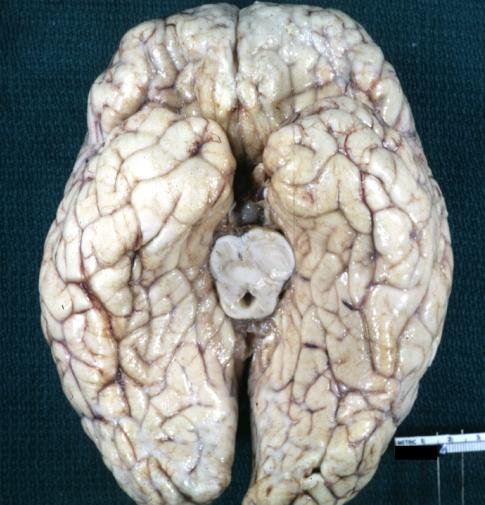
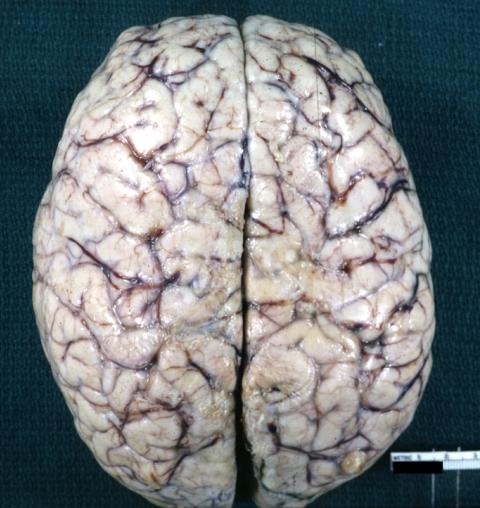
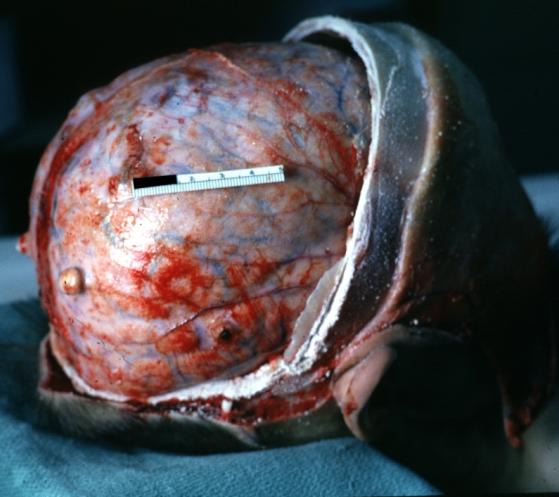
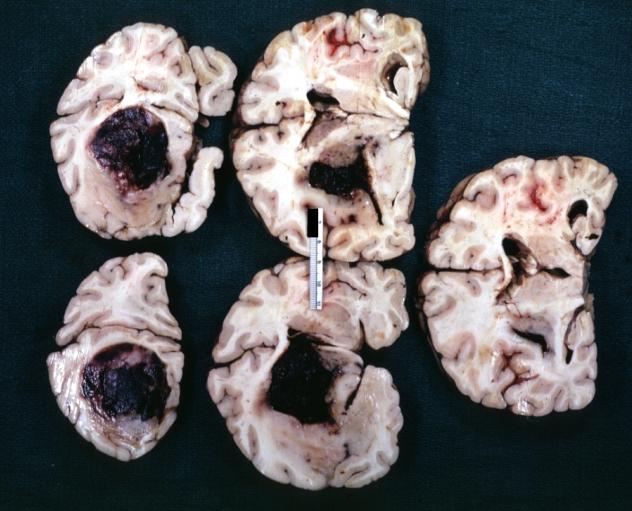
Glioma
Template:DiseaseDisorder infobox Template:Search infobox For patient information click here
Editor-In-Chief: C. Michael Gibson, M.S., M.D. [1]
Associate Editor-In-Chief: Cafer Zorkun, M.D., Ph.D. [2]
Please Take Over This Page and Apply to be Editor-In-Chief for this topic: There can be one or more than one Editor-In-Chief. You may also apply to be an Associate Editor-In-Chief of one of the subtopics below. Please mail us [3] to indicate your interest in serving either as an Editor-In-Chief of the entire topic or as an Associate Editor-In-Chief for a subtopic. Please be sure to attach your CV and or biographical sketch.
Overview
A glioma is a type of primary central nervous system (CNS) tumor that arises from glial cells. The most common site of involvement of gliomas is the brain, but gliomas can also affect the spinal cord or any other part of the CNS, such as the optic nerves.[1]
Classification
By type of cell
Gliomas are named according to the specific type of cell they most closely resemble. The main types of gliomas are:
- Ependymomas — ependymal cells
- Astrocytomas — astrocytes
- Oligodendrogliomas — oligodendrocytes
- Mixed gliomas, such as oligoastrocytomas, contain cells from different types of glia.
By grade
Gliomas are further categorized according to their grade, which is determined by pathologic evaluation of the tumor.
- Low-grade gliomas are well-differentiated (not anaplastic); these are benign and portend a better prognosis for the patient.
- High-grade gliomas are undifferentiated or anaplastic; these are malignant and carry a worse prognosis.
Of numerous grading systems in use, the most common is the World Health Organization (WHO) grading system for astrocytoma. The WHO system assigns a grade from 1 to 4, with 1 being the least aggressive and 4 being the most aggressive. Various types of astrocytomas are given corresponding WHO grades.
- WHO grading system for astrocytomas
- WHO Grade 1 — e.g., pilocytic astrocytoma
- WHO Grade 2 — e.g., diffuse or low-grade astrocytoma
- WHO Grade 3 — e.g., anaplastic (malignant) astrocytoma
- WHO Grade 4 — glioblastoma multiforme (most common glioma in adults)
The prognosis is the worst for grade 4 gliomas, with an average survival time of 12 months. Overall, few patients survive beyond 3 years. [4] [5]
By location
The gliomas can also be roughly classified according to their location:
- infratentorial : mostly in children (70%)
- supratentorial : mostly in adults (70%)
Diagnosis
Symptoms
Symptoms of gliomas depend on which part of the central nervous system is affected. A brain glioma can cause headaches, nausea and vomiting, seizures, and cranial nerve disorders as a result of increased intracranial pressure. A glioma of the optic nerve can cause visual loss. Spinal cord gliomas can cause pain, weakness, or numbness in the extremities. Gliomas do not metastasize by the bloodstream, but they can spread via the cerebrospinal fluid and cause "drop metastases" to the spinal cord.
On May 20th 2008, it was announced that 76 year old Senator Edward Kennedy had a malignant glioma of the left parietal lobe following a seizure.
MRI
CT
-
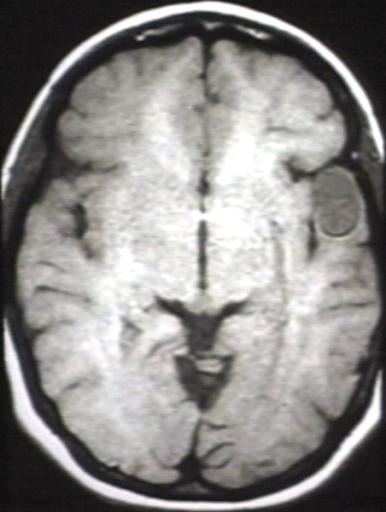
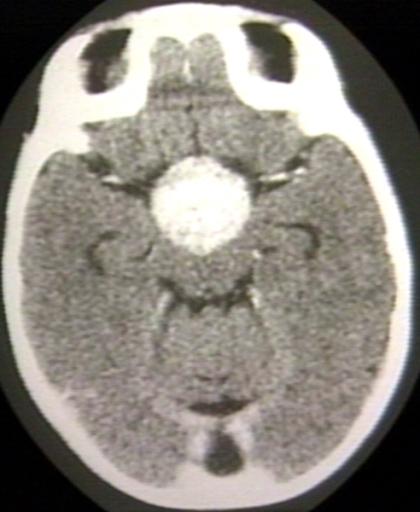
BRAIN: GLIOMA, OPTICOCHIASMATIC; WITH CONTRAST
-
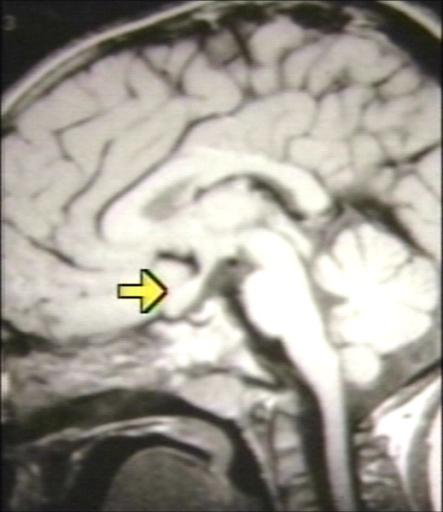
BRAIN: GLIOMA, OPTICOCHIASMATIC; 1 OF 4 WITHOUT CONTRAST
-
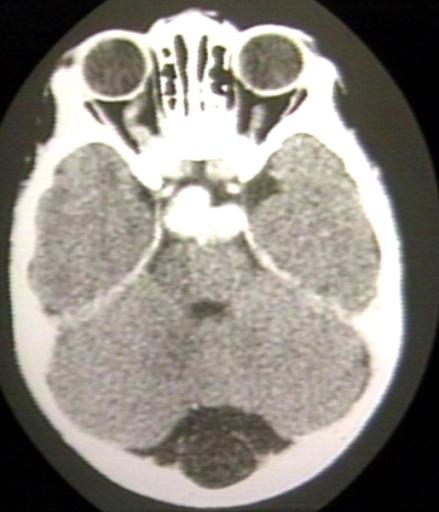
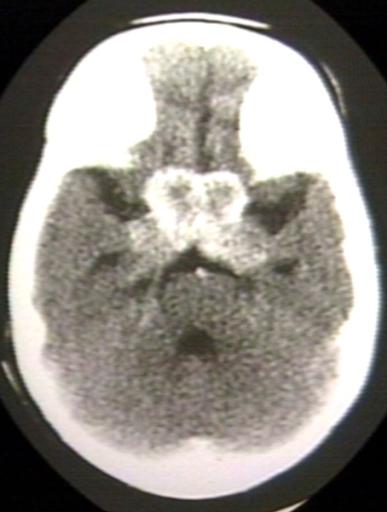
BRAIN: GLIOMA, OPTICOCHIASMATIC; 2 OF 4 WITH CONTRAST
-
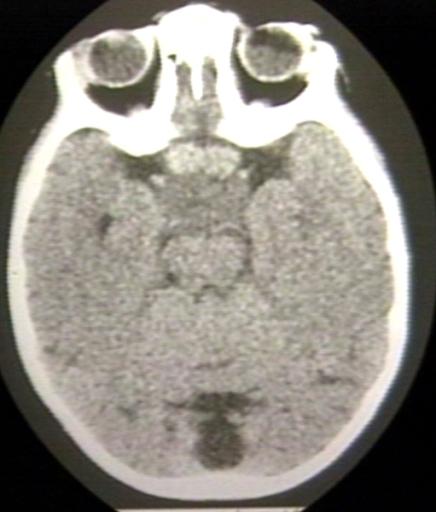
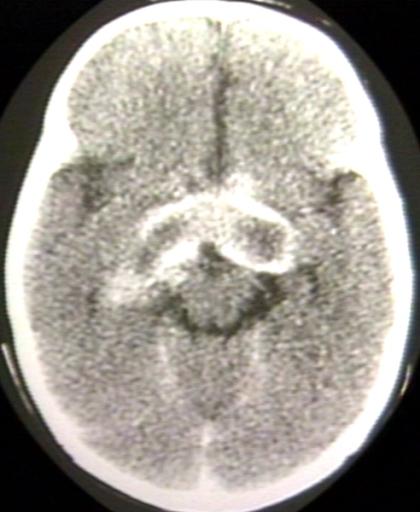
BRAIN: GLIOMA, OPTICOCHIASMATIC; 3 OF 4 WITH CONTRAST
-
BRAIN: GLIOMA, OPTICOCHIASMATIC; 4 OF 4 WITH CONTRAST
Pathology
High-grade gliomas are highly-vascular tumors and have a tendency to infiltrate. They have extensive areas of necrosis and hypoxia. Often tumor growth causes a breakdown of the blood-brain barrier in the vicinity of the tumor. As a rule, high-grade gliomas almost always grow back even after complete surgical excision.
On the other hand, low-grade gliomas grow slowly, often over many years, and can be followed without treatment unless they grow and cause symptoms.
Treatment
Standard therapy
Treatment for brain gliomas depends on the location and the grade. Often, treatment is a combined approach, using surgery, radiation therapy, and chemotherapy. The radiation therapy is in the form of external beam radiation or the stereotactic approach using radiosurgery. Spinal cord tumors can be treated by surgery and radiation. Temozolomide is a chemotherapeutic drug that is able to cross the blood-brain barrier effectively and is being used in therapy.
Experimental therapies
The use of oncolytic viruses or gene therapy using prodrug converting retroviruses and adenoviruses is being studied for the treatment of gliomas.[2][3]
A small number of low-scale clinical studies have shown possible links between prescription of Carphedon and improvement in a number of encephalopathic conditions, including lesions of cerebral blood pathways and certain types of glioma.
