|
|
| (138 intermediate revisions by 14 users not shown) |
| Line 1: |
Line 1: |
| | __NOTOC__ |
| | '''For patient information click [[Hypertrophic cardiomyopathy (patient information)|here]]''' |
| | |
| {{Infobox Disease | | {{Infobox Disease |
| | Name = {{PAGENAME}} | | | Name = {{PAGENAME}} |
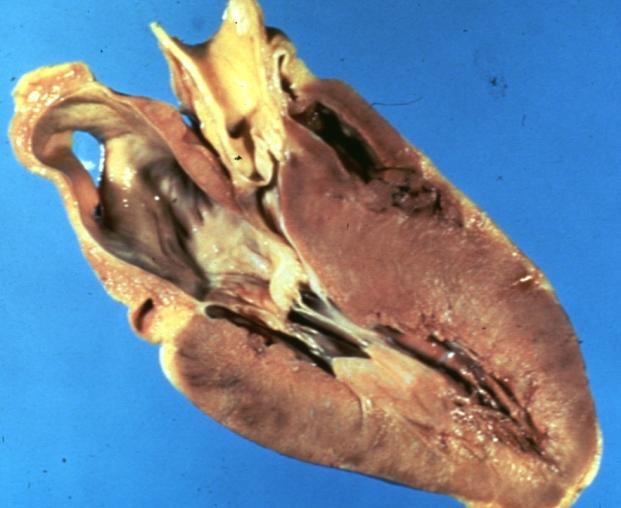
| | Image = 2369.jpg | | | Image = 2369.jpg |
| | Caption = Hypertrophic cardiomyopathy. <br> <small> [http://www.peir.net Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] </small> | | | Caption = Hypertrophic cardiomyopathy. <br> <small> [http://www.peir.net Image courtesy of Professor Peter Anderson DVM PhD and published with permission © PEIR, University of Alabama at Birmingham, Department of Pathology] </small> |
| | DiseasesDB = 6373
| |
| | ICD10 = {{ICD10|I|42|1|i|30}}-{{ICD10|I|42|2|i|30}}
| |
| | ICD9 = {{ICD9|425.4}}
| |
| | ICDO =
| |
| | OMIM =
| |
| | MedlinePlus = 000192
| |
| | eMedicineSubj = med
| |
| | eMedicineTopic = 290
| |
| | eMedicine_mult = {{eMedicine2|ped|1102}} {{eMedicine2|radio|129}}
| |
| | MeshID = D002312
| |
| }} | | }} |
| {{SI}} | | {{Hypertrophic cardiomyopathy}} |
| '''Editors-In-Chief:''' C. Michael Gibson, M.S., M.D. [mailto:mgibson@perfuse.org], Cafer Zorkun, M.D. [mailto:zorkun@perfuse.org], Caitlin J. Harrigan [mailto:charrigan@perfuse.org], Martin S. Maron, M.D., and Barry J. Maron, M.D.
| |
|
| |
| {{Editor Join}}
| |
| | |
| '''''Synonyms and Related Terms:'''''
| |
| '''Hypertrophic cardiomyopathy''' or '''HCM''', '''Asymmetric septal hypertrophy''' or '''ASH''', '''Hypertrophic obstructive cardiomyopathy''' '''HOCM''', '''Idiopathic hypertrophic subaortic stenosis''' or '''IHSS''',
| |
| | |
| A non-obstructive variant of HCM is known as '''apical hypertrophic cardiomyopathy''' <ref name="Rivera-Diaz, Moosvi 1996">Rivera-Diaz J, Moosvi AR. Apical hypertrophic cardiomyopathy. ''South Med J''. 1996 Jul; '''89'''(7):711-3. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8685759 Medline abstract]; [http://www.sma.org/smj/96jul12.htm Full text])</ref>,
| |
| which is also known as '''nonobstructive hypertrophic cardiomyopathy''' and '''Japanese variant hypertrophic cardiomyopathy''' or the '''Yamaguchi variant''' (since the first cases described were all in individuals of Japanese descent).
| |
| | |
| ==Overview==
| |
| '''Hypertrophic cardiomyopathy''', or '''HCM''', is a disease of the [[myocardium]] (the [[muscle]] of the [[heart]]) in which a portion of the myocardium is [[left ventricular hypertrophy|hypertrophied]] (thickened) without any alternate cause such as [[hypertension]], [[amyloid]] or [[aortic stenosis]].<ref name="Richardson, McKenna et al 1996">Richardson P, McKenna W, Bristow M, Maisch B, Mautner B, O'Connell J, Olsen E, Thiene G, Goodwin J, Gyarfas I, Martin I, Nordet P. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the Definition and Classification of cardiomyopathies. ''[[Circulation (journal)|Circulation]]''. 1996 Mar 1; '''93'''(5):841–2. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8598070 Medline abstract]; [http://circ.ahajournals.org/cgi/content/full/93/5/841 Full text])</ref><ref name="Maron 2002">Maron B. Hypertrophic cardiomyopathy: a systematic review. ''[[Journal of the American Medical Association|JAMA]]'' 2002. '''287''':1308–20</ref><ref name="Sherrid Chaudhry et al 2003">Sherrid M, Chaudhry FA, Swistel DG. Obstructive hypertrophic cardiomyopathy. Echocardiography, pathophysiology, and the continuing evolution of surgery for obstruction. ''Annals of Thoracic Surgery'' 2003; '''75''':620–32</ref><ref name="Wigle, Sasson et al 1985">Wigle D, Sasson Z, Henderson MA, Ruddy TD, Fulop J, Rakowski H, Williams WG. Hypertrophic cardiomyopathy. The importance of the site and the extent of hypertrophy. A review. Progress in Cardiovascular Diseases 1985; '''28''':1–83</ref><ref name="Wigle, Rakowski et al 1995">Wigle ED, Rakowski H, Kimball BP, Williams WG. Hypertrophic cardiomyopathy — clinical spectrum and treatment. ''[[Circulation (journal)|Circulation]]'' 1995; '''92''':1680–92</ref><ref name="Maron, McKenna et al 2003">Maron BJ, McKenna WJ, Danielson GK, Kappenberger LJ, Kuhn HJ, Seidman CE, Shah PM, Spencer WH III, Spirito P, Ten Cate FJ, Wigle ED. [[American College of Cardiology]] / [[European Society of Cardiology]] clinical expert consensus document on hypertrophic cardiomyopathy. ''[[Journal of the American College of Cardiology|J Am Coll Cardiol]]''. 2003; '''42''':1687–713
| |
| </ref> Although HCM has gained notoriety as a leading cause of [[sudden cardiac death]] in young athletes,
| |
| <ref name="Maron, Thompson et al 1996">Maron BJ, Thompson PD, Puffer JC, McGrew CA, Strong WB, Douglas PS, Clark LT, Mitten MJ, Crawford MH, Atkins DL, Driscoll DJ, Epstein AE. Cardiovascular preparticipation screening of competitive athletes. A statement for health professionals from the Sudden Death Committee (clinical cardiology) and Congenital Cardiac Defects Committee (cardiovascular disease in the young), American Heart Association. ''[[Circulation (journal)|Circulation]]''. 1996 Aug 15; '''94'''(4):850-6. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8772711 Medline abstract]; [http://circ.ahajournals.org/cgi/content/full/94/4/850 Full text])</ref>
| |
| it should be noted that HCM is a cause of [[sudden cardiac death]] in any age group and may be associated with cardiac morbidity and disabling cardiac symptoms as well.
| |
| | |
| ==Pathophysiology and Etiology==
| |
| ===Histopathologic Abnormalities: Myocardial Disarray===
| |
| | |
| A [[cardiomyopathy]] is any disease that primarily affects the muscle of the heart. In HCM, the normal alignment of muscle cells is disrupted (there is a swirling pattern to the arrangement of the muscle cells), a phenomenon known as [[myocardial disarray]]. HCM is believed to be due to a mutation in one of many [[gene]]s that results in a mutated myosin heavy chain, one of the components of the [[myocyte]] (the muscle cell of the heart). Histopathologically, the cardiac [[sarcomere]] is abnormal resulting in hypertrophy of the [[left ventricle]] in the absence of other disorders that could produce the condition such as [[hypertension]], [[amyloid]] or [[aortic stenosis]].
