can also donate blood (autologous donation).
- For the 2-week period before surgery, the patient should be asked to stop taking drugs that make it harder for the blood to clot. These might cause increased bleeding during the surgery. Some of these drugs are aspirin, ibuprofen (Advil, Motrin), and naproxen (Aleve, Naprosyn).
- The day before the surgery, the patient should shower and shampoo well and wash the whole body below the neck with a special soap.
- The patient may also be asked to take an antibiotic to guard against infection.
- The patient should be informed which drugs he or she should still take on the day of the surgery.
- The patient should stop smoking.
On the day of the surgery:
- An intravenous (IV) line will be placed into a blood vessel in the patient's arm or chest to give fluids and medicines.
- The patient should be asked not to drink or eat anything after midnight the night before surgery. This includes chewing gum and using breath mints. The patient can rinse mouth with water if it feels dry without swallowing.
- Make sure that the patient is taking the drugs that he or she needs to take with a small sip of water.
- Hair near the incision site may be shaved immediately before the surgery.
- The patient should be informed when to arrive to hospital on the day of the surgery.
The procedure
The Procedure can be done either by the traditional open heart surgery or by the Minimally invasive surgery. Before the surgery, the patient will receive general anesthesia. This will make the patient asleep and pain-free during the entire procedure.
- In thetraditional open heart surgery:
- The surgeon will make a 10-inch-long cut in the middle of the chest (sternum).
- Next, the surgeon will separate the breastbone (sternum) to be able to see the heart.
- Most people are connected to a heart-lung bypass machine or bypass pump. The heart is stopped while the patient is connected to this machine. This machine does the work of the heart while the heart is stopped.
- A small cut is made in the left side of the heart so the surgeon can repair or replace the mitral valve.
- In minimally invasive mitral valve surgery; there are several different ways to perform the procedure:
- The heart surgeon may make a 2-inch to 3-inch-long cut in the right part of your chest near the sternum (breastbone). Muscles in the area will be divided so the surgeon can reach the heart. A small cut is made in the left side of the heart so the surgeon can repair or replace the mitral valve.
- In Endoscopic surgery, the surgeon makes one to four small holes in the chest, then he or she uses special instruments and a camera to do the surgery.
- For Robotically-assisted valve surgery, the surgeon makes two to four tiny cuts (about a ½ to a ¾ inch) in the chest. The surgeon uses a special computer to control robotic arms during the surgery. The surgeon sees a three-dimensional view of the heart and mitral valve on the computer. This method is very precise.
The patient may or may not need to be on a heart-lung machine for these types of surgery, but if not, the heart rate will be slowed by medicine or a mechanical device.
If the surgeon can repair the mitral valve, the patient may have:
- Ring annuloplasty: The surgeon repairs the ring-like part around the valve by sewing a ring of metal, cloth, or tissue around the valve.
- Valve repair: The surgeon trims, shapes, or rebuilds one or more of the three leaflets of the valve. The leaflets are flaps that open and close the valve.
If the mitral valve is too damaged, the patient will need a new valve. This is called Replacement surgery. The surgeon will remove the mitral valve and sew a new one into place. There are two types of valves:
1. Mechanical which is made of man-made (synthetic) materials, such as a metal like titanium. These valves last the longest, but the patient will need to take blood-thinning medicine, such as warfarin (Coumadin) or aspirin, for the rest of his or her life.
2. Biological which made of human or animal tissue. These valves last 10 to 12 years, but the patient may not need to take blood thinners for life.
Once the new or repaired valve is working, the surgeon will:
- Close the heart and take you off the heart-lung machine.
- Place catheters (tubes) around the heart to drain fluids that build up.
- Close the sternum with stainless steel wires. It will take about 6 weeks for the bone to heal. The wires will stay inside the body.
The patient may have a temporary pacemaker connected to the heart until his or her natural heart rhythm returns.
The surgeon may also perform coronary artery bypass surgery at the same time, if needed.
Recovery
Recovery at hospital
The patient may spend 4 to 7 days in the hospital after surgery (much less in Minimally invasive mitral valve surgery-3 to 5 days). Then patient will wake up in the intensive care unit (ICU) and recover there for 1 or 2 days. Two to three tubes will be in the patient's chest to drain fluid from around the heart. They are usually removed 1 to 3 days after surgery.
The patient may have a catheter in the bladder to drain urine, and may also have intravenous lines to get fluids. Nurses will closely watch monitors that show information about the vital signs (pulse, temperature, and breathing).
The patient will be moved to a regular hospital room from the ICU. The nurses and doctors will continue to monitor the heart and vital signs until the patient is stable enough to go home. The patient will receive pain medicine to control pain around your surgical cut.
A nurse should help the patient to slowly resume some activity, and the patient should begin a physical therapy program to make the heart and body stronger. A temporary pacemaker may be placed in the patient's heart if the heart rate becomes too slow after surgery.