American scientists are also studying the effects of Leiurus quinquestriatus scorpion (Israeli Yellow Scorpion) venom on glioma. They have successfully isolated the peptide chlorotoxin from the venom of the L. quinquestriatus scorpion by means of gel filtration chromatography. The peptide appears to target glioma-specific chloride ion channels within the cancerous glial cells of the brain, where it binds with a high affinity.
In 2006, German physicians reported on a dose-escalation study for the compound AP 12009 (a phosphorothioate antisense oligodeoxynucleotide specific for the mRNA of human transforming growth factor TGF-beta2) in patients with high-grade gliomas. At the time of the report, the median overall survival had not been obtained and the authors hinted at a potential cure.
As of 2006, additional research started within the past few years is ongoing. Some of the topics included in this research are:
- efficiency of variations in radiotherapy procedures
- drugs to stop the growth of tumors by preventing them to develop blood vessels
- efficiency of combinations of different treatments
- vaccination therapy.
Although there have been individual cases of patients receiving an experimental treatment who still showed no signs of tumor 3 years or even more after the first diagnosis, often a new treatment for GBM will already be considered successful if it significantly increases the percentage of survivors after two years.
A cancer vaccine "Oncophage" is currently showing great promise in clinical trails, 2007.
Pathological Findings
Microscopic Images
-
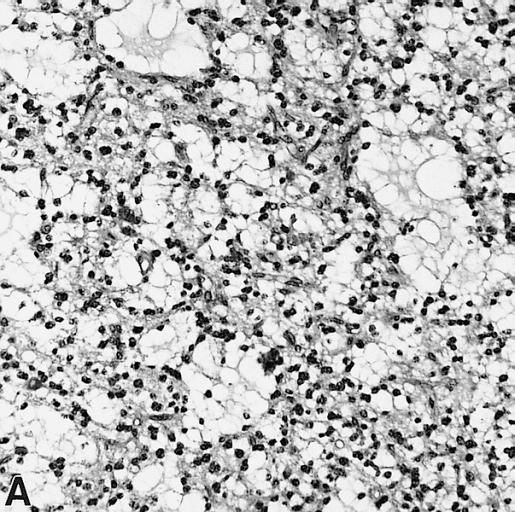
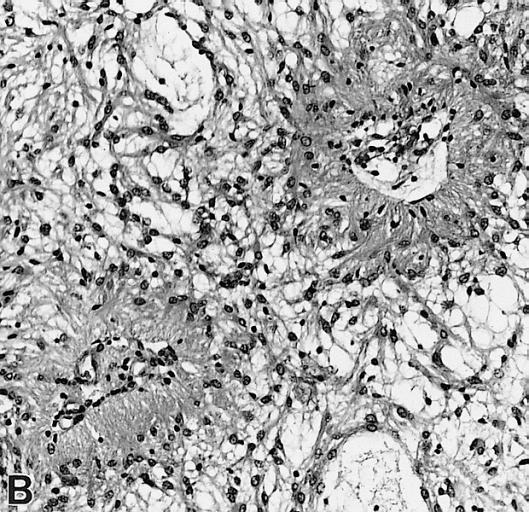
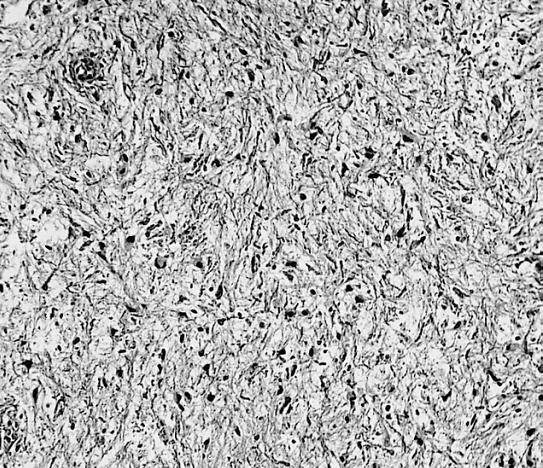
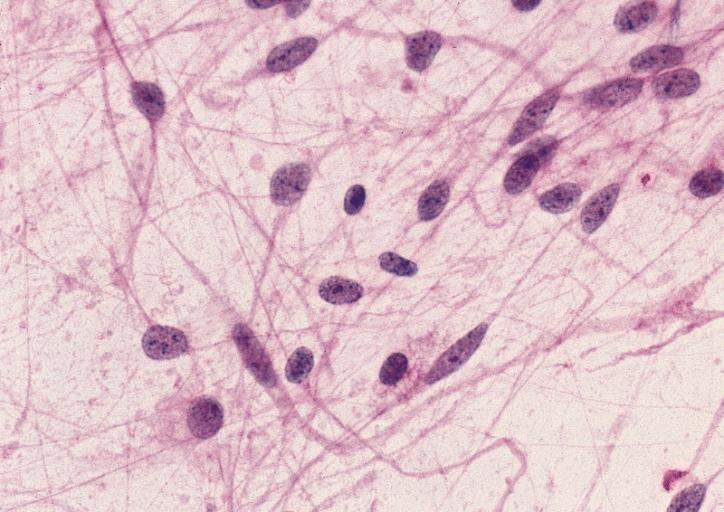
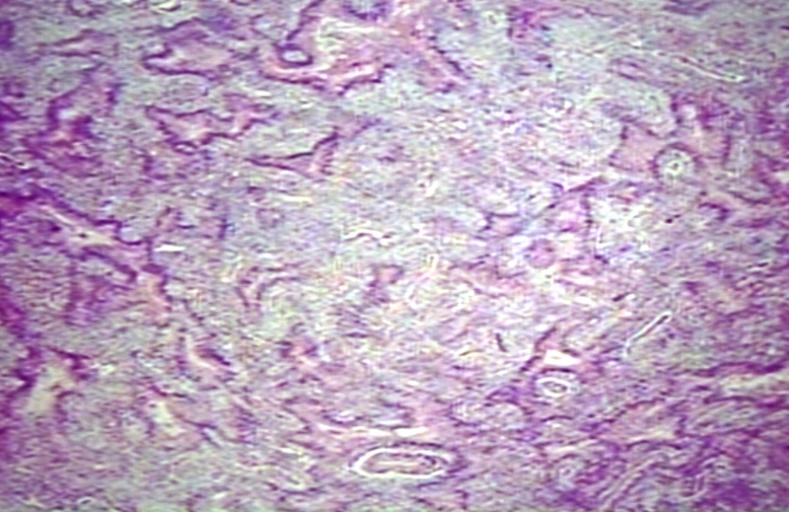
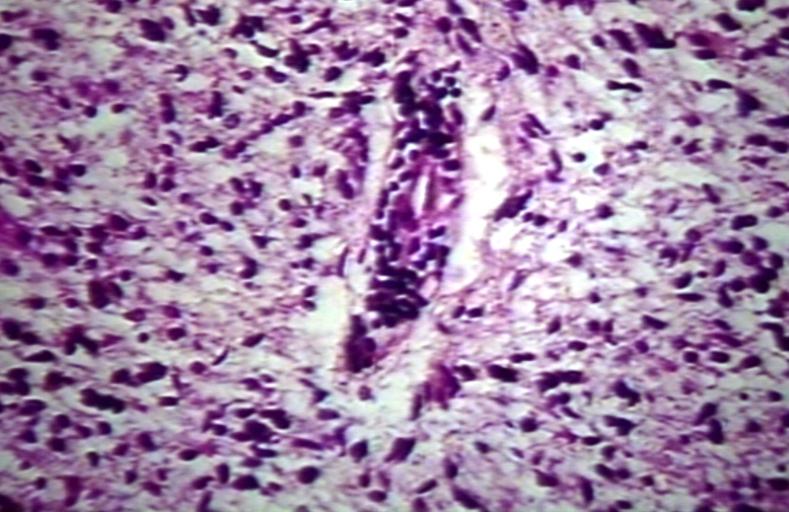
CNS: Pilocytic astrocytoma: variations in histologic appearance. As illustrated, many lesions are composed largely of spongy tissue rich in microcysts. Characteristic of pilocytic astrocytomas in general, the lesion is largely a solid mass of neoplastic cells without an obvious background of infiltrated brain.
-
CNS: Pilocytic astrocytoma: variations in histologic appearance. The perivascular radiating processes in some lesions can create a likeness to an ependymoma. Note the spongy background unusual for ependymomas.
-
CNS: Pilocytic astrocytoma: variations in histologic appearance. Other pilocytic astrocytomas are solid, rather than microcystic, and may be lobular.
-
CNS: Pilocytic astrocytoma: variations in histologic appearance. Rosenthal fibers, usually confined to the solid rather than spongy regions are found in many pilocytic astrocytomas, but are not requisite for the diagnosis.
-
CNS: Pilocytic astrocytoma: variations in histologic appearance. Rosenthal fibers are extremely abundant in some lesions. Particularly in the cerebellum, it can be difficult to distinguish such solid, paucicellular, highly fibrillar pilocytic astrocytomas from reactive gliosis with abundant Rosenthal fiber formation.
-
CNS: Pilocytic astrocytoma: variations in histologic appearance. A loose array of polar cells creates an additional variant of pilocytic astrocytoma.
-
CNS: Pilocytic astrocytoma: variations in histologic appearance. Some pilocytic astrocytomas are traversed by prominent collagenous septa.
-
CNS: Pilocytic astrocytoma: variations in histologic appearance. Unusual pilocytic astrocytomas have an extensive mucinous background without microcysts.
-
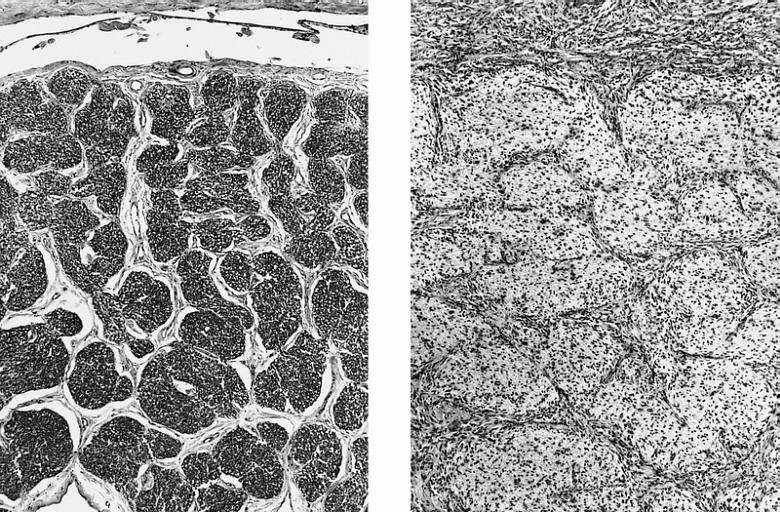
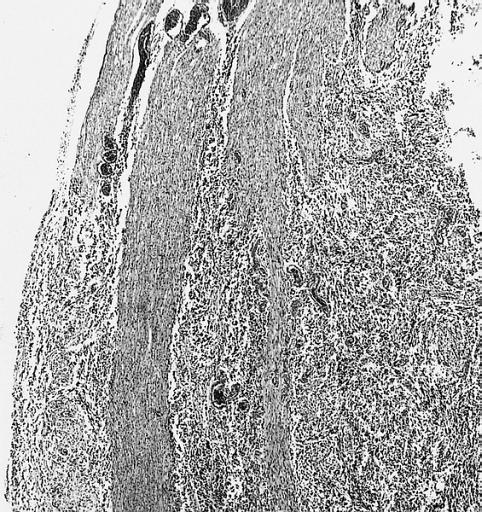
CNS: Comparison of normal optic nerve and pilocytic astrocytoma of the optic nerve. These two figures compare, at the same magnification, the normal optic nerve (left) with one containing a pilocytic astrocytoma (right). The neoplasm enlarges the compartments of the nerve and extends in collar-like fashion into the subarachnoid space.
-
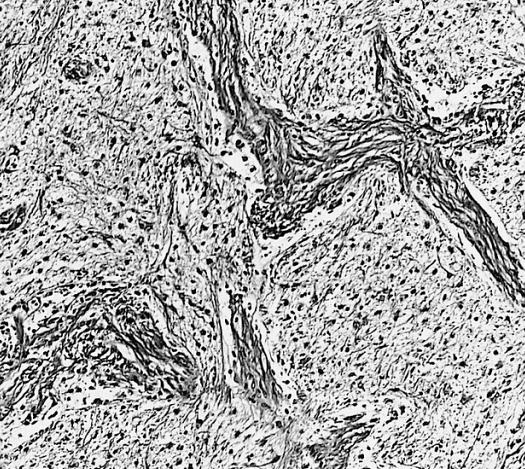
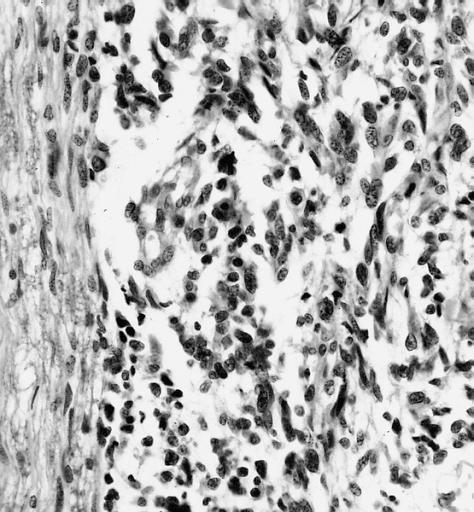
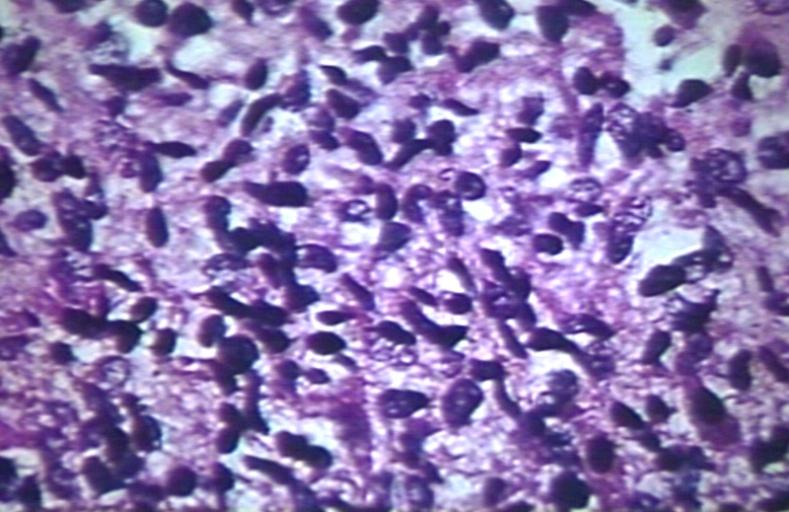
CNS: Pilocytic astrocytoma; The "hair cells" for which this lesion is named are readily seen.