| |
| | |
| <div align="left">
| |
| <gallery heights="115" widths="115">
| |
| Image:438.jpg|Myocardial disarray with swirling pattern of myocytes
| |
| Image:HCP diagram.JPG|Variants of hypertrophic cardiomyopathy
| |
| Image:427.jpg|White areas of fibrosis or scar in a patient with HCM which may contribute in part to arrhythmias
| |
| </gallery>
| |
| </div>
| |
| | |
| ===Histopathologic Abnormalities: Periarteriolar Fibrosis===
| |
| | |
| Compared to normal arterioles on the left, the arterioles from a patient with hyertension (middle) show moderate periarteriolar thickening and fibrosis. Shown on the right is a patient with HCM in which there is even more signficant periarteriolar thickening and fibrosis. This thickening of the wall of the intramyocardial arterioles leads to an increased wall/lumen ratio, subendocardial ischemia and impaired coronary flow reserve. Patients who subsequently died in one series had abnormal coronary flow reserve on PET scanning at baseline indicating that ischemia may play a role, at least in part, in subsequent mortality.
| |
| | |
| <div align="left">
| |
| <gallery heights="115" widths="115">
| |
| Image:Arteriole-normal.jpg|Normal arteriole
| |
| Image:Arteriole-hypertensive.jpg|Hypertensive arteriole with wall thickening and myocyte hypertrophy
| |
| Image:Arteriole-hcm.jpg|Arteriole in HCM patient with periarteriole fibrosis and thicknening
| |
| </gallery>
| |
| </div>
| |
| | |
| ===Anatomic Abnormalities: Septal and Ventricular Hypertrophy===
| |
| | |
| Individuals with HCM have some degree of [[left ventricular hypertrophy]]. In approximately 2/3rds of cases this is an asymmetric hypertrophy, involving the inter-ventricular septum, and is known as [[asymmetric septal hypertrophy]] ([http://www.cardiomyopathy.org/html/which_card_hcm.htm ASH]). This is in contrast to the concentric hypertrophy seen in [[aortic stenosis]] or [[hypertension]].
| |
| | |
| The degree of ventricular involvement is variable ranging from diffuse involvement of both ventricles to isolated involvement of a portion of one segment of the [[LV]].
| |
| | |
| Data from two large registries indicates that;
| |
| *55% of cases involve the septum and anterolateral free wall,
| |
| *20% involve the entire septum alone,
| |
| *10% are limited to the basal septum and 15% are limited to the apical or distal LV (Yamaguchi variant).
| |
| | |
| Some genetic variants may manifest very little overt [[LVH]] but have an increased risk of [[sudden cardiac death]] (SCD).
| |
| | |
| ===Functional Abnormalities: Dynamic Outflow Obstruction===
| |
| Depending on the degree of obstruction of the outflow of [[blood]] from the [[left ventricle]] of the heart, HCM can be defined as obstructive or non-obstructive. About 25% of individuals with HCM demonstrate an obstruction to the outflow of blood from the left ventricle during rest. In other individuals obstruction only occurs under certain conditions. This is known as dynamic outflow obstruction, because the degree of obstruction is variable and is dependent on the amount of blood in the ventricle immediately before ventricle [[systole (medicine)|systole]] (contraction).
| |
| | |
| Dynamic outflow obstruction (when present in HCM) is usually due to systolic anterior motion (SAM) of the anterior leaflet of the [[mitral valve]]. Systolic anterior motion of the mitral valve (SAM) was initially thought to be due to the septal subaortic bulge, narrowing the outflow tract, causing high velocity flow and a Venturi effect — a local underpressure in the outflow tract. Low pressure was thought to suck the mitral valve anteriorly into the septum. But SAM onset is observed to be a low velocity phenomenon: SAM begins at velocities no different from those measured in normals
| |
| <ref name="Jiang, Levine et al 1987">Jiang L, Levine RA, King ME, Weyman AE. An integrated mechanism for systolic anterior motion of the mitral valve in hypertrophic cardiomyopathy based on echocardiographic observations. ''Am Heart J'' 1987; '''113''':633–44</ref>
| |
| <ref name="Sherrid, Gunsburg et al 2000">Sherrid MV, Gunsburg DZ, Moldenhauer S, Pearle G. Systolic anterior motion begins at low left ventricular outflow tract velocity in obstructive hypertrophic cardiomyopathy. ''[[Journal of the American College of Cardiology|J Am Coll Cardiol]]'' 2000; '''36''':1344–54</ref>.
| |
| Hence, the magnitude and importance of Venturi forces in the outflow tract are much less than previously thought, and Venturi forces cannot be the main force that initiates SAM.
| |
| | |
| Recent echocardiographic evidence indicates that drag, the pushing force of flow is the dominant hydrodynamic force on the mitral leaflets
| |
| <ref name="Jiang, Levine et al 1987"/>
| |
| <ref name="Sherrid, Gunsburg et al 2000"/>
| |
| <ref name="Sherrid, Chu et al 1993">Sherrid MV, Chu Ck, DeLia E, Mogtader A, Dwyer Jr. EM, An echocardiographic study of the fluid mechanics of obstruction in hypertrophic cardiomyopathy. ''[[Journal of the American College of Cardiology|J Am Coll Cardiol]]'' 1993; '''22''':816–25</ref>
| |
| <ref name="Levine, Vlahakes et al 1995">Levine RA, Vlahakes GJ, Lefebvre X, et al. Papillary muscle displacement causes systolic anterior motion of the mitral valve. ''[[Circulation (journal)|Circulation]]'' 1995; '''91''':1189–95</ref>
| |
| <ref name="Messmer 1994">Messmer BJ. Extended myectomy for hypertrophic obstructive cardiomyopathy. ''Ann Thorac Surg'' 1994; '''58''':575–7</ref>
| |
| <ref name="Schoendube, Klues et al 1995">Schoendube FA, Klues HG, Reith S, Flachskampf FA, Hanrath P, Messmer BJ. Long-term clinical and echocardiographic follow-up after surgical correction of hypertrophic obstructive cardiomyopathy with extended myectomy and reconstruction of the subvalvular mitral apparatus. ''[[Circulation (journal)|Circulation]]'' 1995; '''92''':II-122–7</ref>.
| |
| In obstructive HCM the mitral leaflets are often large
| |
| <ref name="Klues, Maron et al 1992">Klues HG, Maron BJ, Dollar AL, Roberts WC. Diverstiy of structural mitral valve alterations in hypertrophic cardiomyopathy. ''[[Circulation (journal)|Circulation]]'' 1992; '''85''':1651–60</ref>
| |
| and are anteriorly positioned in the LV cavity
| |
| <ref name="Jiang, Levine et al 1987"/>
| |
| <ref name="Henry, Clark et al 1975">Henry WL, Clark CE, Griffith JM, Epstein SE. Mechanism of left ventricular outflow obstruction in patients with obstructive asymmetric septal hypertrophy (idiopathic hypertrophic subaortic stenosis). ''Am J Cardiol'' 1975; '''35''':337–45</ref>
| |
| due to anteriorly positioned papillary muscles<ref name="Jiang, Levine et al 1987"/> that at surgery are often "agglutinated" onto the LV anterior wall by abnormal attachments
| |
| <ref name="Messmer 1994"/>
| |
| <ref name="Schoendube, Klues et al 1995"/>.
| |
| | |
| The mid-septal bulge aggravates the malposition of the valve and redirects outflow so that it comes from a lateral and posterior direction<ref name="Sherrid, Chu et al 1993"/>. The abnormally directed outflow may be visualized behind and lateral to the enlarged mitral valve, where it catches it, and pushes it into the septum <ref name="Jiang, Levine et al 1987"/>
| |
| <ref name="Sherrid, Gunsburg et al 2000"/>
| |
| <ref name="Sherrid, Chu et al 1993"/>
| |
| <ref name="Levine, Vlahakes et al 1995"/>.
| |
| There is a crucial overlap between the inflow and outflow portions of the left ventricle
| |
| <ref name="Schwammenthal and Levine 1996">Schwammenthal E, Levine RA. Dynamic subaortic obstruction: a disease of the mitral valve suitable for surgical repair? ''[[Journal of the American College of Cardiology|J Am Coll Cardiol]]'' 1996; '''28''':203–6</ref>.