Recovery at home
The patient should be informed about the following:
- Taking care for his or her healing incisions.
- Recognizing signs of infection or other complications.
- Coping with after-effects of surgery.
- Followup appointments, medicines, and situations when he or she should call the doctor right away.
- When he or she can go back to daily routine, such as working, driving, and physical activity.
After-effects of heart surgery are normal. They may include muscle pain, chest pain, or swelling. Other after-effects may include loss of appetite, problems sleeping, constipation, and mood swings and depression. After-effects usually go away over time.
Less recovery time is needed for off-pump heart surgery and minimally invasive heart surgery.
Ongoing care
Ongoing care after valve surgery may include periodic checkups with the doctor. During these visits, the patient may have blood tests, an EKG (electrocardiogram), echocardiography, or a stress test. These tests will show how the patient's heart is working after the surgery.
Routine tests should be done to make sure the patient is getting the right amount of the blood-thinning medicine in case of mechanical valve placement.
The patient may be advised to change his or her lifestyle, this includes: quitting smoking, making changes to diet, being physically active, and reducing and managing stress.
Surgical outcome
The results of mitral valve repair are excellent in the centers that regularly perform this surgery.
Techniques for minimally invasive heart valve surgery have improved greatly over the past 10 years. These techniques are safe for most patients, and they reduce recovery time and pain.
Valve repair versus valve replacement
Advantages of Mitral valve repair include:
- Lower operative mortality rate [12][13]
- Improves left ventricular EF and function [14].
- Preserves native heart valve and avoids the use of a prosthetic heart valve with its complications.
- Has good overall outcome with good survival rates [15][10][16].
- Lower risk for endocarditis.
- Avoids long term use of anticoagulants.
Mechanical versus Biological valves
Mechanical heart valves do not fail often. They last from 12 to 20 years. However, blood clots develop on them. If a blood clot forms, the patient may have a stroke. Bleeding can occur, but this is rare. Biological valves tend to fail over time [17][18], but they have a lower risk of blood clots.
Possible complications
Risks for any surgery
- Blood clots in the legs that may travel to the lungs.
- Blood loss.
- Breathing problems.
- Infection, including in the lungs, kidneys, bladder, chest, or heart valves.
- Reactions to medicines.
Possible risks from having open-heart surgery
- Heart attack or stroke.
- Heart rhythm problems.
- Infection in the cut, which is more likely to happen in people who are obese, have diabetes, or have already had this surgery.
- Memory loss and loss of mental clarity, or "fuzzy thinking."
- Post-pericardiotomy syndrome, which is a low-grade fever and chest pain. This could last for up to 6 months.
Prosthetic heart valves are associated with a variety of complications
- Structural deterioration, particularly with bioprosthetic valves.
- Valve obstruction due to thrombosis or pannus formation.
- Systemic embolization.
- Bleeding.
- Endocarditis and other infections.
- Left ventricular systolic dysfunction, which may be preexisting.
- Hemolytic anemia.
Videos
- Minimally invasive mitral valve surgery (Right thoracotomy approach video)
<youtube v=EnJQh_W3r3A/>
- Robotic mitral valve repair surgery animation-(1)
<youtube v=VrIxRfWDOm8/>
- Robotic mitral valve repair surgery animation-(2)
<youtube v=GYAmSH2zwic/>
External links
http://en.wikipedia.org/wiki/Mitral_valve#cite_note-0
http://www.nlm.nih.gov/medlineplus/ency/article/000176.htm
http://www.nhlbi.nih.gov/health/health-topics/topics/hs/before.html
http://www.mayoclinic.org/mitral-valve-disease/
http://www.nlm.nih.gov/medlineplus/ency/article/007411.htm
http://www.nhlbi.nih.gov/health/health-topics/topics/hs/during.html
http://www.nhlbi.nih.gov/health/health-topics/topics/hs/after.html
References
- ↑ Shinoda H, Stern PH (1992). "Diurnal rhythms in Ca transfer into bone, Ca release from bone, and bone resorbing activity in serum of rats". Am J Physiol. 262 (2 Pt 2): R235–40. PMID 1539731.
- ↑ Nazari S, Carli F, Salvi S, Banfi C, Aluffi A, Mourad Z; et al. (2000). "Patterns of systolic stress distribution on mitral valve anterior leaflet chordal apparatus. A structural mechanical theoretical analysis". J Cardiovasc Surg (Torino). 41 (2): 193–202. PMID 10901521.
- ↑ Pai RG, Varadarajan P, Tanimoto M (2003). "Effect of atrial fibrillation on the dynamics of mitral annular area". J Heart Valve Dis. 12 (1): 31–7. PMID 12578332.
- ↑ 4.0 4.1 4.2 4.3 4.4 4.5 Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD; et al. (2008). "2008 Focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the 1998 Guidelines for the Management of Patients With Valvular Heart Disease): endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". Circulation. 118 (15): e523–661. doi:10.1161/CIRCULATIONAHA.108.190748. PMID 18820172.