-
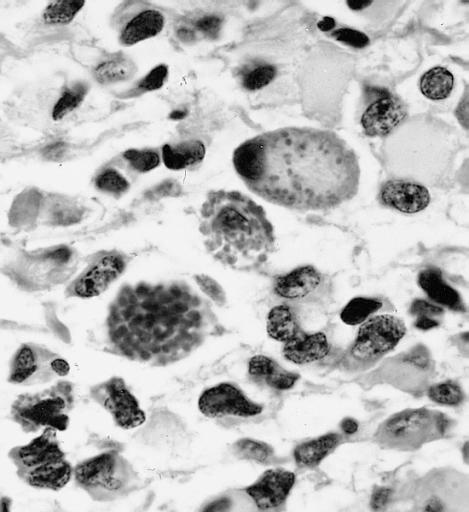
CNS: Pilocytic astrocytoma; Nuclear hyperchromasia and pleomorphism are common. Note the typical cellular elongation, and, at the center of the illustration, the eosinophilic granular body that populates pilocytic astrocytomas and certain other slowly growing gliomas.
-
CNS: Pilocytic astrocytoma; Intracytoplasmic Rosenthal fibers are prominent in some pilocytic neoplasms.
-
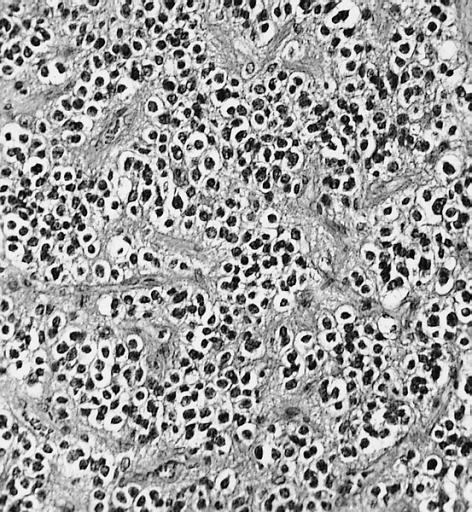
Brain: Malignant ependymoma: Micro med mag H&E tumor cells.
-
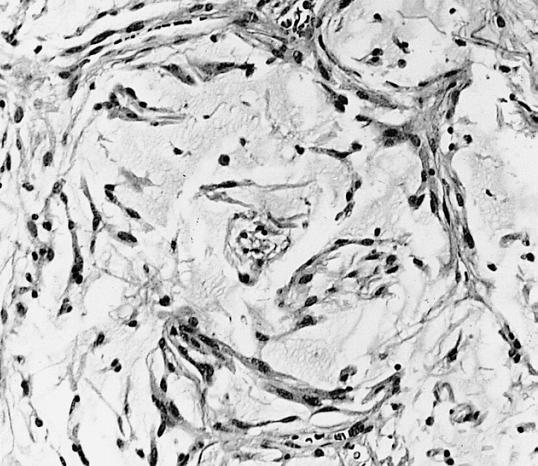
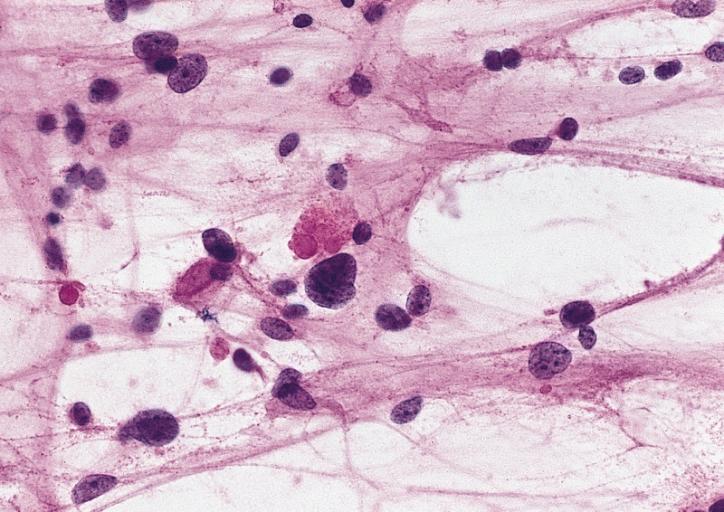
CNS: Oligodendroglioma; Occasional oligodendrogliomas contain cells with minute, refractile eosinophilic bodies representing miniature Rosenthal fibers.
-
CNS: Oligodendroglioma; The cells of some oligodendrogliomas acquire sufficient cytoplasm and process formation to become decidedly astrocytic, but their nuclei retain the roundness, uniformity, and chromatin distribution typical of oligodendroglioma.
-
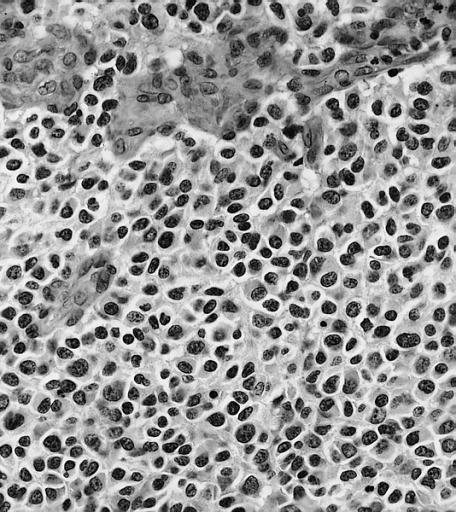
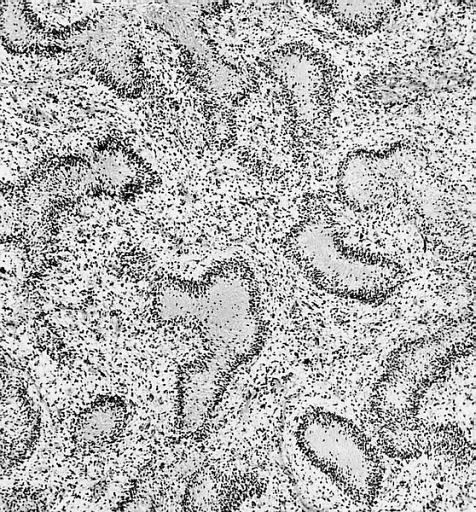
Anaplastic oligodendroglioma: Anaplastic oligodendrogliomas are highly cellular and associated with vascular proliferation.
-
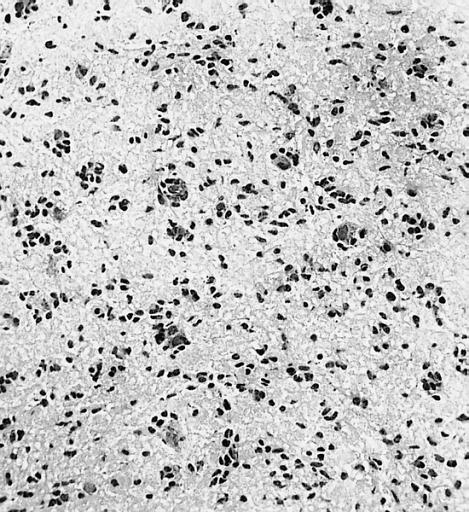
CNS: Oligodendroglioma (frozen section); Oligodendrogliomas in frozen sections lack the distinctive halos so often seen in permanent sections. Cellular monomorphism and infiltration of cerebral cortex with perineuronal satellitosis suggest the correct diagnosis.
-
CNS: Clear cell ependymoma; Perinuclear clearing similar to that seen in oligodendrogliomas is a prominent feature of the clear cell variant. Note the vague perivascular pseudorosettes. The lesion was a discrete occipital intraventricular mass.
-
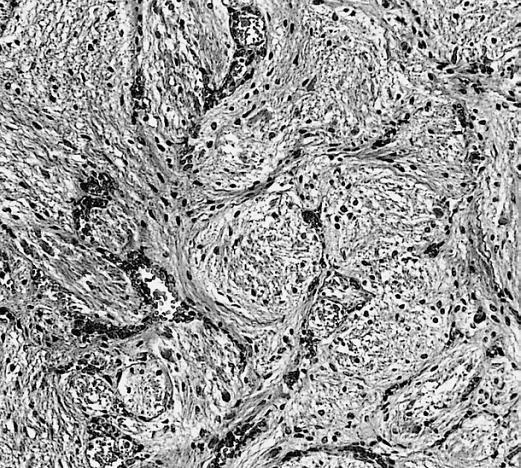
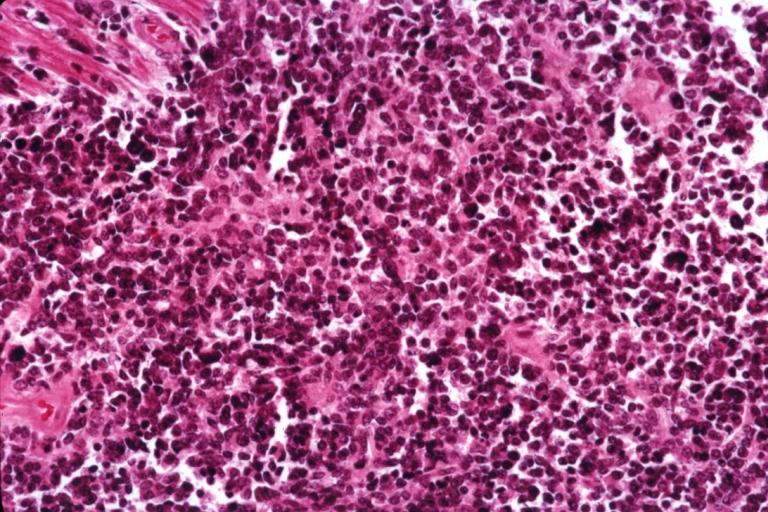
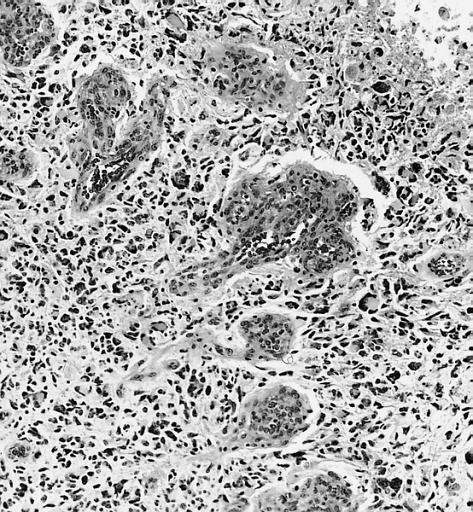
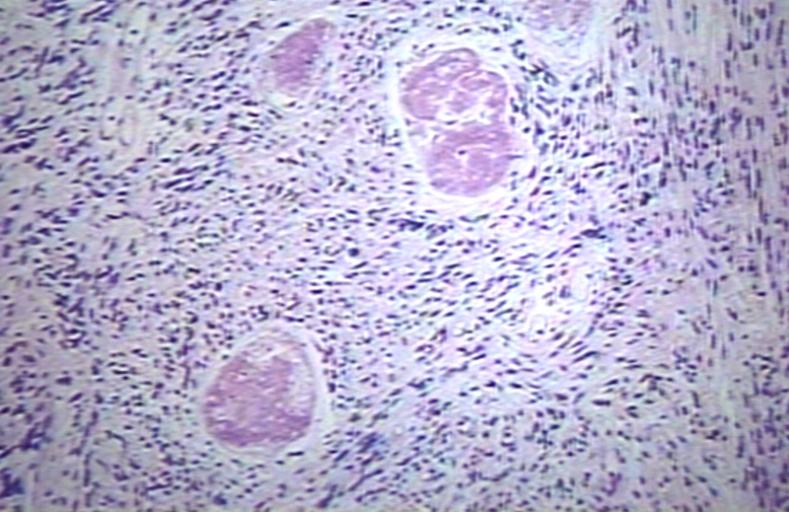
CNS: Glioblastoma multiforme; Brain: Glioblastoma multiforme. Grade I-Ii: Micro med mag with H&E, tumor well shown
-
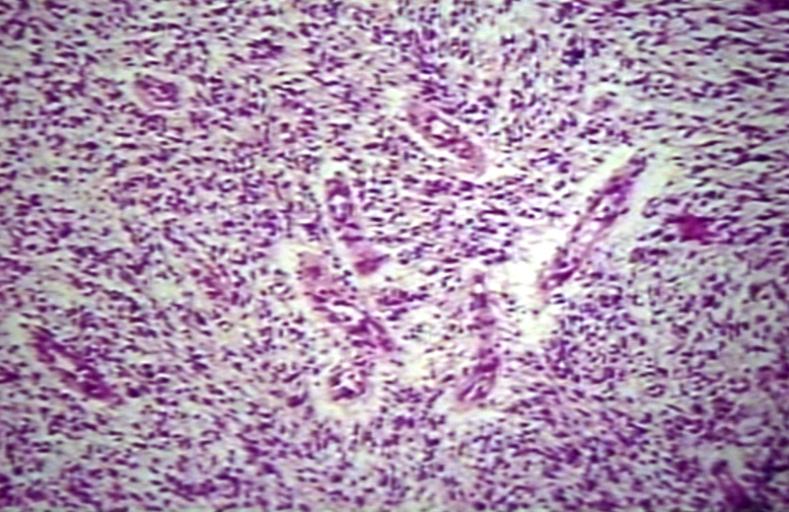
CNS: Glioblastoma multiforme arising in an astrocytoma. At higher magnification, gemistocytic astrocytoma with microcystic change is apparent at the bottom of the illustration and cellular nodules of glioblastoma multiforme are seen at the top. The 6-year history of symptoms attested to the initially low-grade nature of this astrocytic tumor.