| |
| As SAM progresses in early systole the angle between outflow and the protruding mitral leaflet increases. A greater surface area of the leaflets is now exposed to drag which amplifies the force on the leaflets – drag increases with increasing angle relative to flow<ref name="Sherrid, Chu et al 1993"/>. An analogy is an open door in a drafty corridor: the door starts by moving slowly and then accelerates as it presents a greater surface area to the wind and finally it slams shut. The necessary conditions that predispose to SAM are: anterior position of the mitral valve in the LV, altered LV geometry that allows flow to strike the mitral valve from behind, and chordal slack
| |
| <ref name="Jiang, Levine et al 1987"/>
| |
| <ref name="Sherrid, Gunsburg et al 2000"/>
| |
| <ref name="Sherrid, Chu et al 1993"/>
| |
| <ref name="Levine, Vlahakes et al 1995"/>.
| |
| SAM may considered anteriorly directed mitral prolapse
| |
| <ref name="Sherrid, Gunsburg et al 2000"/>
| |
| <ref name="Sherrid, Chu et al 1993"/>
| |
| <ref name="Levine, Vlahakes et al 1995"/>.
| |
| In both conditions the mitral valve is enlarged and is displaced in systole by the pushing force of flow resulting in mitral regurgitation.
| |
| | |
| Because the mitral valve leaflet doesn't get pulled into the LVOT until after the aortic valve opens, the initial upstroke of the arterial pulse will be normal. When the mitral valve leaflet gets pushed into the LVOT, the arterial pulse will momentarily collapse and be followed by a second rise, as the left ventricular pressure overcomes the increased obstruction that SAM of the mitral valve causes. This can be seen on the physical examination as a double tap upon palpation of the apical impulse and as a double pulsation upon palpation of the carotid pulse, known as ''[[pulsus bisferiens]]''.
| |
| | |
| ===Functional Abnormalities:Arrhythmogenesis===
| |
| | |
| HCM also causes disruptions of the electrical functions of the heart and can be associated with [[sudden cardiac death]].
| |
| | |
| Primary arrhythmias, the presence of scar or fibrosis, hemodynamic instability with diminished [[stroke volume]], and/or ischemia have been implicated in the etiology of sudden cardiac death in HCM.
| |
| | |
| It must be emphasized that atrial arrhythmias (which are commonly detected on ambulatory monitoring) can lead to ischemia and hemodynamic compromise leading to sudden death in these patients as well.
| |
| | |
| Assessment of autonomic function in patients with HCM often reveals abnormal responses of heart rate and blood pressure to exercise in two-thirds, which was associated with a more malignant clinical course, suggesting that autonomic imbalance may also be important in the genesis of sudden cardiac death in these patients.
| |
| | |
| ==Epidemiology and Demographics==
| |
| While most literature so far focuses on European, American, and Japanese populations, HCM appears in all racial groups. The incidence of HCM is about 0.2% to 0.5% of the general population.
| |
| | |
| ==Genetics==
| |
| | |
| HCM is the most common genetically transmitted cardiovascular disease. Penetrance of HCM is incomplete and age-related. The disease may be sporadic but affected family members are discovered in 13% of cases. More than 70 mutations involving at least 7 chromosomes encoding structural proteins of the myocyte have been discovered. These mutations have varying degrees of penetrance and even the same mutation may have variable expression, implying superimposed effects of other genes or environmental influences.
| |
| | |
| Hypertrophic cardiomyopathy is inherited as an [[autosomal dominant]] trait and is attributed to mutations in one of a number of genes that encode for one of the [[sarcomere]] [[protein]]s including beta-cardiac myosin heavy chain (the first gene identified), cardiac actin, cardiac troponin T, alpha-tropomyosin, cardiac troponin I, cardiac myosin-binding protein C, and the myosin light chains. Currently there are more than 400 mutations in these genes. The prognosis is variable, based on the gene mutation. In individuals without a family history of HCM, the most common cause of the disease is a [[mutation|de novo mutation]] of the gene that produces the β-myosin heavy chain.
| |
| | |
| An insertion/deletion polymorphism in the [[gene]] encoding for [[angiotensin converting enzyme]] (ACE) alters the clinical [[phenotype]] of the disease. The D/D (deletion/deletion) genotype of ACE is associated with more marked hypertrophy of the left ventricle and may be associated with higher risk of adverse outcomes
| |
| <ref name="Doolan, Nguyen et al 2004">Doolan G, Nguyen L, Chung J, Ingles J, Semsarian C. Progression of left ventricular hypertrophy and the angiotensin-converting enzyme gene polymorphism in hypertrophic cardiomyopathy. ''Int J Cardiol''. 2004 Aug; '''96'''(2):157–63. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=15314809 Medline abstract])</ref> <ref name="Marian, Yu et al 1993">Marian AJ, Yu QT, Workman R, Greve G, Roberts R. Angiotensin-converting enzyme polymorphism in hypertrophic cardiomyopathy and sudden cardiac death. ''Lancet''. 1993 Oct 30; '''342'''(8879):1085–6. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=8105312 Medline abstract])</ref>.
| |
| | |
| ===Specific Chromosomal Abnormalities===
| |
|
| |
|
| ====β [[Myosin]] Heavy Chain-Chromosome 14 q11.2-3====
| | '''Editor-In-Chief:''' [[C. Michael Gibson, M.S., M.D.]] [mailto:charlesmichaelgibson@gmail.com] {{AE}} {{Soroush}} |
|
| |
|
| Accounts for approximately 35%-45% of cases. Significant [[LVH]] ([[left ventricular hypertrophy]]) is usually noted. The Arg403Gln mutation is associated with an extremely poor prognosis with average age of death at 33 years, while the Val606Met mutation is associated with a better prognosis.
| | {{SK}} Hypertrophic cardiomyopathy or HCM, Asymmetric septal hypertrophy or ASH, Hypertrophic obstructive cardiomyopathy, HOCM, Idiopathic hypertrophic subaortic stenosis or IHSS, familial isolated hypertrophic obstructive cardiomyopathy, familial isolated hypertrophic subaortic stenosis, familial hypertrophic subaortic stenosis, idiopathic hypertrophic subaortic stenosis, familial hypertrophic obstructive cardiomyopathy, idiopathic hypertrophic obstructive cardiomyopathy, primitive hypertrophic obstructive cardiomyopathy, primitive hypertrophic subaortic stenosis, muscular subaortic stenosis, apical hypertrophic cardiomyopathy, which is also known as nonobstructive hypertrophic cardiomyopathy and Japanese variant hypertrophic cardiomyopathy or Yamaguchi syndrome. |
|
| |
|
| ====Cardiac Myosin Binding Protein-C-Chromosome 11==== | | ==[[Hypertrophic cardiomyopathy overview|Overview]]== |
| | ==[[Hypertrophic cardiomyopathy historical perspective|Historical Perspective]]== |
| | ==[[Hypertrophic cardiomyopathy classification|Classification]]== |
|
| |
|
| Accounts for another 15%-35% but has a reduced penetrance so may actually be more. Patients generally present later in life and in general, have a better prognosis than with the prior 2 mutations. Up to 60% at age 50 years have no [[LVH]].
| | ==[[Hypertrophic cardiomyopathy pathophysiology|Pathophysiology]]== |
|
| |
|
| ====Cardiac Troponin T-Chromosome 11==== | | ==[[Hypertrophic cardiomyopathy differential diagnosis|Differentiating Hypertrophic Cardiomyopathy from Other Diseases]]== |
| | ==[[Hypertrophic cardiomyopathy causes|Causes]]== |
|
| |
|
| Accounts for approximately 15% of cases. Substantially less hypertrophy is noted but histology demonstrates the characteristic myocyte disarray of HCM. Most mutations of this gene are associated with markedly reduced survival.
| | ==[[Hypertrophic cardiomyopathy epidemiology and demographics|Epidemiology and Demographics]]== |
| | ==[[Hypertrophic cardiomyopathy risk factors|Risk Factors]]== |
| | ==[[Hypertrophic cardiomyopathy screening|Screening]]== |
|
| |
|
| ====Other Genetic Abnormalities====
| |
|
| |
|
| Other genes encoding alpha tropomyosin, myosin regulatory light chain, cardiac [[troponin I]] and cardiac [[troponin C]] have been implicated. A mutation on Chromosome 7 is associated with HCM and the [[WPW]] ([[Wolff-Parkinson-White syndrome]]).