- ↑ Rosenhek R, Rader F, Klaar U, Gabriel H, Krejc M, Kalbeck D; et al. (2006). "Outcome of watchful waiting in asymptomatic severe mitral regurgitation". Circulation. 113 (18): 2238–44. doi:10.1161/CIRCULATIONAHA.105.599175. PMID 16651470.
- ↑ "ACC/AHA guidelines for the management of patients with valvular heart disease. A report of the American College of Cardiology/American Heart Association. Task Force on Practice Guidelines (Committee on Management of Patients with Valvular Heart Disease)". Journal of the American College of Cardiology. 32 (5): 1486–588. 1998. PMID 9809971. Retrieved 2011-03-16. Unknown parameter
|month=ignored (help) - ↑ Gillinov AM, Faber C, Houghtaling PL, Blackstone EH, Lam BK, Diaz R; et al. (2003). "Repair versus replacement for degenerative mitral valve disease with coexisting ischemic heart disease". J Thorac Cardiovasc Surg. 125 (6): 1350–62. PMID 12830055.
- ↑ Bonow RO, Carabello BA, Chatterjee K, de Leon AC, Faxon DP, Freed MD; et al. (2008). "2008 focused update incorporated into the ACC/AHA 2006 guidelines for the management of patients with valvular heart disease: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to revise the 1998 guidelines for the management of patients with valvular heart disease). Endorsed by the Society of Cardiovascular Anesthesiologists, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons". J Am Coll Cardiol. 52 (13): e1–142. doi:10.1016/j.jacc.2008.05.007. PMID 18848134.
- ↑ Yau TM, El-Ghoneimi YA, Armstrong S, Ivanov J, David TE (2000). "Mitral valve repair and replacement for rheumatic disease". J Thorac Cardiovasc Surg. 119 (1): 53–60. PMID 10612761.
- ↑ 10.0 10.1 Mohty D, Orszulak TA, Schaff HV, Avierinos JF, Tajik JA, Enriquez-Sarano M (2001). "Very long-term survival and durability of mitral valve repair for mitral valve prolapse". Circulation. 104 (12 Suppl 1): I1–I7. PMID 11568020.
- ↑ Lin SS, Lauer MS, Asher CR, Cosgrove DM, Blackstone E, Thomas JD; et al. (2001). "Prediction of coronary artery disease in patients undergoing operations for mitral valve degeneration". J Thorac Cardiovasc Surg. 121 (5): 894–901. doi:10.1067/mtc.2001.112463. PMID 11326232.
- ↑ Tribouilloy CM, Enriquez-Sarano M, Schaff HV, Orszulak TA, Bailey KR, Tajik AJ; et al. (1999). "Impact of preoperative symptoms on survival after surgical correction of organic mitral regurgitation: rationale for optimizing surgical indications". Circulation. 99 (3): 400–5. PMID 9918527.
- ↑ Krayenbuehl HP (1986). "Surgery for mitral regurgitation. Repair versus valve replacement". Eur Heart J. 7 (8): 638–43. PMID 3769948.
- ↑ Enriquez-Sarano M, Schaff HV, Orszulak TA, Tajik AJ, Bailey KR, Frye RL (1995). "Valve repair improves the outcome of surgery for mitral regurgitation. A multivariate analysis". Circulation. 91 (4): 1022–8. PMID 7850937.
- ↑ Lee EM, Shapiro LM, Wells FC (1997). "Superiority of mitral valve repair in surgery for degenerative mitral regurgitation". Eur Heart J. 18 (4): 655–63. PMID 9129898.
- ↑ Thourani VH, Weintraub WS, Guyton RA, Jones EL, Williams WH, Elkabbani S; et al. (2003). "Outcomes and long-term survival for patients undergoing mitral valve repair versus replacement: effect of age and concomitant coronary artery bypass grafting". Circulation. 108 (3): 298–304. doi:10.1161/01.CIR.0000079169.15862.13. PMID 12835220.
- ↑ Hammermeister KE, Sethi GK, Henderson WG, Oprian C, Kim T, Rahimtoola S (1993). "A comparison of outcomes in men 11 years after heart-valve replacement with a mechanical valve or bioprosthesis. Veterans Affairs Cooperative Study on Valvular Heart Disease". N Engl J Med. 328 (18): 1289–96. doi:10.1056/NEJM199305063281801. PMID 8469251.
- ↑ Hammermeister K, Sethi GK, Henderson WG, Grover FL, Oprian C, Rahimtoola SH (2000). "Outcomes 15 years after valve replacement with a mechanical versus a bioprosthetic valve: final report of the Veterans Affairs randomized trial". J Am Coll Cardiol. 36 (4): 1152–8. PMID 11028464.