-
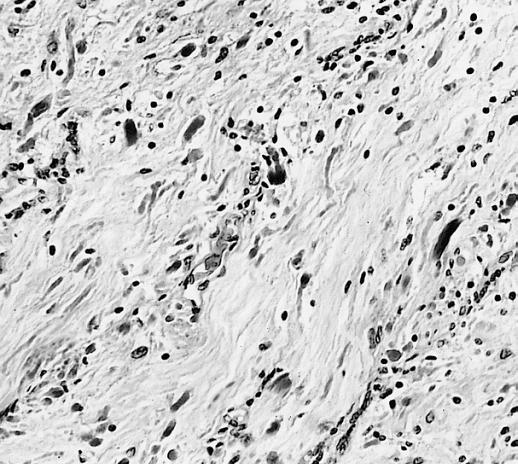
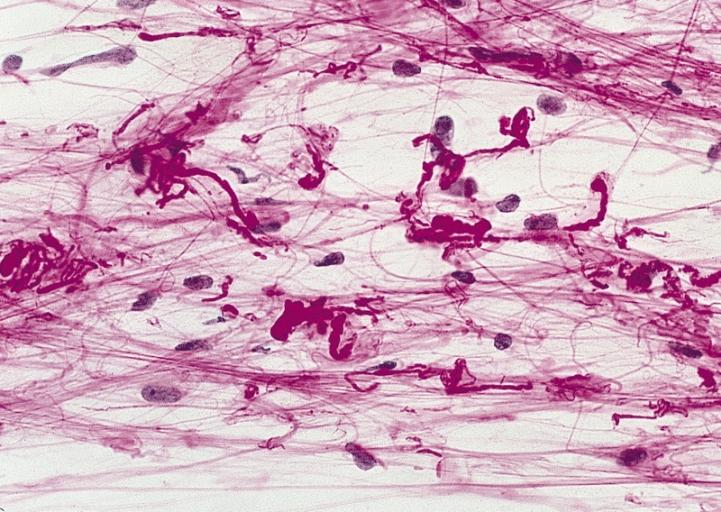
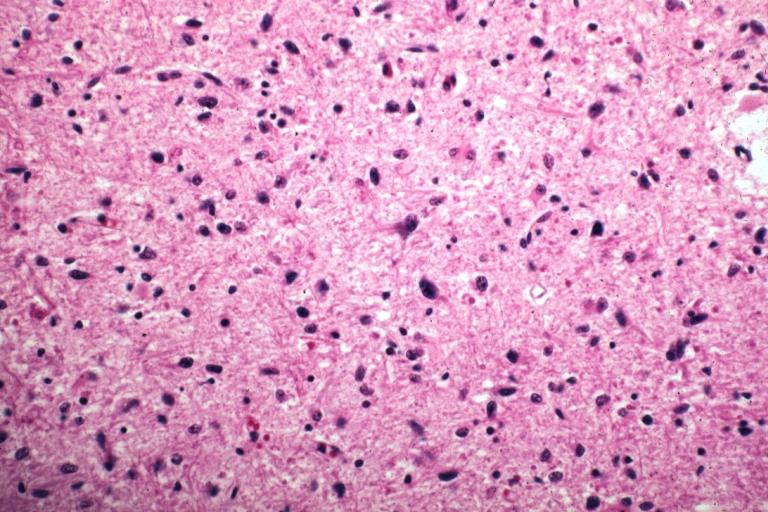
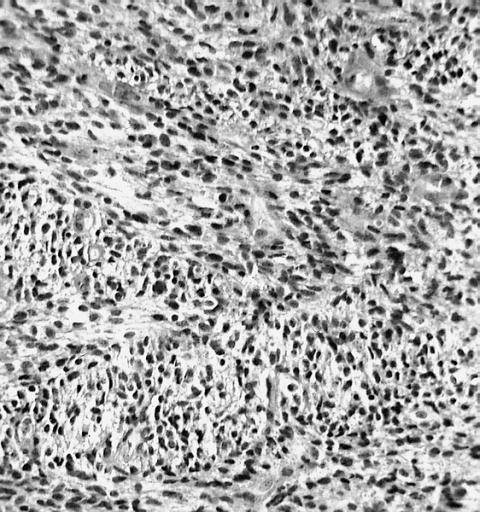
CNS: Glioblastoma multiforme; Characteristic of most glioblastomas are small cells with elongated nuclei and bipolar processes. As here, the chromatin is generally not markedly dense nor are nucleoli usually prominent.
-
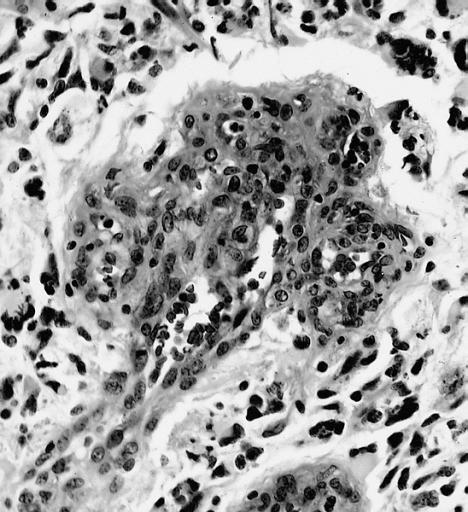
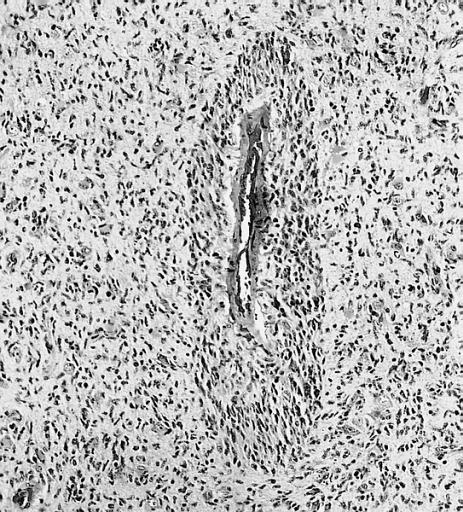
CNS: Glioblastoma multiforme; Vascular proliferation, a common feature of glioblastoma, produces tufts which often grow directionally. Here, as is often the case, they are oriented toward a focus of necrosis (top right).
-
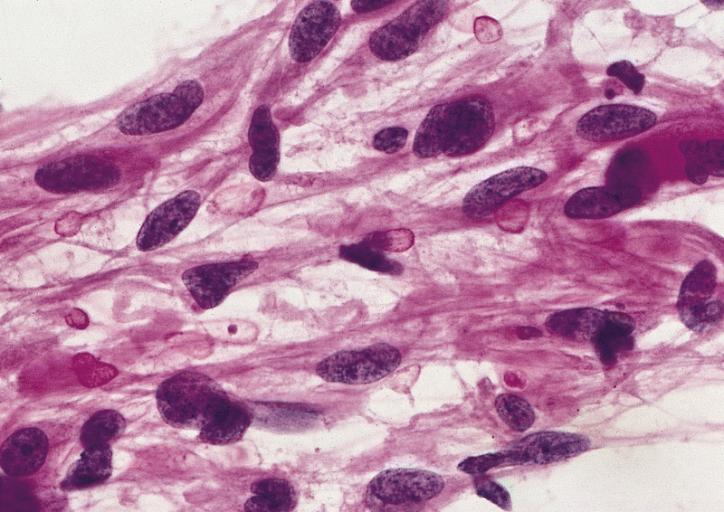
CNS: Glioblastoma multiforme; At high magnification, the neovascular tuft is a mass which, as can be confirmed by immunohistochemistry, is formed of both endothelial cells and smooth muscle cells (pericytes).
-
CNS: Glioblastoma multiforme; In many instances, necrosis is surrounded by a distinctive collar of cells, which are often smaller than those in surrounding neoplastic tissue. The phenomenon is referred to as pseudopalisading.
-
CNS: Cerebrospinal dissemination of glioblastoma multiforme; As seen at low (left) and high (right) magnification, the small undifferentiated-appearing cells of this glioblastoma are drop metastases colonizing the nerve roots of the cauda equina.
-
CNS: Cerebrospinal dissemination of glioblastoma multiforme; As seen at low (left) and high (right) magnification, the small undifferentiated-appearing cells of this glioblastoma are drop metastases colonizing the nerve roots of the cauda equina.
-
CNS: Glioblastoma multiforme; Higher magnification reveals the small cell nature of such tumors.
-
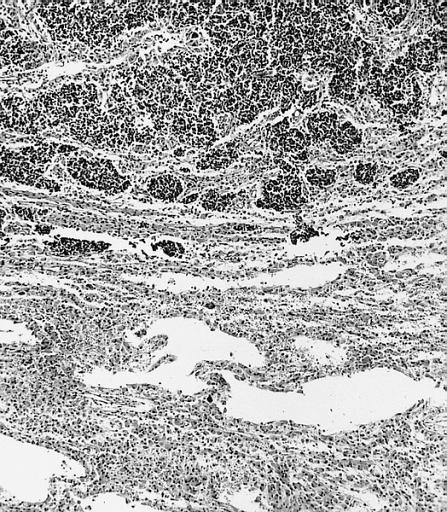
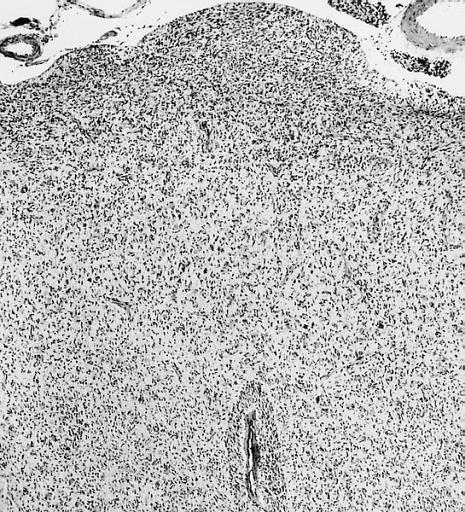
CNS: Glioblastoma multiforme; Some glioblastomas are especially infiltrative of the cerebral cortex where subpial, perivascular, and perineuronal accumulations are prominent.
-
CNS: Glioblastoma multiforme; Although this densely cellular and largely undifferentiated lesion technically merits a diagnosis of anaplastic astrocytoma, it is, for practical purposes, a glioblastoma.