| | ==[[Hypertrophic cardiomyopathy natural history|Natural History, Complications and Prognosis]]== |
| | |
| ==Screening==
| |
| | |
| * Screening for HCM is a controversial subject in the medical community, as HCM is the leading cause of [[sudden death]] in athletes.
| |
| * In Italy, all competitive athletes are required to go through pre-participation screening of HCM
| |
| * This particular program has been in existence since 1982, and screening generally includes:
| |
| *:* 12-lead ECG
| |
| *:* General and cardiovascular physical examination, including blood pressure measurements
| |
| *:* Family history
| |
| * Over 3 million competitive athletes are evaluated each year, and athletes judged to be free of cardiovascular disease receive a certificate enabling them to participate in competitive athletics<cite>italyref1</cite>
| |
| * In a study done in Italy over a 9-year period (1990-1998), 4450 competitive athletes were screened for HCM. Based on echocardiographic evaluation, 4397 of the athletes were excluded from a clinical diagnosis of HCM<cite>italyref1</cite>
| |
| *:* 41 of the 4450 athletes showed [[LV]] hypertrophy, with increased wall thicknesses, with four athletes being on the border, with maximal [[LV]] wall thickness of 13 mm
| |
| | |
| ==Natural History==
| |
| | |
| ===Sudden Cardiac Death===
| |
| | |
| The incidence of [[sudden cardiac death]] (SCD) in patients with HCM is 2 to 4 percent per year in adults, and a 4 to 6 percent per year in children and adolescents. A review of 78 patients with HCM who died suddenly or survived a cardiac arrest episode showed that 71 percent were younger than 30 years of age, 54 percent were without functional limitation, and 61 percent were performing sedentary or minimal physical activity at the time of cardiac arrest.
| |
| | |
| ====Predictors of Sudden Cardiac Death====
| |
| | |
| There are few predictors of SCD in patients with HCM.
| |
| *Onset of symptoms in childhood
| |
| *A clinical history of spontaneous, sustained monomorphic VT or sudden death in family members.
| |
| *History of impaired consciousness
| |
| *Atrial arrhythmias
| |
| *Development of [[systolic dysfunction]]
| |
| *[[Non-sustained ventricular tachycardia]] (NSVT) in patients with symptoms
| |
| *[[Left ventricular]] wall thickness >30 mm. A recent report of 480 patients showed that left ventricular wall thickness was useful in identifying patients at high risk for sudden cardiac death. However, sudden cardiac death can occur in children and adolescents in the absence of left ventricular hypertrophy as well.
| |
| | |
| ====Prognosis in Survivors of Sudden Cardiac Death====
| |
| | |
| Survivors of SCD have a poor prognosis. Event free survival at 1,5 and 10 years was 83, 65 and 53 percent respectively.
| |
| | |
| ====Role of Electrophysiologic Testing====
| |
| | |
| The prognostic value of electrophysiologic testing in the absence of spontaneous, sustained ventricular tachycardia is limited, and in fact, the study itself may be dangerous. Sustained ventricular tachyarrhythmias, predominantly rapid polymorphic ventricular tachycardia, have been induced in 27 to 43 percent of patients with HCM at electrophysiologic study, but their prognostic significance is controversial. The predictive value of asymptomatic nonsustained ventricular tachycardia is also limited. Paced electrogram fractionation in hypertrophic cardiomyopathy may helpful in determining which patients are at risk for [[ventricular fibrillation]].
| |
| | |
| The absence of inducible, sustained monomorphic ventricular tachyarrhythmias, absence of nonsustained ventricular tachycardia on ambulatory ECG, and no history of ''impaired consciousness'' (i.e., cardiac arrest or syncope) identified a subset (22 percent) of patients with HCM with a low (<1 percent) risk for sudden cardiac death.
| |
|
| |
|
| ==Diagnosis== | | ==Diagnosis== |
| | | [[Hypertrophic cardiomyopathy diagnostic study of choice|Diagnostic Study of Choice]]| [[Hypertrophic cardiomyopathy symptoms|History and Symptoms]] | [[Hypertrophic cardiomyopathy physical examination|Physical examination]] | [[Hypertrophic cardiomyopathy laboratory findings|Laboratory findings]] | [[Hypertrophic cardiomyopathy electrocardiogram|Electrocardiogram]] | [[Hypertrophic cardiomyopathy x ray|X Ray]] | [[Hypertrophic cardiomyopathy echocardiography and ultrasound|Echocardiography and Ultrasound]] | [[Hypertrophic cardiomyopathy CT scan|CT Scan]] | [[Hypertrophic cardiomyopathy MRI|MRI]] |[[Hypertrophic cardiomyopathy other imaging findings|Other Imaging Findings]] | [[Hypertrophic cardiomyopathy other diagnostic studies|Other Diagnostic Studies]]. |
| Clinical diagnosis of HCM is most easily and readily established with 2-dimensional echocardiography, by imaging the hypertrophied but nondilated LV chamber, in the absence of another cardiac or systemic disease capable of producing the magnitude of hypertrophy evident.
| |
| | |
| ===Differential Diagnosis===
| |
| HCM must be distinguished from the following disorders:
| |
| | |
| * [[Hypertensive heart disease]]
| |
| * [[Aortic stenosis]]
| |
| * [[Cardiac amyloidosis]]
| |
| | |
| ===Symptoms===
| |
| The clinical course of HCM is variable. Many patients are asymptomatic or mildly symptomatic. The [[symptom]]s of HCM include dyspnea (shortness of breath), [[chest pain]] (sometimes known as ''[[Angina pectoris|angina]]''), uncomfortable awareness of the [[heart beat]] ([[palpitations]]), [[lightheadedness]], fatigue, [[fainting]] (called [[syncope]]) and [[sudden cardiac death]]. [[Dyspnea]] is largely due to increased stiffness of the [[left ventricle]], which impairs filling of the ventricles and leads to elevated pressure in the left ventricle and left atrium. Symptoms are not closely related to the presence or severity of an outflow tract gradient
| |
| <ref name="Braunwauld 2005">Braunwauld E. The Cardiomyopathies, in Braunwald's ''Heart Disease'', 7th ed, D Zipes, et al (eds). Philadelphia, Saunders, 2005</ref>. Oftentimes, symptoms mimic those of [[congestive heart failure]] (esp. activity intolerance & dyspnea), but it must be noted that treatment is very different. To treat with diuretics (a mainstay of CHF treatment) will exacerbate symptoms in hypertrophic cardiomyopathy by decreasing ventricular volume and increasing outflow resistance.
| |
| | |
| Risk factors for sudden death in individuals with HCM include a young age at first diagnosis (age < 30 years), an episode of aborted sudden death, a family history of HCM with sudden death of relatives, specific mutations in the genes encoding for troponin T and myosin, sustained [[supraventricular tachycardia|supraventricular]] or [[ventricular tachycardia]], recurrent [[syncope]], ventricular septal wall thickness over 3 cm, hypotensive response to exercise, [[syncope]] (especially in children), and bradyarrhythmias (slow rhythms of the heart)<ref name="Maron, Cecchi et al 1994">Maron BJ, Cecchi F, McKenna WJ. Risk factors and stratification for sudden cardiac death in patients with hypertrophic cardiomyopathy. ''[[British Heart Journal|Br Heart J]]''. 1994 Dec; '''72'''(6 Suppl):S13–8. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=7873317 Medline abstract]) and the Task Force on Sudden Cardiac Death of the European Society of Cardiology [http://www.guideline.gov/summary/summary.aspx?doc_id=2977 link] Note: Guideline withdraw</ref>
| |
| | |
| ===Physical examination===
| |
| | |
| ====Heart====
| |
| On physical examination, (as shown in the table below) maneuvers that decrease LV filling augment the murmur and maneuvers that increase afterload or filling decrease the murmur. The murmur is characteristically a crescendo-decrescendo systolic murmur. There may be paradoxically split S2, and S3 or S4 as well as mitral regurgitation. HCM can be differentiated from AS by the fact that the murmur of AS does not change substantially with maneuvers. The character of the pulse in AS is ''parvus et tardus'', while a bisferiens pulse is noted in HCM. S2 is louder in HCM than AS.
| |
| | |
| {| style="margin: inherit auto;"
| |
| |+ '''Differentiating hypertrophic cardiomyopathy and valvular aortic stenosis'''
| |
| |- style="background:#efefef;"
| |
| ! scope="col" style="width: 33%" |
| |
| ! scope="col" style="width: 33%" | Aortic stenosis
| |
| ! scope="col" style="width: 33%" | Hypertrophic cardiomyopathy
| |
| |- style="background:#dfefff;"
| |
| | colspan="3" | Echocardiography
| |
| |-
| |
| ! scope="row" | Aortic valve calcification
| |
| | Common || No
| |
| |-
| |
| ! scope="row" | Dilated ascending aorta
| |
| | Common || Rare
| |
| |-
| |
| ! scope="row" | Ventricular hypertrophy
| |
| | Concentric [[left ventricular hypertrophy|LVH]]
| |
| | Asymmetric, often involving the septum
| |
| |-
| |
| | colspan="3" style="background:#dfefff;" | Physical examination
| |
| |-
| |
| ! scope="row" | Murmur of [[Aortic insufficiency|AI]]
| |
| | Common || No
| |
| |- | |
| ! scope="row" | Pulse pressure after [[premature ventricular contraction|PVC]]
| |
| | Increased || Decreased
| |
| |-
| |
| ! scope="row" | [[Valsalva maneuver]]
| |
| | Decreased intensity of murmur || Increased intensity of murmur | |
| |-
| |
| ! scope="row" | Carotid pulsation
| |
| | Normal or [[Pulsus tardus et parvus|tardus et parvus]]
| |
| | Brisk, jerky, or bisferiens pulse (a collapse of the pulse followed by a secondary rise) | |
| |}
| |
| | |
| The physical findings of HCM are associated with the dynamic outflow obstruction that is often present with this disease.
| |
| | |
| Upon [[auscultation]], the cardiac [[heart murmur|murmur]] will sound similar to the murmur of [[aortic stenosis]]. However, this murmur will increase in intensity with any maneuver that decreases the volume of blood in the left ventricle (such as standing or the strain phase of a [[Valsalva maneuver]]).
| |
| | |
| If dynamic outflow obstruction exists, physical examination findings that can be elicited include the pulsus bisferiens and the double apical impulse with each ventricular contraction. These findings, when present, can help differentiate HCM from [[aortic stenosis]]. In addition, if the individual has [[premature ventricular contraction]]s (PVCs), the change in the carotid pulse intensity in the beat after the PVC can help differentiate HCM from aortic stenosis. In individuals with HCM, the pulse pressure will decrease in the beat after the PVC, while in aortic stenosis, the pulse pressure will increase.
| |
| | |
| === Diagnostic testing ===
| |
| A [[diagnosis]] of hypertrophic cardiomyopathy is based upon a number of features of the disease process. While there is use of [[echocardiogram|echocardiography]], [[cardiac catheterization]], or cardiac [[MRI]] in the diagnosis of the disease, other important factors include [[electrocardiogram|ECG]] findings and if there is any family history of HCM or unexplained sudden death in otherwise healthy individuals.
| |
| | |
| ===Electrocardiogram===
| |
| Large septal q waves may be present reflective of the septal hypertrophy. In the Yamaguchi variant of apical hypertrophic cardiomyopathy there may be deeply inverted T waves in precordial leads V2-V6 and II, III, aVL (see example).
| |
| | |
| <div align="center">
| |
| <gallery heights="125" widths="225">
| |
| Image:HCP ecg.JPG|A variant of apical hypertrophic cardiomyopathy. Deeply inverted T waves in precordial leads V2-V6 and II, III, aVL.
| |
| </gallery>
| |
| </div>
| |
| | |
| ===Echocardiography===
| |
| | |
| Echo with doppler is the primary proceedure used to diagnose hypertrophic cardiomyopathy. Proper examination should evaluate <ref>Otto, Catherine. ''Textbook of Clinical Echocardiography''. 3rd Edition, 2004</ref>:
| |
| *Left ventricular asymmetric hypertrophy
| |
| **Parasternal long axis shows relationshipo of the septal hypertrophy and the outflow tract
| |
| *Left ventricular diastolic dysfunction
| |
| **LV inflow across the mitral valve
| |
| **LA inflow in the pulmonary vein
| |
| **Myocardial Doppler tissue velocity
| |
| **Isovolumetric relaxation time
| |
| *Dynamic outflow tract obstruction
| |
| **SAM (systolic anterior motion) of the mitral leaflet
| |
| **Mid-systolic closure of the aortic valve
| |
| **Late peaking, high velocity flow in the outflow tract
| |
| **Variability of obstruction with maneuvers (exercise, amyl nitrate inhalation, and post-PVC beats)
| |
| *Doppler Techniques
| |
| **Use continuous wave doppler to measure the systolic flow velocity in the LV outflow tract and mid-cavity (both at rest and during maneuvers)
| |
| | |
| Because of the turbulent, high-velocity jet in the left ventricular outflow tract (LVOT), the anterior mitral leaflet moves anteriorly in systole, exacerbating the outflow tract obstruction, and promoting mitral regurgitation. The following images show classic systolic anterior motion (SAM) of the mitral valve leaflets:
| |
| | |
| '''On parasternal long-axis view'''
| |
| | |
| <googlevideo>-4301014230430356751</googlevideo>
| |
| | |
| '''On parasternal short-axis view'''
| |
| | |
| <googlevideo>8393870190048992602</googlevideo>
| |
| | |
| ===Cardiac MRI===
| |
| | |
| ====Late Myocardial Enhancement====
| |
| | |
| Late myocardial enhancement has been associated with myocardial fibrosis and may allow for earlier detection of hypertrophic cardiomyopathy than is currently available with echocardiography and ECG.
| |
| | |
| * Choudhury et al studied 21 patients with previously diagnosed hypertrophic cardiomyopathy<ref>Choudhury et al. Myocardial scarring in asymptomatic or mildly symptomatic patients with hypertrophic cardiomyopathy. J. Am Coll Card. 2002; 40: 2156.</ref>. They noted:
| |
| :* Late myocardial enhancement following gadolinium administration in a patchy intramyocardial distribution.
| |
| :* Typically occurred in the hypertrophied regions, predominantly in the middle third of the ventricular wall in a patchy, multi focal distribution.
| |
| :* If enhancement occurred, it occurred at the junctions of the intraventricular septum and right ventricular free wall.
| |
|
| |
| * Moon et al looked at whether the extent of hyperenhancement on MR in patients with HCM would be associated with the risk of heart failure and sudden death <ref>Moon et al., Toward Clinical Risk Assessment in Hypertrophic Cardiomyopathy with Gadolinium Cardiovascular Magnetic Resonance. J Am Coll Card. 2003; 41; 1561.</ref> The study involved 53 patient were selected for presence or absence of an increased clinical risk of sudden death and/or progressive adverse left ventricular remodeling.
| |
| :* Myocardial hyperenhancement was present in 79% of patients.
| |
| :* They found no evidence of abnormal myocardium on non-contrast images.
| |
| :* There was more hyperenhancement in patients with progressive disease than without.
| |
| :* There was greater hyperenhancement in patients with ≥ 2 risk factors for sudden death.
| |
| :* Patients with diffuse hyperenhancement had ≥ 2 risk factors for sudden death vs patients with confluent hyperenhancement.
| |
| | |
| Of note, other investigators have discovered that in carriers without signs of hypertrophy on [[EKG]] or [[echocardiography]], Cardiac MR can detect the presence of crypts in the LV wall which may progress to hypertrophy.
| |
| | |
| ====Left Ventricular Hypertrophy====
| |
| | |
| MR is helpful in visualizing the asymmetric thickening of the interventricular septum in patients with HCM. However, it may be more helpful than other forms of imaging to differentiate the variant types of hypertrophic cardiomyopathy.<ref>Germans, T et al. Structural Abnormalities of the inferoseptal left ventricular wall detected by Cardiac Magnetic Resonance Imaging in carriers of Hypertrophic Cardiomyopathy mutations. J Am Coll Cardiol. 2006: 48; 2518.</ref>
| |
| | |
| ====Mitral Regurgitation and Systolic Anterior Motion====
| |
|
| |
| MR can be helpful in evaluating the extent of systolic anterior motion of the mitral valve.
| |
|
| |
| ====Obstruction====
| |
| | |
| MR can be help visualize turbulence in left ventricular outflow tract created by obstruction in patients with obstructive hypertrophic cardiomyopathy.