-
Brain: Glioblastoma multiforme; pallisading
-
Brain: Glioblastoma multiforme; vascular proliferation
-
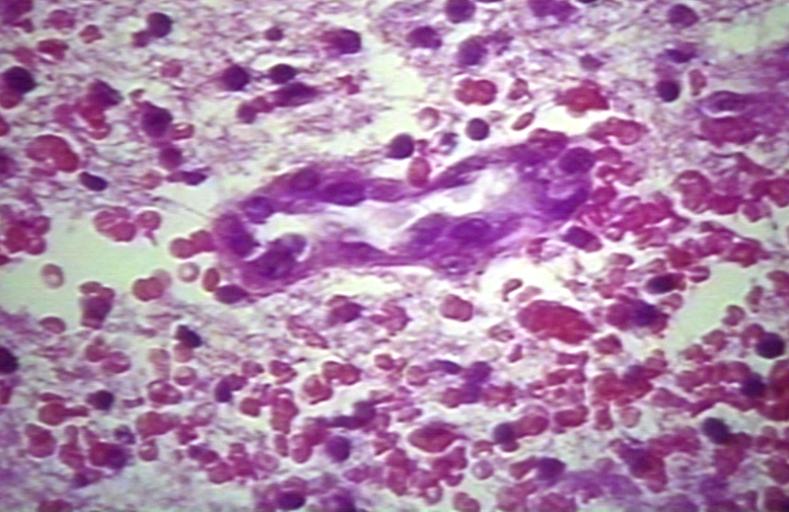
Brain: Glioblastoma multiforme; Plump and juicy endothelial cells, extravasated blood
-
Brain: Glioblastoma multiforme; perivascular lymphocytes
-
Brain: Glioblastoma multiforme; thrombosed vessel
-
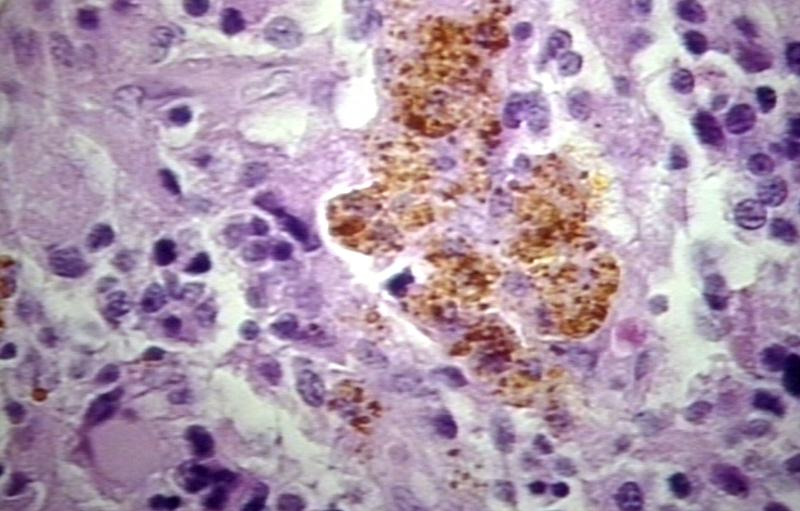
Brain: Glioblastoma multiforme; Hemosiderin in glioblastoma
Gross Images
-
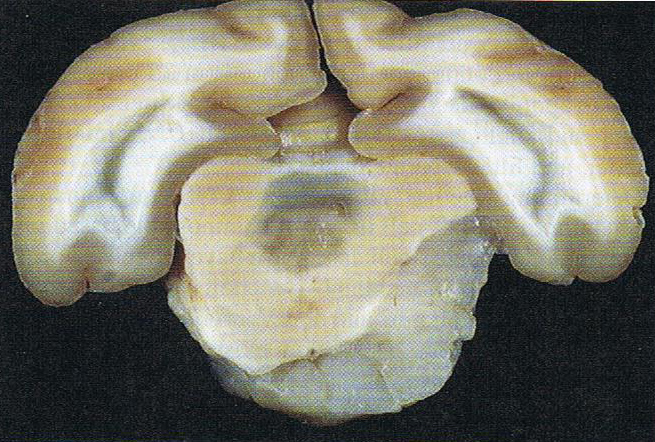
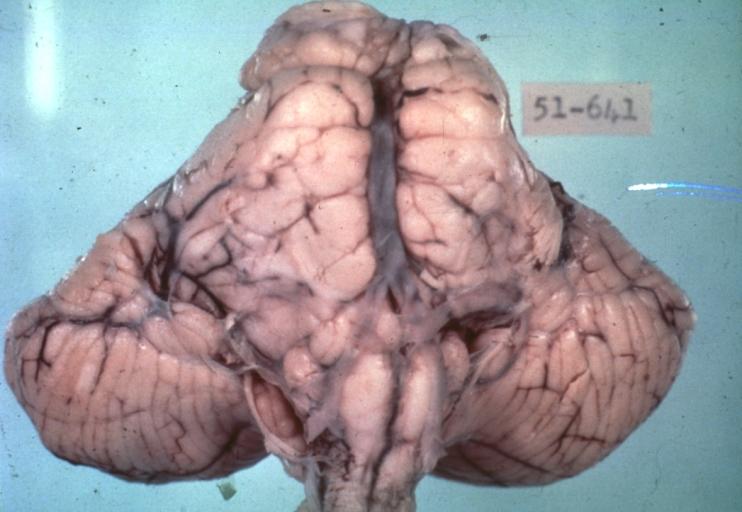
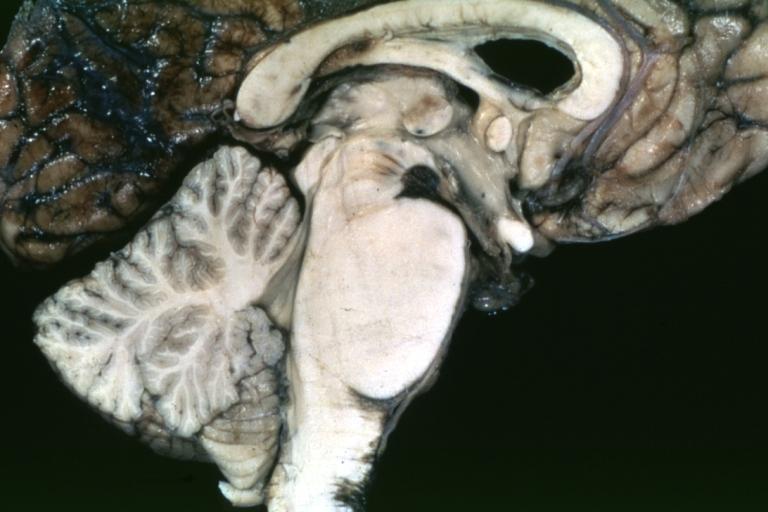
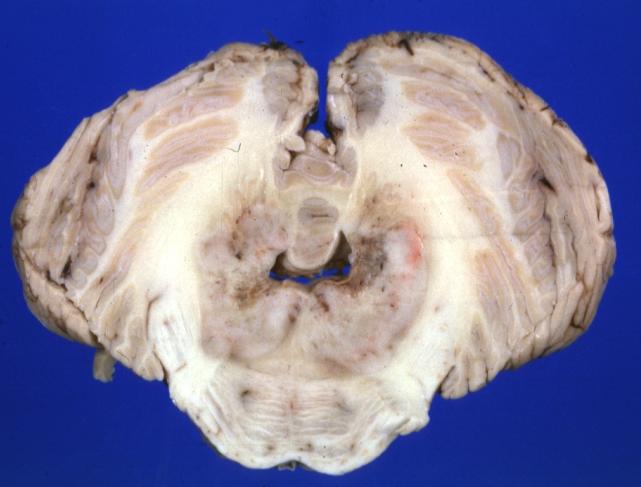
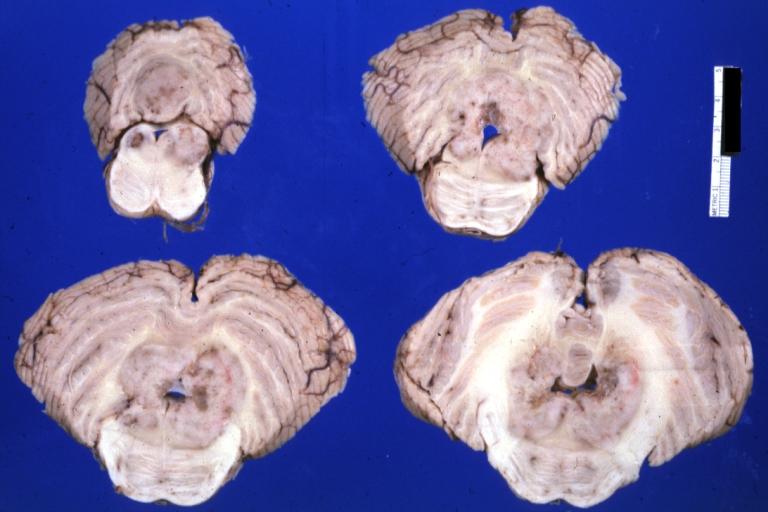
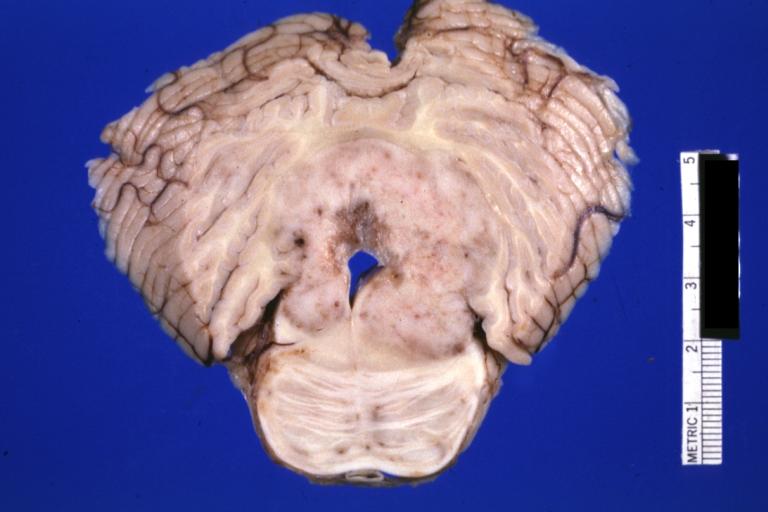
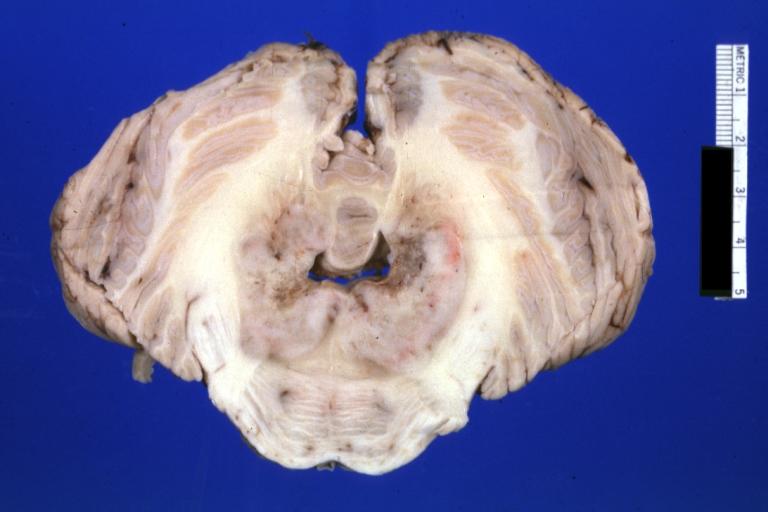
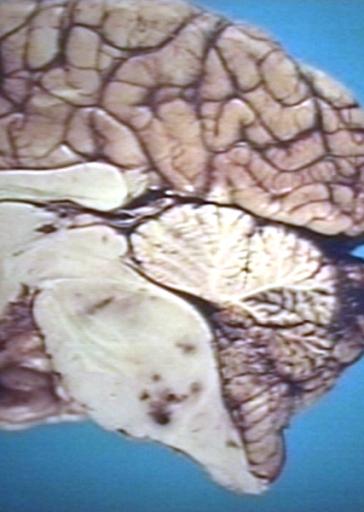
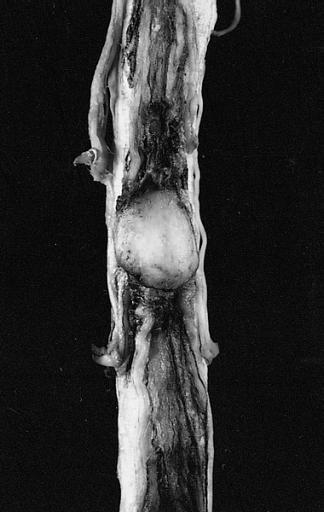
Brain: Pontine Glioma: Gross; fixed tissue, anterior view of brain stem and cerebellum with bosselated tumor adjacent to basilar artery
-
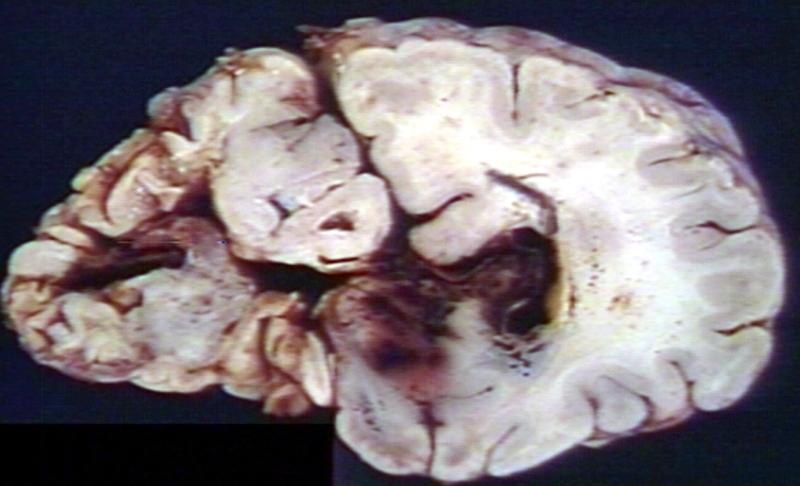
Brain: Pontine Glioma: Gross; fixed tissue, sagittal section brain stem and cerebellum
-
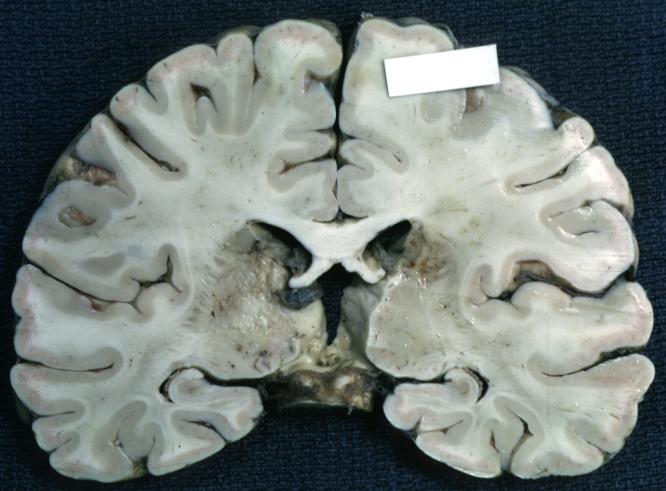
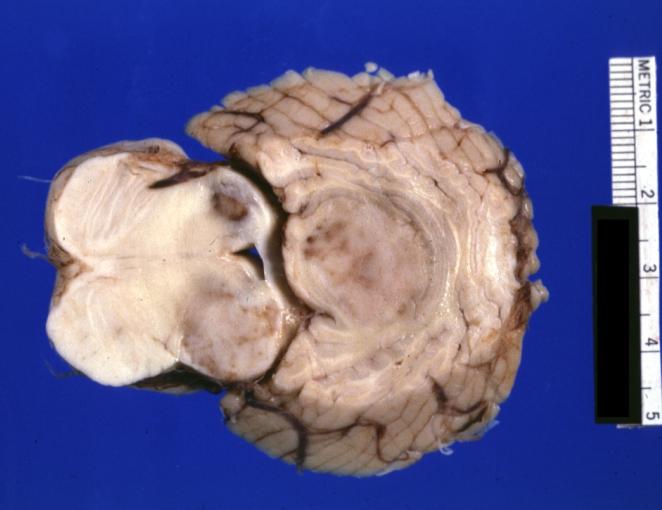
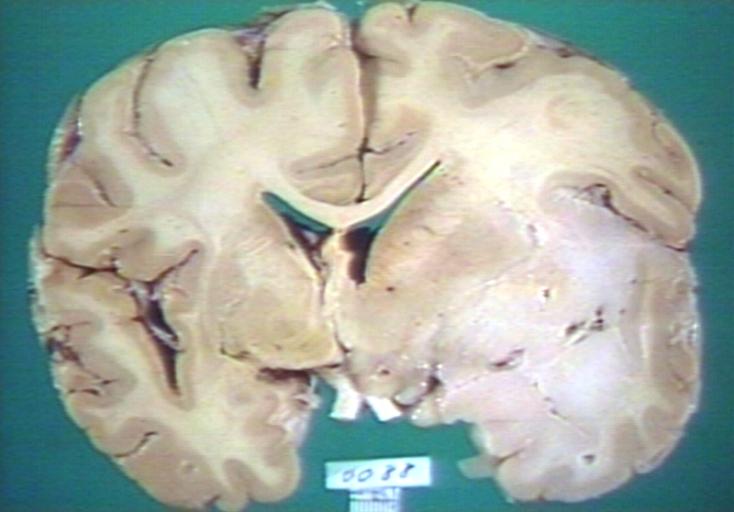
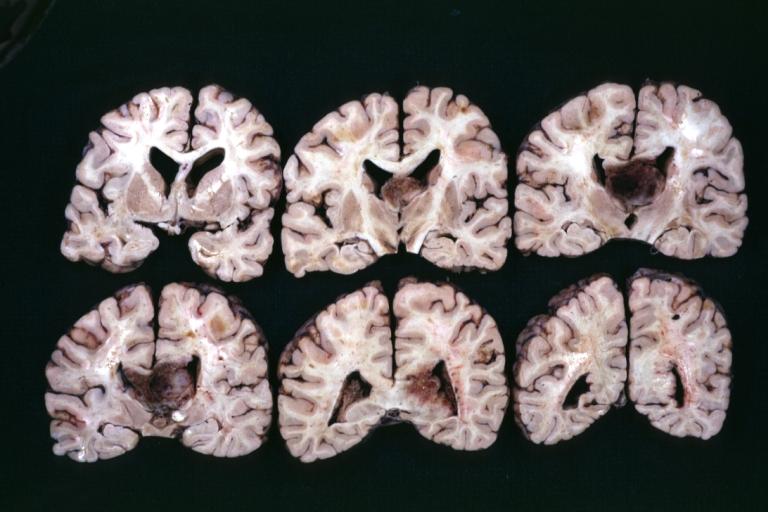
Brain: Glioma: Gross; fixed tissue, horizontal section brain stem and cerebellum with obvious gelatinous appearing neoplasm a pontine glioma
-
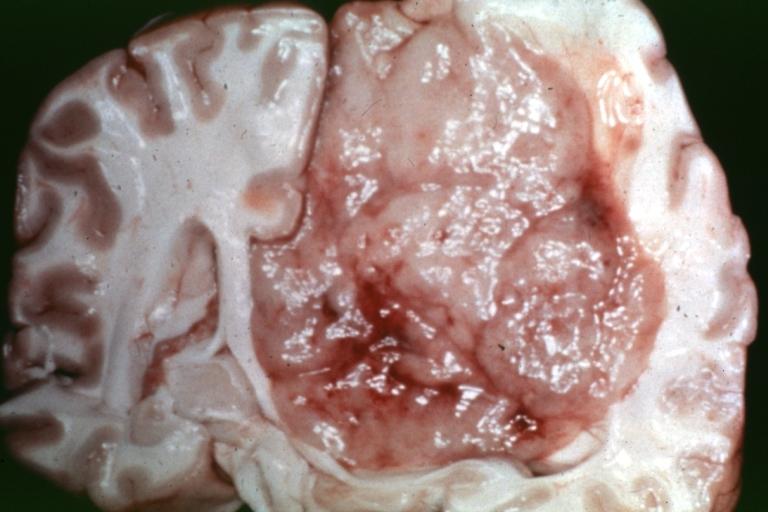
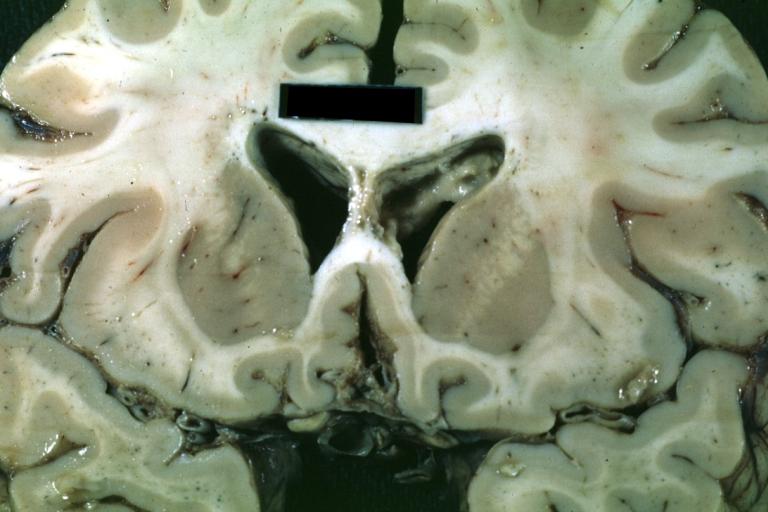
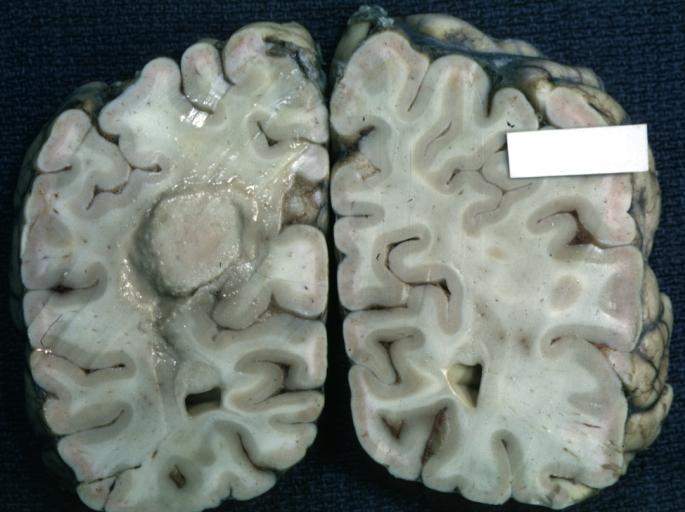
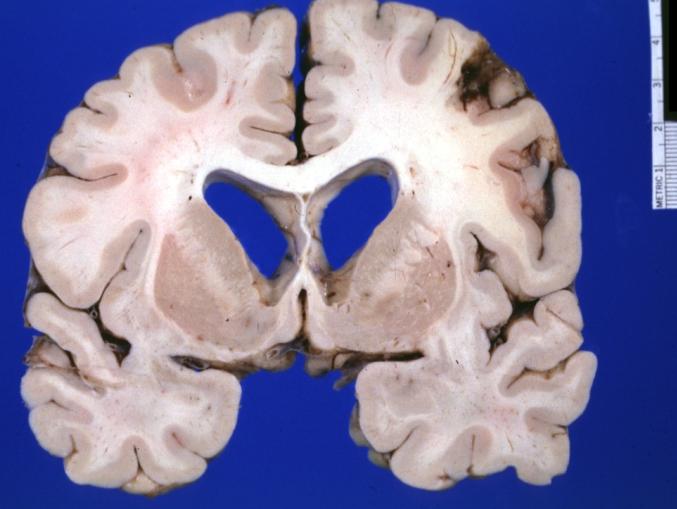
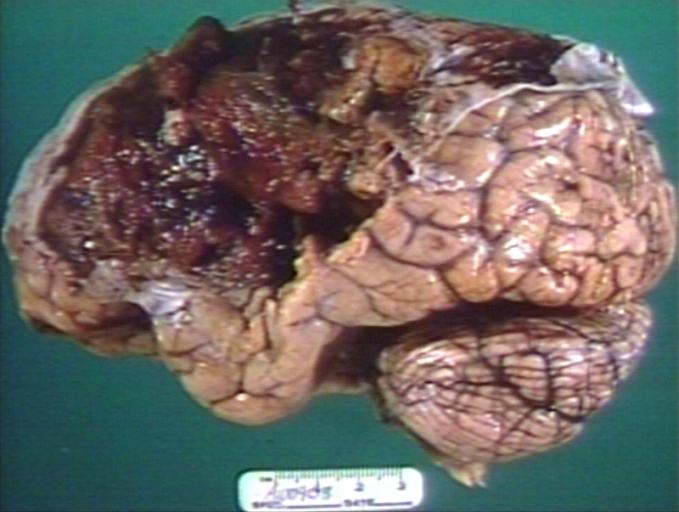
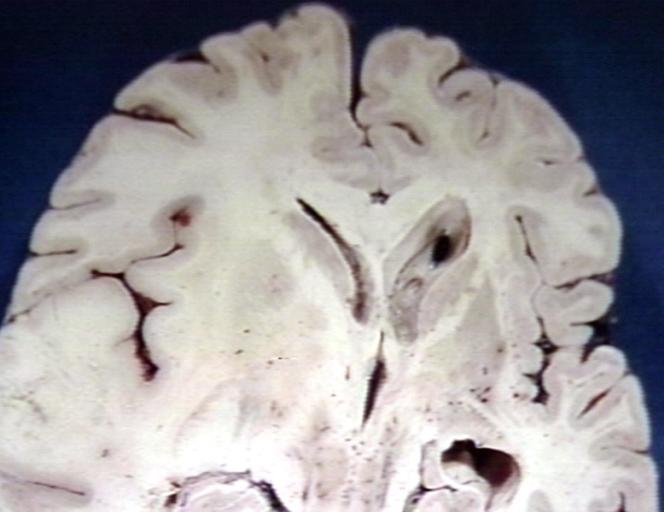
Brain: Oligodendroglioma: Gross; natural color, large, well circumscribed lesion in left frontal lobe
-
Brain: Glioma: Gross; fixed tissue, horizontal sections brain stem and cerebellum showing large pontine glioma
-
Brain: Pontine Glioma and Diffuse Meningeal Gliomatosis: Gross; fixed tissue, view of cerebral hemispheres from inferior with brain stem and cerebellum removed. Pontine asymmetry is easily seen due to low grade astrocytoma and meningeal gliomatosis is easily seen over frontal lobes
-
Brain: Pontine Glioma and Diffuse Meningeal Gliomatosis in 7 yo boy: Gross; fixed tissue, view of cerebral hemispheres from vertex meningeal gliomatosis.