| |
|
| |
| <youtube v=xP3gvVFGUaU/>
| |
| | |
| ===CT===
| |
| | |
| Echocardiographic findings reflect the clinical and anatomic findings described above, e.g. LVH, diastolic dysfunction, MR, LA enlargement, elevated PAP.
| |
| | |
| Characteristic of obstructive HM is '''systolic anterior motion''' of the [[mitral valve]] (SAM). The anterior leaflet is pulled toward the LVOT during systole via the Venturi effect, leading to obstruction, a gradient and MR.
| |
| | |
| ===Positron Emission Tomography===
| |
| Positron Emission Tomography (PET) studies have demonstrated that coronary flow reserve is reduced in patients with HCM. Those patients who subsequently died had a greater reduction in coronary flow reserve at baseline. It has been hypothesized that this ischemia may mediate in part the higher risk in [[sudden cardiac death]].
| |
| | |
| ===Cardiac Catheterization===
| |
| | |
| Upon [[cardiac catheterization]], [[catheter]]s can be placed in the left ventricle and the ascending [[aorta]], to measure the pressure difference between these structures. In normal individuals, during ventricular [[systole]], the pressure in the ascending aorta and the left ventricle will equalize, and the aortic valve is open. In individuals with [[aortic stenosis]] or with HCM with an outflow tract gradient, there will be a pressure gradient (difference) between the left ventricle and the aorta, with the left ventricular pressure higher than the aortic pressure. This gradient represents the degree of obstruction that has to be overcome in order to eject blood from the left ventricle.
| |
| | |
| The '''Brockenbrough–Braunwald–Morrow sign''' is observed in individuals with HCM with outflow tract gradient. This sign can be used to differentiate HCM from aortic stenosis. In individuals with aortic stenosis, after a [[premature ventricular contraction]] (PVC), the following ventricular contraction will be more forceful, and the pressure generated in the left ventricle will be higher. Because of the fixed obstruction that the stenotic aortic valve represents, the post-PVC ascending aortic pressure will increase as well. In individuals with HCM, however, the degree of obstruction will increase more than the force of contraction will increase in the post-PVC beat. The result of this is that the left ventricular pressure increases and the ascending aortic pressure ''decreases'', with an increase in the LVOT gradient.
| |
| | |
| [[Image:Hypertrophic_Cardiomyopathy_-_Intraventricular_Pressure_Tracing.png|thumb|400px|center|Pressure tracings demonstrating the Brockenbrough–Braunwald–Morrow sign<br><small>AO = Descending aorta; LV = Left ventricle; ECG = Electrocardiogram.<br>After the third [[electrocardiogram|QRS complex]], the ventricle has more time to fill. Since there is more time to fill, the left ventricle will have more volume at the end of [[diastole]] (increased [[Preload (cardiology)|preload]]). Due to the [[Frank-Starling law of the heart|Frank–Starling law of the heart]], the contraction of the left ventricle (and pressure generated by the left ventricle) will be greater on the subsequent beat (beat #4 in this picture). Because of the dynamic nature of the outflow obstruction in HCM, the obstruction increases ''more'' that the left ventricular pressure increase. This causes a fall in the aortic pressure as the left ventricular pressure rises (seen as the yellow shaded area in the picture).<br></small>]]
| |
| | |
| While the Brockenbrough–Braunwald–Morrow sign is most dramatically demonstrated using simultaneous intra-cardiac and intra-aortic catheters, it can be seen on routine physical examination as a decrease in the pulse pressure in the post-PVC beat in individuals with HCM.
| |
| | |
| ==Pathological Findings==
| |
| | |
| ===Gross Pathological Findings===
| |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:2367.jpg|Cardiomyopathy: Intermediate between hypertrophic and dilated
| |
| Image:17412.jpg|Hypertrophic cardiomyopathy, concentric
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:4648.jpg|Hypertrophic Obstructive Cardiomyopathy: Gross natural color opened left ventricular outflow tract with subaortic shelf and marked endocardial thickening matching the contour of the anterior mitral leaflet
| |
| Image:416.jpg|Cardiomyopathy: Gross apical slice of left and right ventricles concentric hypertrophy with cavitary obliteration sudden unexpected death obstructive cardiomyopathy
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:2369.jpg|Cardiomyopathy Asymmetrical Septal Hypertrophy
| |
| Image:455.jpg|Cardiomyopathy: Gross excellent view of mitral valve atrial surface showing thickening which is fibrous in body of valve and myxoid at area of free margin changes presumed secondary to insufficiency due to anterior motion
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:428.jpg|Cardiomyopathy: Gross close up view of a ventricle slice
| |
| Image:427.jpg|Cardiomyopathy: Gross ventricular slices hypertrophy and extensive myocardial fibrosis a unique case of global fiber disarray with atrophy and fibrosis
| |
| </gallery>
| |
| </div>
| |
| | |
| ===Microscopic Pathological Findings===
| |
| | |
| Histopathologically, small vessels have hypertrophy of the tunica media. Combined with increased wall tension, decreased vasodilator reserve and inadequate capillary density, there is a mismatch between blood supply and demand. Over time, it is thought that there is repeated ischemia followed by fibrosis and eventually, dilation and systolic dysfunction (“burned out hypertrophy”).
| |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:3380.jpg|Micro med mag H&E mid-mural myocardium with hypertrophy and interstitial fibrosis atrophy is present marked increase in interstitial fibroblastic cells
| |
| Image:3381.jpg|Micro high mag H&E myofiber hypertrophy and interstitial fibrosis with marked increase in interstitial fibroblastic cells
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:3480.jpg|Micro med mag H&E myofiber hypertrophy some atrophy interstitial fibrosis with many fibroblastic cells
| |
| Image:3481.jpg|Micro high mag H&E hypertrophied fibers with some evidence of atrophy and marked interstitial fibrosis with many fibroblastic type cells
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:3482.jpg|Micro low mag H&E shows myofiber hypertrophy and interstitial fibrosis
| |
| Image:439.jpg|Cardiomyopathy: Micro H&E low mag interventricular septum at junction of normal myofiber orientation with asymmetrical hypertrophy (an excellent example)
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:440.jpg|Cardiomyopathy: Micro H&E low mag marked myofiber disarray asymmetrical hypertrophy
| |
| Image:441.jpg|Cardiomyopathy: Micro trichrome high mag marked myofiber disarray
| |
| </gallery>
| |
| </div>
| |
| | |
| | |
| <div align="left">
| |
| <gallery heights="175" widths="175">
| |
| Image:442.jpg|Cardiomyopathy: Micro H&E med mag excellent example myofiber disarray
| |
| Image:438.jpg|Cardiomyopathy: Micro H&E high mag excellent example myofiber disarray
| |
| </gallery>
| |
| </div>
| |
|
| |
|
| ==Treatment== | | ==Treatment== |
| In all patients with hypertrophic cardiomyopathy risk stratification is essential to attempt to ascertain which patients are at risk for sudden cardiac death
| |
| <ref name="Maron 2002"/>
| |
| <ref name="Wigle, Rakowski et al 1995"/>.
| |
| In those patients deemed to be at high risk the benefits and infrequent complications of defibrillator therapy are discussed; devices have been implanted in as many as 15% of patients at HCM centers. Treatment symptoms of obstructive HCM is directed towards decreasing the left ventricular outflow tract gradient and symptoms of dyspnea, chest pain and syncope.
| |
|
| |
| ===Pharmacotherapy===
| |
| Medical therapy is successful in the majority of patients. The first medication that is routinely used is beta-blockade ([[metoprolol]], [[atenolol]], [[bisoprolol]], [[propranolol]])<ref name="Maron 2002"/>. If symptoms and gradient persist disopyramide may be added to the [[beta-blocker]]
| |
| <ref name="Sherrid, Barac et al 2005">Sherrid MV, Barac I, McKenna WJ, Eliott M, Dickie S, Chojnowska L, Casey S, Maron BJ. Multicenter study of the efficacy and safety of disopyramide in obstructive hypertrophic cardiomyopathy. ''[[Journal of the American College of Cardiology|J Am College of Cardiol]]'' 2005; '''45''':1251–58</ref>.