-
Brain: Pontine Glioma and Diffuse Meningeal Gliomatosis: Gross; in situ dural nodule
-
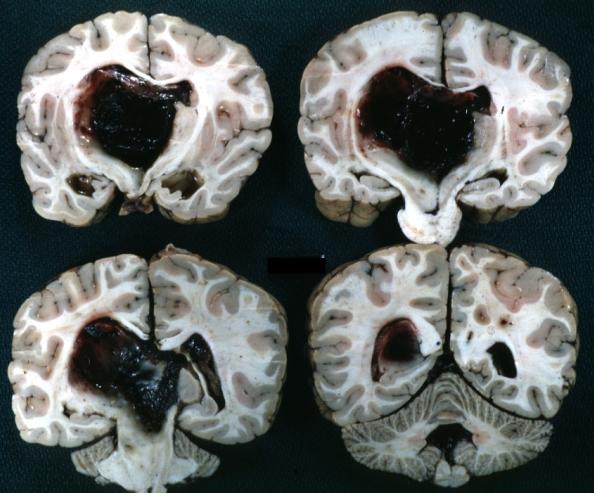
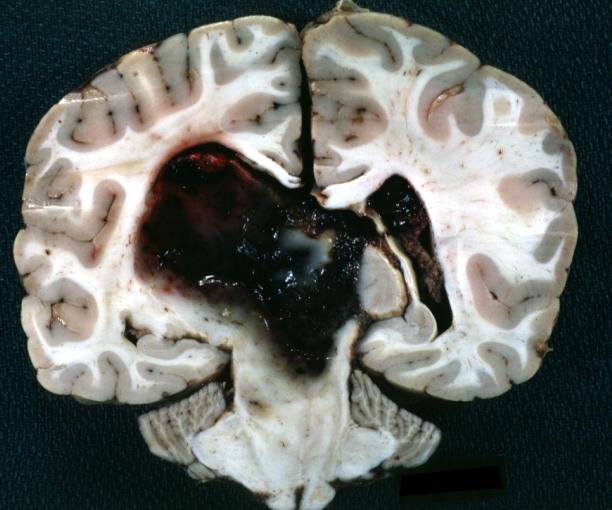
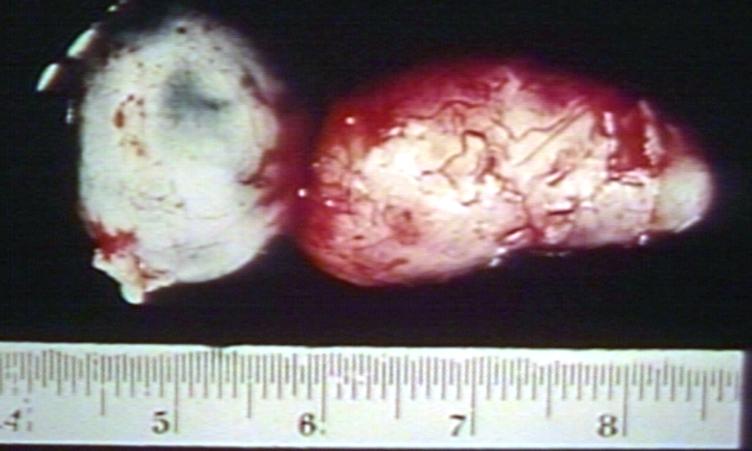
Brain: Oligodendroglioma: Gross; fixed tissue, multiple coronal sections, cerebral hemispheres with large tumor and hemorrhage into tumor
-
Brain: Oligodendroglioma: Gross; fixed tissue, coronal section, cerebral hemispheres, large hemorrhagic lesion in one hemisphere
-
Brain: Oligodendroglioma: Gross; fixed tissue, ischemic tissue, anterior to tumor mass
-
Brain: Oligodendroglioma: Gross; natural color, coronal section, cerebral hemispheres, large lesion, left parieto occipital white matter
-
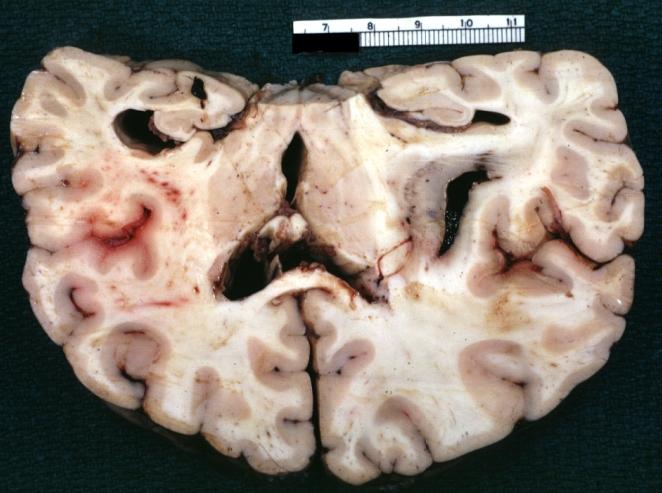
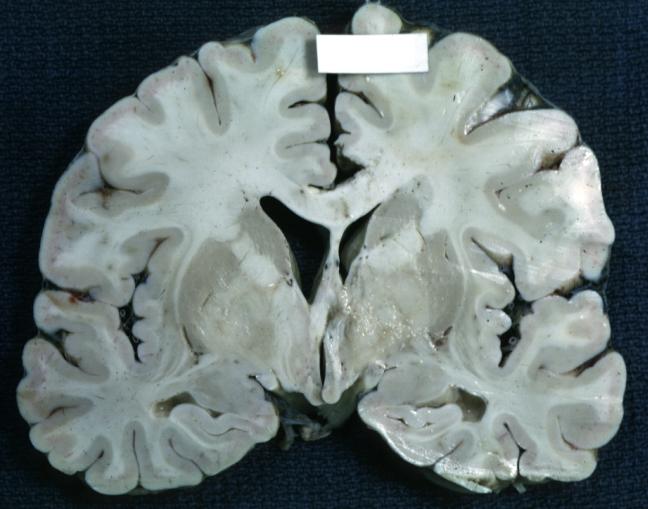
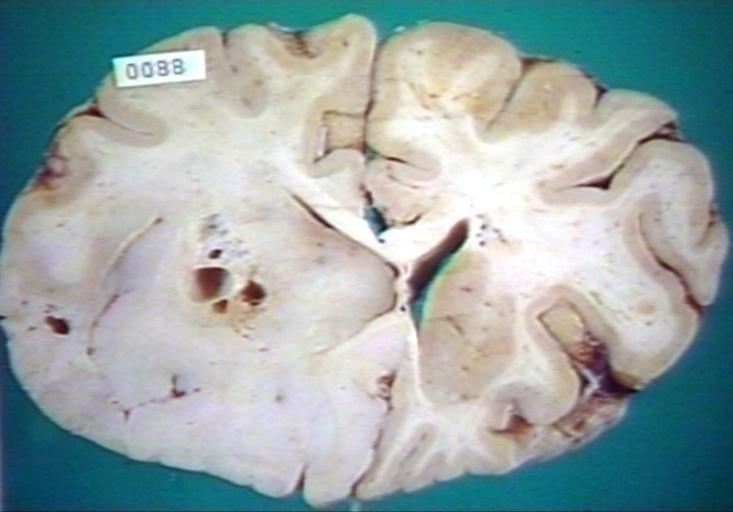
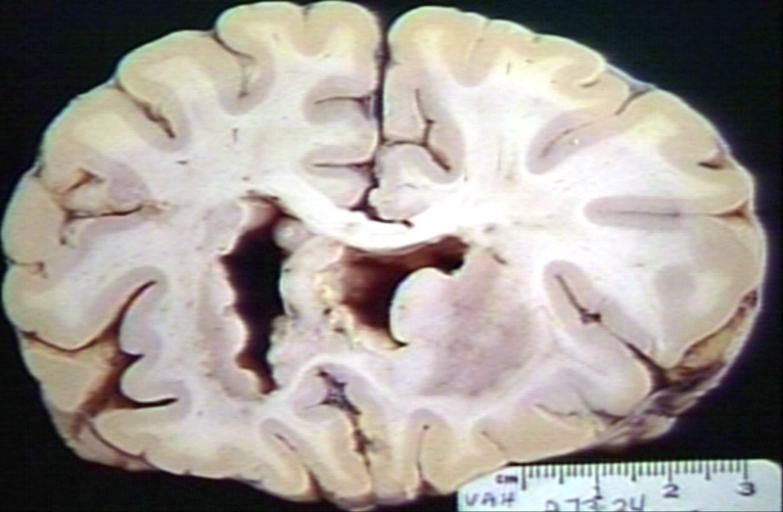
Brain: Gliomatosis Cerebri: Gross; fixed tissue, coronal sections, cerebral hemispheres, lesion is in temporal lobes and hypothalamus
-
Brain: Ventriculitis: Gross; fixed tissue, case of glioma with meningitis, a nice view of ventriculitis in one lateral ventricle
-
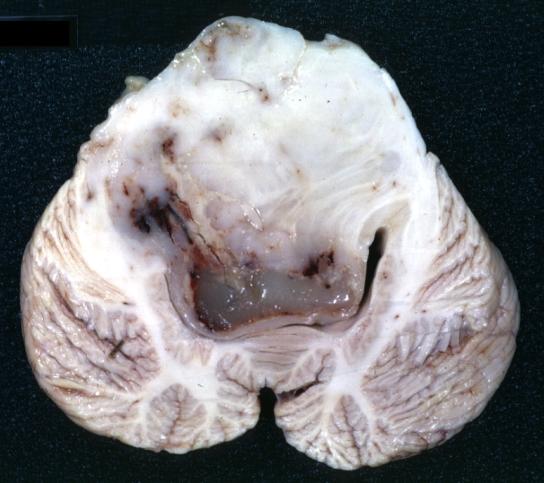
Brain: Glioma Thalamic Grade Ii-Iii: Gross; fixed tissue, four coronal sections, cerebral hemispheres, very large hemorrhagic lesion
-
Brain: Glioma Thalamic Grade Ii-Iii: Gross; fixed tissue, coronal section, cerebral hemispheres with large hemorrhagic lesion
-
Brain: Glioma Thalamic Grade Ii-Iii: Gross fixed tissue coronal section cerebral hemispheres lesions appears to be in choroid plexus of lateral ventricle in this picture. There is blood in fourth ventricle
-
Brain: Cerebral Sarcoma or Microglioma: Gross; fixed tissue, coronal section, cerebral hemispheres (58 yo man)
-
Brain: Cerebral Sarcoma or Microglioma: Gross; fixed tissue, coronal section, cerebral hemispheres
-
Brain: Cerebral Sarcoma or Microglioma: Gross; fixed tissue, coronal section, cerebral hemispheres
-
Brain: Infarct Subcortical: Gross; fixed tissue, close-up view of old small subcortical infarct, a case of microglioma
-
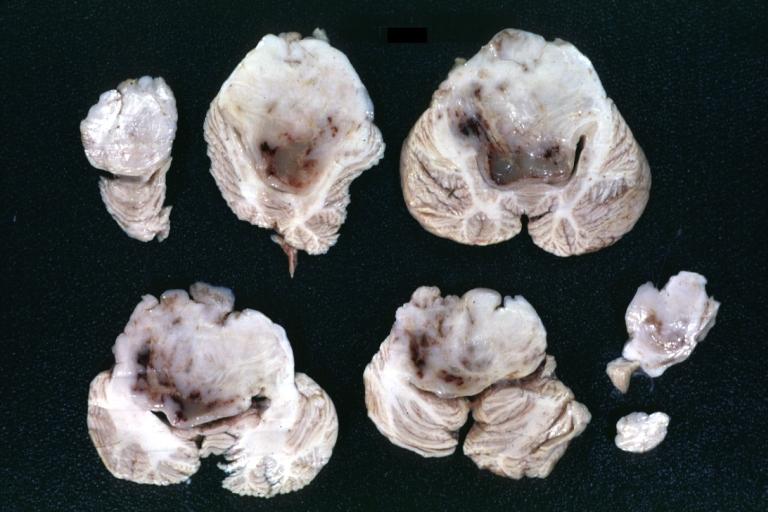
Brain: Microglioma: Gross; fixed tissue; cerebellum and fourth ventricle with periventricular tumor invasion
-
Brain: Microglioma: Gross fixed tissue horizontal sections cerebellum and brain stem with periventricular neoplastic infiltrate
-
Brain: Microglioma: Gross fixed tissue horizontal section midbrain and cerebellum at mid pons level periventricular tumor infiltration
-
Brain: Microglioma: Gross fixed tissue horizontal section rostral pons and cerebellum
-
Brain: Microglioma: Gross fixed tissue horizontal section rostral pons and cerebellum periventricular tumor invasion
-
Brain: Microglioma: Gross fixed tissue coronal section cerebral hemispheres with mild ventricular dilation
-
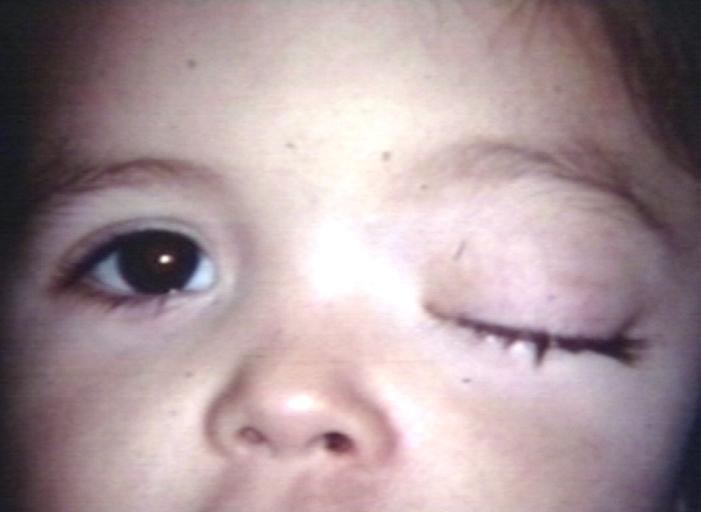
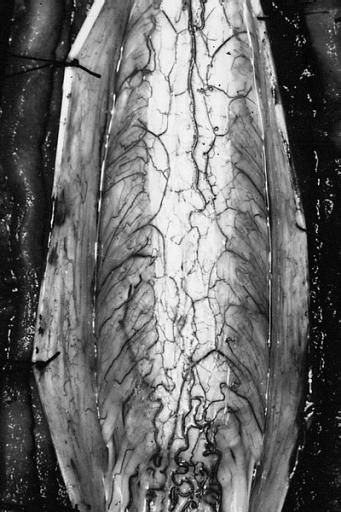
Glioma: Optic Nerve
-
Brain: Oligodendroglioma, Frontal Lobe
-
Brain: Oligodendroglioma, Mixed Astrocytoma & Oligodendroglioma
-
Brain: Glioma, Grade II Anaplastic
-
Brain: Glioma, Brain stem, Low Grade
-
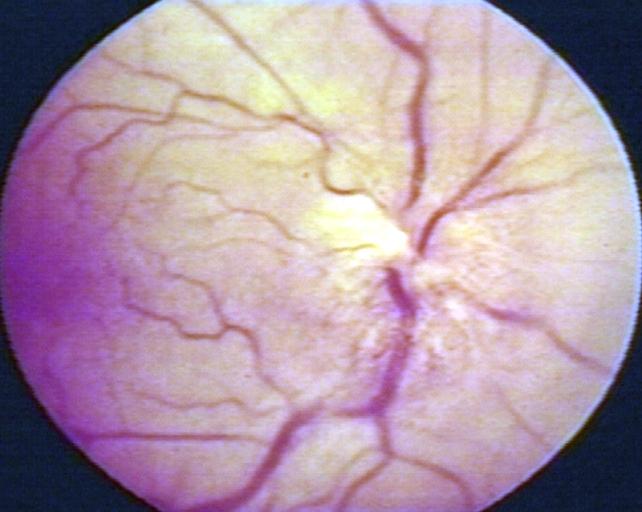
Fundoscopy: Eye; Optic Nerve Glioma, Optic Nerve
-
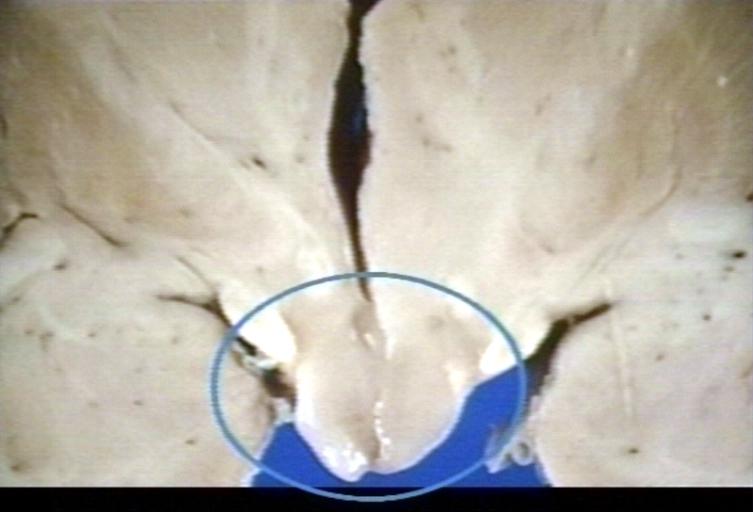
Brain: Glioma, Hypothalamic, Circle Around Region of Tumor
-
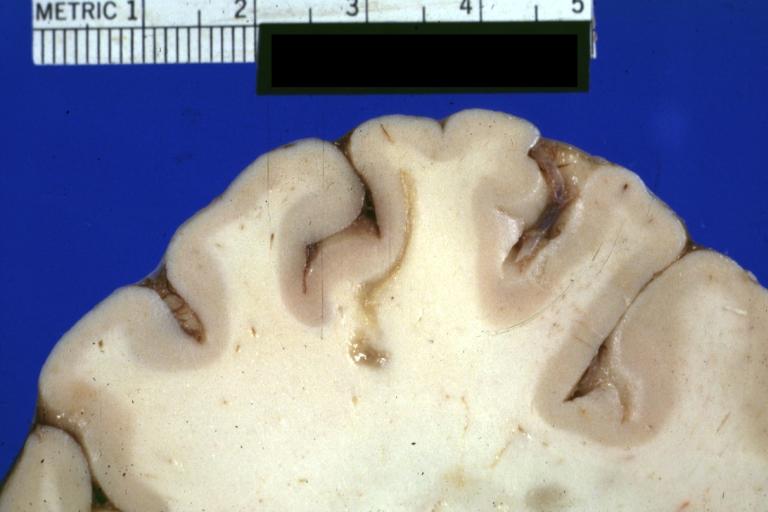