| |
| Alternately a calcium channel blocker such as [[verapamil]] may be substituted for beta-blockade. It should be stressed that most patient's symptoms may be managed medically without needing to resort to inteventions such as surgical septal myectomy, alcohol septal ablation or pacing. Severe symptoms in non-obstructive HCM may actually be more difficult to treat because there is no obvious target (obstruction) to treat. Medical therapy with verapamil, beta-blockade may improve symptoms. [[Diuretics]] should be avoided, as they reduce the intravascular volume of blood, decreasing the amount of blood available to distend the left ventricular outflow tract, leading to an increase in the obstruction to the outflow of blood in the left ventricle <ref name="Wynne and Braunwald 1997">Wynne J, Braunwald E. Hypertrophic cardiomyopathy. In: Braunwald E, ed. Heart disease: a textbook of cardiovascular medicine. 5th ed. Philadelphia: WB Saunders; 1997.</ref>.
| |
|
| |
| As a summary:
| |
|
| |
| * The asymptomatic patient without risk factors for SCD ([[sudden cardiac death]][) does not require therapy, even in the presence of NSVT. The symptomatic patient can be treated with negative inotropes such as [[calcium channel blocker]]s and/or [[beta-blockers]]. [[Atrial fibrillation]] should be treated aggressively. Some use [[Disopyramide]] to maintain NSR (normal sinus rhythm) because of its negative inotropic effects. Amiodarone is the best medicine to maintain NSR and has been associated with symptomatic improvement in patients with HCM.
| |
| * These patients require [[endocarditis]] prophylaxis.
| |
|
| |
| ===Interventional Cardiology and Device Based Therapy===
| |
|
| |
| ====Alcohol septal ablation====
| |
| [[Alcohol septal ablation]], introduced by Ulrich Sigwart in 1994, is a percutaneous technique that involves injection of alcohol into the first septal perferator of the [[coronary circulation|left anterior descending artery]]. This is a technique with results similar to the surgical septal myectomy procedure but is less invasive, since it does not involve general anaesthesia and opening of the chest wall and pericardium (which are done in a septal myomectomy). In a select population with symptoms secondary to a high outflow tract gradient, alcohol septal ablation can reduce the symptoms of HCM.<ref name="Maron 2002"/><ref name="Wigle, Rakowski et al 1995"/><ref name="Brilakis and Nishimura 2003">Brilakis ES, Nishimura RA. Severe pulmonary hypertension in a patient with hypertrophic cardiomyopathy: response to alcohol septal ablation. ''[[Heart (journal)|Heart]]''. 2003 Jul; '''89'''(7):790. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=12807862 Medline abstract])</ref>
| |
|
| |
| When performed properly, an alcohol septal ablation induces a controlled [[myocardial infarction|heart attack]], in which the portion of the interventricular septum that involves the left ventricular outflow tract is infarcted and will contract into a scar.
| |
|
| |
| ====Ventricular pacing====
| |
| The use of a [[artificial pacemaker|pacemaker]] has been advocated in a subset of individuals, in order to cause asynchronous contraction of the left ventricle. Since the pacemaker activates the interventricular septum before the left ventricular free wall, the gradient across the left ventricular outflow tract may decrease. This form of treatment has been shown to provide less relief of symptoms and less of a reduction in the left ventricular outflow tract gradient when compared to surgical myectomy
| |
| <ref name="Ommen, Nishimura et al 1999">Ommen SR, Nishimura RA, Squires RW, Schaff HV, Danielson GK, Tajik AJ. Comparison of dual-chamber pacing versus septal myectomy for the treatment of patients with hypertropic obstructive cardiomyopathy: a comparison of objective hemodynamic and exercise end points. ''[[Journal of the American College of Cardiology|J Am Coll Cardiol]]''. 1999 Jul; '''34'''(1):191–6. ([http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=pubmed&dopt=Abstract&list_uids=10400010 Medline abstract])</ref>.
| |
|
| |
|
| ===Surgery===
| | *'''[[Hypertrophic cardiomyopathy medical therapy|Medical therapy]]''', '''[[Hypertrophic cardiomyopathy interventions|Interventions]], [[Hypertrophic cardiomyopathy surgery|Surgery]], [[Hypertrophic cardiomyopathy primary prevention|Primary Prevention]], [[Hypertrophic cardiomyopathy secondary prevention|Secondary Prevention]], Cost Effectiveness of Therapy, Future or Investigational Therapy'''<br /> |
| The role of ICD (implantable cardiac defibrillator) placement in HCM is controversial. It offers the best potential benefit for survival and should probably be implanted in survivors of SCD and those deemed at high risk by clinical parameters. Nonetheless, the impact on prognosis is unclear because tachyarrhythmias may not always be the mechanism for syncope and sudden death. In addition, older patients may be self-selected “survivors” that stand to gain less from ICD placement. One recent retrospective study showed that at an average follow-up of 128 patients at 3.1 years, 23 percent had shocks for VT (ventricular tachycardia) and 25% had inappropriate shocks. Of those receiving the ICD prophylactically, 5% were shocked per year. This study did not evaluate the role of clinical predictors, evaluate total mortality and was a non-randomized retrospective design that does not establish the need for ICD placement in all patients with HCM or superiority to amiodarone therapy.
| |
|
| |
|
| ====Indications for Surgery==== | | ==Resources== |
| * Surgical myomectomy has significant morbidity and mortality associated and is reserved for symptomatic patients who fail medical therapy.
| |
| * Cardiac catheterization and ethanol ablation is currently under investigation.
| |
| * Pacing in HCM is controversial.
| |
| *:* The idea is to change the sequence of activation of the ventricle and atria, altering the geometry of the heart and decreasing LVOT gradient.
| |
| *:* The AV interval must be shortened to do this, but not at the expense of diastolic filling.
| |
| *:* Outcomes for dual-chamber pacing in HCM are variable and it does not decrease the risk of SCD.
| |
| | |
| ====Surgical myectomy====
| |
| Surgical [[septal myectomy]] is the gold standard for relief of symptoms for patients who do not experience relief of symptoms from medications
| |
| <ref name="Maron 2002"/>
| |
| <ref name="Sherrid Chaudhry et al 2003"/>
| |
| <ref name="Wigle, Rakowski et al 1995"/>
| |
| <ref name="Maron, McKenna et al 2003"/>
| |
| <ref name="Sherrid, Barac et al 2005"/>
| |
| <ref name="Morrow 1978">Morrow AF. Hypertrophic subaortic stenosis. Operative methods utilized to relieve left ventricular outflow obstruction. ''J Thorac Cardiovasc Surg'' 1978; '''76''':423–30</ref>.
| |
| It has been performed successfully for more than 25 years. Surgical septal myectomy uniformly decreases left ventricular outflow tract obstruction and improves symptoms, and in experienced centers has a surgical mortality of 1%. It involves a midline thoracotomy (general anesthesia, opening the chest, and cardiopulmonary bypass) and removing a portion of the interventricular septum<ref name="Maron 2002"/>. Surgical myectomy resection focused just on the subaortic septum, to increase the size of the outflow tract to reduce Venturi forces may be inadequate to abolish systolic anterior motion (SAM) of the anterior leaflet of the mitral valve. With this limited sort of resection the residual mid-septal bulge still redirects flow posteriorly: SAM persists because flow still gets behind the mitral valve. It is only when the deeper portion of the septal bulge is resected that flow is redirected anteriorly away from the mitral valve, abolishing SAM
| |
| <ref name="Sherrid Chaudhry et al 2003"/>
| |
| <ref name="Nakatani, Schwammenthal et al 1996">Nakatani S, Schwammenthal E, Lever HM, Levine RA, Lytle BW, Thomas JD. New insights into the reduction of mitral valve systolic anterior motion after ventricular septal myectomy in hypertrophic obstructive cardiomyopathy. ''Am Heart J'' 1996; '''131''':294–300</ref>.
| |
| With this in mind, a modification of the Morrow myectomy termed extended myectomy, mobilization and partial excision of the papillary muscles has become the excision of choice
| |
| <ref name="Sherrid Chaudhry et al 2003"/>
| |
| <ref name="Messmer 1994"/>
| |
| <ref name="Schoendube, Klues et al 1995"/>
| |
| <ref name="Balaram, Sherrid et al 2005">Balaram SK, Sherrid MV, DeRose JJ, Hillel Z, Winson G, Swistel DG. Beyond extended myectomy for hypertrophic cardiomyopathy: The RPR (Resection–Plication–Release) Repair. ''Annals of Thoracic Surgery'' 2005; '''80''':217–23</ref>.