CNS: Pilocytic Astrocytoma of the Spinal Cord. The fusiform expansion of the spinal cord produced by this pilocytic astrocytoma is not, on external examination alone, distinguishable from that produced by a nonresectable diffuse glioma.
-
Brain: Glioma, Pontine
-
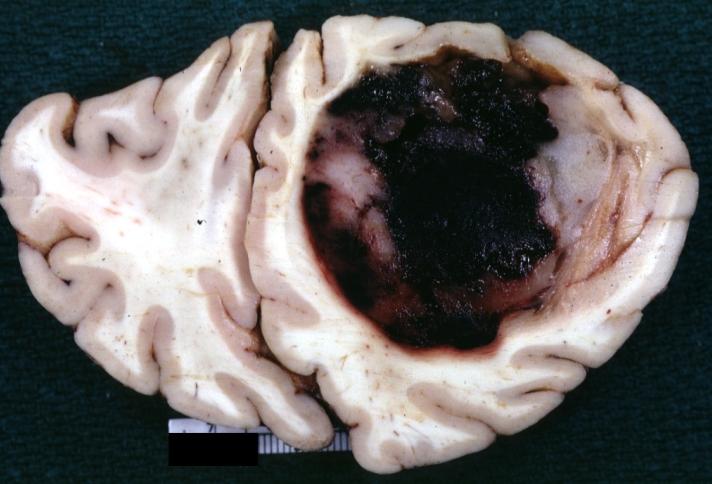
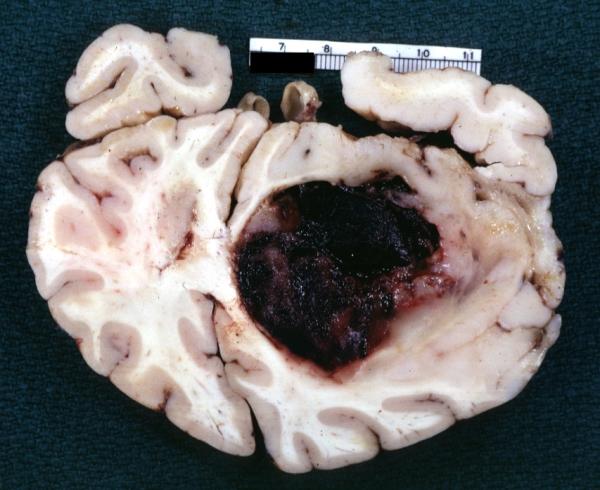
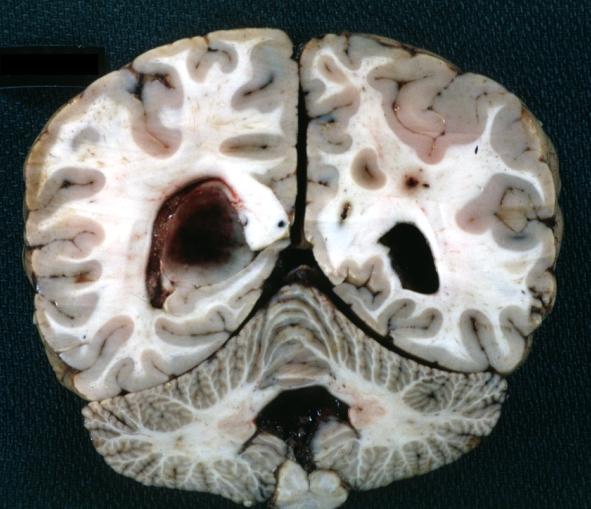
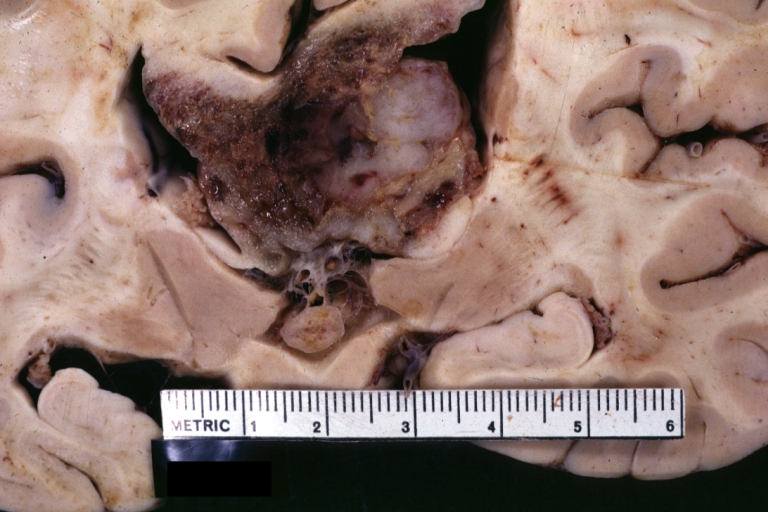
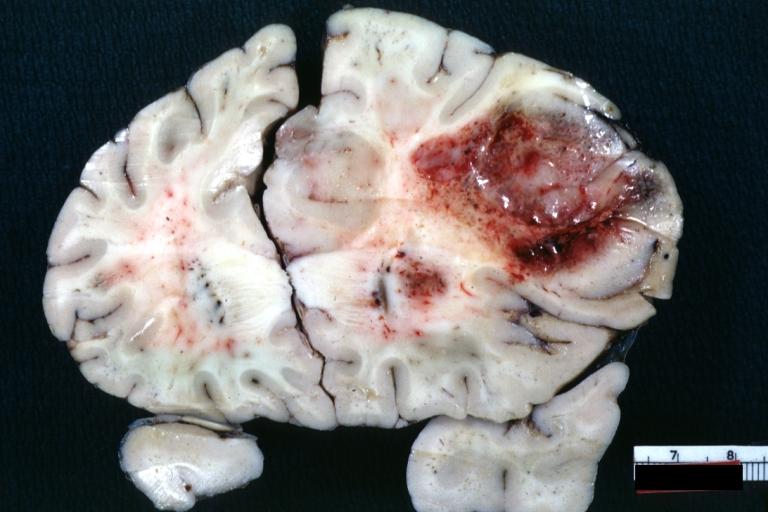
Brain: Glioblastoma Multiforme: Gross fixed tissue close-up large necrotic tumor mass in septum pellucidum
-
Brain: Glioblastoma Multiforme: Gross fixed tissue coronal section of the brain with a large necrotic tumor mass in septum pellucidum diagnosed as astrocytoma grade III
-
Brain: Glioblastoma Multiforme: Gross natural color large hemorrhagic lesion in right centrum semiovale
-
CNS: Malignant pilocytic astrocytoma: A 29-year-old woman died 2 years after a diagnosis of "atypical pilocytic astrocytoma" of the pineal region. At autopsy, multiple tumor implants were present in the craniospinal subarachnoid spaces.
References
- ↑ Mamelak A.N., and Jacoby, D.B. Targeted delivery of antitumoral therapy to glioma and other malignancies with synthetic chlorotoxin (TM-601) Expert Opin. Drug Drliv. (2007) 4(2):175-186.
- ↑ Gromeier M, Wimmer E (2001). "Viruses for the treatment of malignant glioma". Curr. Opin. Mol. Ther. 3 (5): 503–8. PMID 11699896.
- ↑ Rainov N, Ren H (2003). "Gene therapy for human malignant brain tumors". Cancer journal (Sudbury, Mass.). 9 (3): 180–8. PMID 12952303.
Recent Publications
- Su Y, Zhang X, Gu J, Zhang C, Tian Z, Zhang J. JSI-124 Inhibits Glioblastoma Multiforme Cell Proliferation through G2/M Cell Cycle Arrest and Apoptosis Augment. Cancer Biol Ther. 2008 May 10;7(8). [Epub ahead of print] PMID 18487947 [PubMed - as supplied by publisher]
- Emblem KE, Nedregaard B, Nome T, Due-Tonnessen P, Hald JK, Scheie D, Borota OC, Cvancarova M, Bjornerud A. Glioma grading by using histogram analysis of blood volume heterogeneity from MR-derived cerebral blood volume maps. Radiology. 2008 Jun;247(3):808-17. PMID 18487536 [PubMed - in process]
- Capuani S, Porcari P, Fasano F, Campanella R, Maraviglia B. (10) B-editing (1) H-detection and (19) F MRI strategies to optimize boron neutron capture therapy. Magn Reson Imaging. 2008 May 15. [Epub ahead of print] PMID 18486394 [PubMed - as supplied by publisher]
- Chung IS, Son YI, Ko YJ, Baek CH, Cho JK, Jeong HS. Peritumor injections of purified tumstatin delay tumor growth and lymphatic metastasis in an orthotopic oral squamous cell carcinoma model. Oral Oncol. 2008 May 15. [Epub ahead of print] PMID 18485794 [PubMed - as supplied by publisher]
- Ahmed AE, Jacob S, Nagy AA, Abdel-Naim AB. Dibromoacetonitrile-induced protein oxidation and inhibition of proteasomal activity in rat glioma cells. Toxicol Lett. 2008 Apr 8. [Epub ahead of print] PMID 18485629 [PubMed - as supplied by publisher]
External links
- Template:GPnotebook
- Treatment Options for Glioblastoma and other Gliomas (.pdf format)
- German Brain Tumor Association
- WHO Classification
- Experimental Anti-cancer Drug Kills Brain Tumor Stem Cells (Science Daily)
- Statin Plus Cancer Drug Deliver Combo Punch to Brain Cancer Cells (Medical News Today, Jan 2007)
Template:Tumors Template:Epithelial neoplasms Template:Nervous tissue tumors Template:SIB