| |
| In selected patients with particularly large redundant mitral valves, anterior leaflet plication may be added to complete separation of the mitral valve and outflow
| |
| <ref name="Balaram, Sherrid et al 2005"/>
| |
| <ref name="McIntosh, Maron et al 1992">McIntosh CL, Maron BJ, Cannon RO, Klues H. Initial results of combined anterior mitral valve plication and ventricular septal myotomy–myectomy for relief of left ventricular outflow obstruction in patients with hypertrophic cardiomyopathy. ''[[Circulation (journal)|Circulation]]'' 1992; '''86''':II 60–7</ref>.
| |
| | |
| ====Cardiac transplantation====
| |
| In cases that are refractory to all other forms of treatment, [[heart transplant|cardiac transplantation]] is an option.
| |
| | |
| ==Recommendations for Pacing in Patients With Hypertrophic Cardiomyopathy (DO NOT EDIT) <ref name="Epstein"> Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008; 117: 2820–2840. PMID 18483207 </ref>==
| |
| {{cquote|
| |
| ===Class I===
| |
| 1. Permanent [[pacemaker|pacing]] is indicated for [[SND]] or [[AV block]] in patients with [[HCM]] as described previously (see Section 2.1.1, “[[Sick sinus syndrome#Treatment|Sinus Node Dysfunction]],” and Section 2.1.2, “[[Atrioventricular block#Surgery and Device Based Therapy |Acquired Atrioventricular Block in Adults]]”). ''(Level of Evidence: C)''
| |
| | |
| ===Class IIb===
| |
| 1. Permanent [[pacemaker|pacing]] may be considered in medically refractory symptomatic patients with [[HCM]] and significant resting or provoked LV outflow tract obstruction. ''(Level of Evidence: A)'' As for Class I indications, when risk factors for [[SCD]] are present, consider a DDD [[ICD]] (see Section 3, “Indications for Implantable Cardioverter-Defibrillator Therapy”).
| |
| | |
| ===Class III===
| |
| 1. Permanent [[pacemaker]] implantation is not indicated for patients who are asymptomatic or whose symptoms are medically controlled. ''(Level of Evidence: C)''
| |
| | |
| 2. Permanent [[pacemaker]] implantation is not indicated for symptomatic patients without evidence of LV outflow tract obstruction. ''(Level of Evidence: C)''}}
| |
| | |
| ==Related disorders==
| |
| '''Feline hypertrophic cardiomyopathy''' is the most common [[heart disease]] in cats; the disease process and genetics are believed to be similar to the disease in [[human]]s.<ref name="Kittleson, Meurs et al 1999">{{cite journal | author = Kittleson M, Meurs K, Munro M, Kittleson J, Liu S, Pion P, Towbin J | title = Familial hypertrophic cardiomyopathy in maine coon cats: an animal model of human disease | journal = Circulation | volume = 99 | issue = 24 | pages = 3172-80 | year = 1999 | id = PMID 10377082}}</ref>
| |
| The first genetic mutation (in cardiac myosin binding protein C) responsible for feline hypertrophic cardiomyopathy was discovered in 2005 in Maine Coon cats.<ref name="Meurs, Sanchez et al 2005">{{cite journal | author = Meurs K, Sanchez X, David R, Bowles N, Towbin J, Reiser P, Kittleson J, Munro M, Dryburgh K, Macdonald K, Kittleson M | title = A cardiac myosin binding protein C mutation in the Maine Coon cat with familial hypertrophic cardiomyopathy | journal = Hum Mol Genet | volume = 14 | issue = 23 | pages = 3587-93 | year = 2005 | id = PMID 16236761}}</ref> A test for this mutation is available.<ref>[http://www.vetmed.wsu.edu/deptsVCGL/ Veterinary Cardiac Genetics Laboratory of the College of Veterinary Medicine]</ref> About one third of Maine Coon cats tested for the mutation have been shown to be either heterozygous and homozygous for the mutation, although many of these cats have no clinical signs of the disease. Some Maine Coon cats with clinical evidence of hypertrophic cardiomyopathy test negative for this mutation, strongly suggesting that a second mutation exists in the breed. The cardiac myosin binding protein C mutation has not been found in any other breed of cat with HCM.
| |
| | |
| ==Sources==
| |
| * The ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities <ref name="Epstein"> Epstein AE, DiMarco JP, Ellenbogen KA, Estes NAM III, Freedman RA, Gettes LS, Gillinov AM, Gregoratos G, Hammill SC, Hayes DL, Hlatky MA, Newby LK, Page RL, Schoenfeld MH, Silka MJ, Stevenson LW, Sweeney MO. ACC/AHA/HRS 2008 guidelines for device-based therapy of cardiac rhythm abnormalities: executive summary: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices). Circulation. 2008; 117: 2820–2840. PMID 18483207 </ref>
| |
| | |
| == References ==
| |
| {{reflist|2}}
| |
| | |
| ===Additional References (to be updated and added)===
| |
| | |
| #PMID 16831826
| |
| #PMID 11886323
| |
| #PMID 10359738
| |
| #PMID 9826323
| |
| #PMID 10853000
| |
| #PMID 9052657
| |
| #Pinto DS, Josephson ME: Sudden Cardiac Death. In Fuster, V. (ed.): Hurst’s the Heart, 10th ed. McGraw-Hill, 2000.
| |
| #PMID 15139989
| |
| | |
| == External links ==
| |
| * [http://www.4hcm.org/ Hypertrophic Cardiomyopathy Association] | | * [http://www.4hcm.org/ Hypertrophic Cardiomyopathy Association] |
| * [http://www.cardiomyopathy.org/ Cardiomyopathy Association] | | * [http://www.cardiomyopathy.org/ Cardiomyopathy Association] |
| * [http://hcm.stanfordhospital.com/ Information from the Stanford Hypertrophic Cardiomyopathy Center]
| |
| * [http://www.hcmresearchfoundation.org/ Hypertrophic Cardiomyopathy Research Foundation] | | * [http://www.hcmresearchfoundation.org/ Hypertrophic Cardiomyopathy Research Foundation] |
| * [http://www.mayoclinic.org/hypertrophic-cardiomyopathy/physiciansguide.html Physician's Guide to Treating HCM] | | * [https://hcmcare.com HCM Care] |
| * [http://rad.usuhs.mil/medpix/medpix.html?mode=single&recnum=2385 MedPix]
| |
| * [http://members.aol.com/jchinitz/hcm/ Feline Hypertrophic Cardiomyopathy]
| |
| * [http://my.clevelandclinic.org/heart/webchat/hypertrophic_cardiomyopathy_transcript.aspx Cleveland Clinic Webchat - Hypertrophic Cardiomyopathy (HCM) Webchat with Dr. Harry Lever]
| |
| | |
| ==Additional Reading==
| |
| | |
| * Moss and Adams' Heart Disease in Infants, Children, and Adolescents Hugh D. Allen, Arthur J. Moss, David J. Driscoll, Forrest H. Adams, Timothy F. Feltes, Robert E. Shaddy, 2007 ISBN 0781786843
| |
| * Braunwald's Heart Disease, Libby P, 8th ed., 2007, ISBN 978-1-41-604105-4
| |
| * Hurst's the Heart, Fuster V, 12th ed. 2008, ISBN 978-0-07-149928-6
| |
| * Willerson JT, Cardiovascular Medicine, 3rd ed., 2007, ISBN 978-1-84628-188-4
| |
| | |
| {{Circulatory system pathology}}
| |
| {{Electrocardiography}}
| |
| {{SIB}}
| |
| | |
| [[de:Hypertrophische Kardiomyopathie]]
| |
| [[nl:HOCM]]
| |
| [[pl:Kardiomiopatia przerostowa]]
| |
| [[ru:Гипертрофическая кардиомиопатия]]
| |
|
| |
|
| [[Category:Cardiomyopathy]] | | [[Category:Cardiomyopathy]] |
| [[Category:Cardiology]] | | [[Category:Cardiology]] |
| | [[Category:Disease]] |
| | [[Category:Overview complete]] |
| | [[Category:Template complete]] |
|
| |
|
| {{WikiDoc Help Menu}} | | {{WikiDoc Help Menu}} |
| {{WikiDoc Sources}} | | {{WikiDoc Sources}} |